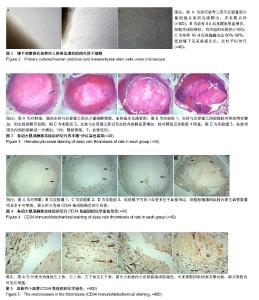
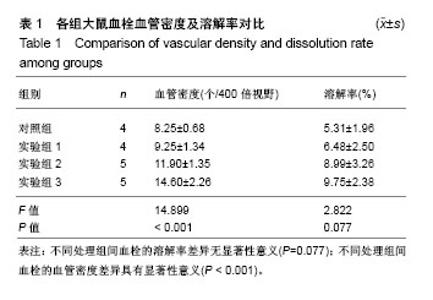
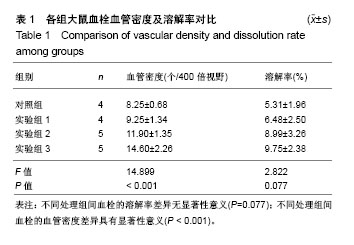
| [1] Vedovetto V, Dalla Valle F, Milan M, et al. Residual vein thrombosis and trans-popliteal reflux in patients with and without the post-thrombotic syndrome. Thromb Haemost. 2013;110(4):854-855.[2] Sarolo L, Turatti G, Milan M, et al. Trans-popliteal reflux in limbs with and without deep-vein thrombosis of the same subject: cross-sectional study. Thromb Res. 2017;154:53-54.[3] Altmann J, Sharma S, Lang IM. Advances in our understanding of mechanisms of venous thrombus resolution. Expert Rev Hematol. 2016;9(1):69-78.[4] Mukhopadhyay S, Johnson TA, Sarkar R, et al. Serpins in venous thrombosis and venous thrombus resolution. Methods Mol Biol. 2018;1826:197-211.[5] Schonfelder T, Brandt M, Kossmann S,et al. Lack of T-bet reduces monocytic interleukin-12 formation and accelerates thrombus resolution in deep vein thrombosis. Sci Rep. 2018; 8(1):3013.[6] McGuinness CL, Humphries J, Waltham M, et al. Recruitment of labelled monocytes by experimental venous thrombi. Thromb Haemost. 2001;85(6):1018-1024.[7] Modarai B, Burnand KG, Humphries J, et al. The role of neovascularisation in the resolution of venous thrombus. Thromb Haemost. 2005;93(5):801-809.[8] Li WD, Li XQ. Endothelial progenitor cells accelerate the resolution of deep vein thrombosis. Vascul Pharmacol. 2016; 83:10-16.[9] Evans CE, Grover SP, Humphries J, et al. Antiangiogenic therapy inhibits venous thrombus resolution. Arterioscler Thromb Vasc Biol. 2014;34(3):565-570.[10] Choi M, Lee HS, Naidansaren P, et al. Proangiogenic features of Wharton's jelly-derived mesenchymal stromal/stem cells and their ability to form functional vessels. Int J Biochem Cell Biol. Mar 2013;45(3):560-570.[11] Gorkun AA, Shpichka AI, Zurina IM, et al. Angiogenic potential of spheroids from umbilical cord and adipose-derived multipotent mesenchymal stromal cells within fibrin gel. Biomed Mater. 22 2018;13(4):044108.[12] Boroujeni ME, Gardaneh M. Umbilical cord: an unlimited source of cells differentiable towards dopaminergic neurons.Neural Regen Res. 2017;12(7):1186-1192.[13] Bullard JD, Lei J, Lim JJ, et al. Evaluation of dehydrated human umbilical cord biological properties for wound care and soft tissue healing. J Biomed Mater Res B Appl Biomater. 2018. doi: 10.1002/jbm.b.34196.[14] Rabbani S, Soleimani M, Sahebjam M, et al. Simultaneous delivery of wharton's jelly mesenchymal stem cells and insulin-like growth factor-1 in acute myocardial infarction. Iran J Pharm Res. 2018;17(2):426-441.[15] Yousefifard M, Nasirinezhad F, Shardi Manaheji H, et al. Human bone marrow-derived and umbilical cord-derived mesenchymal stem cells for alleviating neuropathic pain in a spinal cord injury model. Stem Cell Res Ther. 2016;7:36.[16] 徐海环,董化江,商崇智,等.脐带间充质干细胞移植急性脑创伤大鼠损伤区域微血管密度的变化[J].中国组织工程研究,2017, 21(33):5320-5324.[17] Yaghoobi K.Neural regeneration after spinal cord injury treatment by lavandula angustifolia and human umbilical mesanchymal stem cell transplantation.Neural Regen Res. 2017;12(1):68-69.[18] Li XY, Zheng ZH, Li XY, et al. Treatment of foot disease in patients with type 2 diabetes mellitus using human umbilical cord blood mesenchymal stem cells: response and correction of immunological anomalies. Curr Pharm Des. 2013;19(27): 4893-4899.[19] 李茂,游成东,黄文.人脐带间充质干细胞移植治疗小鼠皮肤溃疡[J].中国组织工程研究,2018,22(33):5315-5320.[20] 杨晓清,张沐,杨兵,等.人脐带华通氏胶间充质干细胞的分离、培养、鉴定及冻存、复苏[J].南通大学学报(医学版),2010,30(6): 413-415, 419.[21] 白云城,赵学凌,周如丹,等.大鼠下腔静脉血栓模型的建立及手术技巧[J].南京医科大学学报(自然科学版),2014,34(3):394-399.[22] Alias S, Redwan B, Panzenboeck A, et al. Defective angiogenesis delays thrombus resolution: a potential pathogenetic mechanism underlying chronic thromboembolic pulmonary hypertension. Arterioscler Thromb Vasc Biol. Apr 2014;34(4):810-819.[23] Wakefield TW, Linn MJ, Henke PK, et al. Neovascularization during venous thrombosis organization: a preliminary study. J Vasc Surg. 1999;30(5):885-892.[24] Chabasse C, Siefert SA, Chaudry M, et al. Recanalization and flow regulate venous thrombus resolution and matrix metalloproteinase expression in vivo. J Vasc Surg Venous Lymphat Disord. 2015;3(1):64-74.[25] 付健,唐博,陈以宽,等.新型大鼠下腔静脉血栓模型的建立及血栓溶解演变过程研究[J].解放军医学杂志,2015,40(8): 610-615.[26] Aguilera V, Briceno L, Contreras H, et al. Endothelium trans differentiated from Wharton's jelly mesenchymal cells promote tissue regeneration: potential role of soluble pro-angiogenic factors. PLoS One. 2014;9(11):e111025.[27] Kupcova Skalnikova H. Proteomic techniques for characterisation of mesenchymal stem cell secretome. Biochimie. 2013;95(12):2196-2211.[28] Au P, Tam J, Fukumura D, et al. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111(9):4551-4558.[29] Edwards SS, Zavala G, Prieto CP, et al. Functional analysis reveals angiogenic potential of human mesenchymal stem cells from Wharton's jelly in dermal regeneration. Angiogenesis. 2014;17(4):851-866.[30] Chen J, Liu Z, Hong MM, et al. Proangiogenic compositions of microvesicles derived from human umbilical cord mesenchymal stem cells. PLoS One. 2014;9(12):e115316.[31] Arutyunyan I, Fatkhudinov T, Kananykhina E, et al. Role of VEGF-A in angiogenesis promoted by umbilical cord-derived mesenchymal stromal/stem cells: in vitro study. Stem Cell Res Ther. 2016;7:46.[32] Zhu J, Liu Q, Jiang Y, et al. Enhanced angiogenesis promoted by human umbilical mesenchymal stem cell transplantation in stroked mouse is Notch1 signaling associated. Neuroscience. 2015;290:288-299.[33] Kamolz LP, Keck M, Kasper C. Wharton's jelly mesenchymal stem cells promote wound healing and tissue regeneration. Stem Cell Res Ther. 2014;5(3):62.[34] Francois S, Eder V, Belmokhtar K, et al. Synergistic effect of human bone morphogenic protein-2 and mesenchymal stromal cells on chronic wounds through hypoxia-inducible factor-1 alpha induction. Sci Rep. 2017;7(1):4272.[35] Mahmoudian-Sani MR, Rafeei F, Amini R, et al. The effect of mesenchymal stem cells combined with platelet-rich plasma on skin wound healing. J Cosmet Dermatol. 2018;17(5): 650-659. |