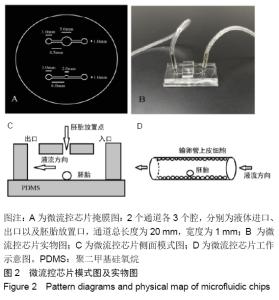
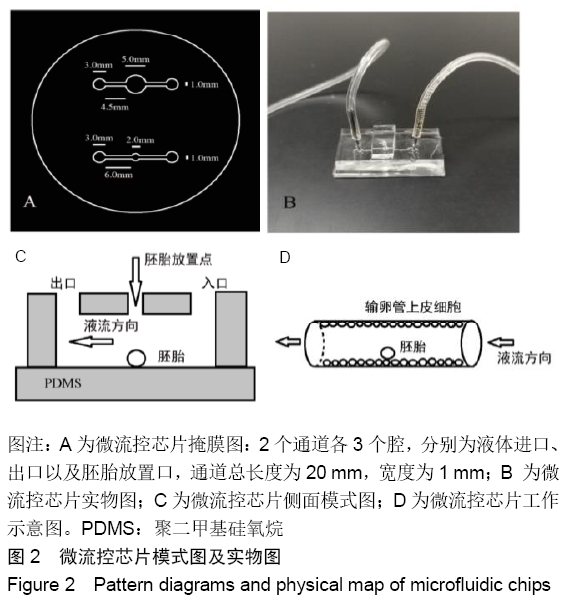
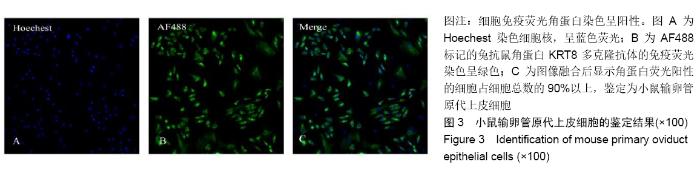
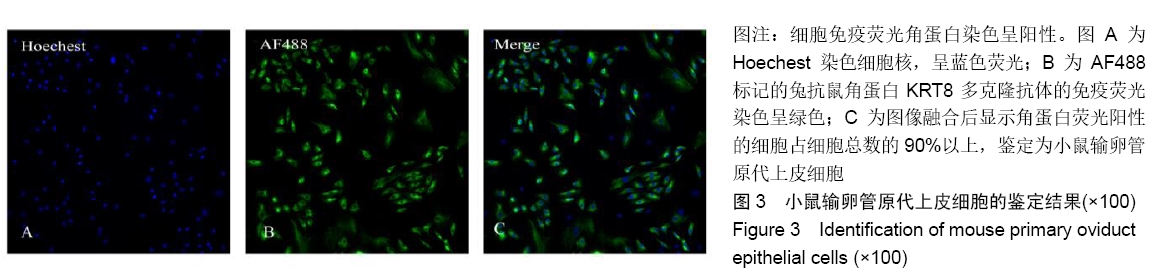
[1] POOL TB.An update on embryo culture for human assisted reproductive technology: media, performance, and safety. Semin Reprod Med.2005;23(4):309-318.
[2] KIDDER BL.In vitro maturation and in vitro fertilization of mouse oocytes and preimplantation embryo culture.Methods Mol Biol. 2014;1150:191-199.
[3] GARDNER DK, KELLEY RL.Impact of the IVF laboratory environment on human preimplantation embryo phenotype.J Dev Orig Health Dis. 2017;8(4):418-435.
[4] WALE PL, GARDNER DK.The effects of chemical and physical factors on mammalian embryo culture and their importance for the practice of assisted human reproduction. Hum Reprod Update. 2016;22(1):2-22.
[5] PICTON HM, ELDER K, HOUGHTON FD, et al. Association between amino acid turnover and chromosome aneuploidy during human preimplantation embryo development in vitro. Mol Hum Reprod. 2010;16(8):557-569.
[6] SUAREZ SS, WU M. Microfluidic devices for the study of sperm migration. Mol Hum Reprod. 2017;23(4):227-234.
[7] SMITH GD, TAKAYAMA S. Application of microfluidic technologies to human assisted reproduction. Mol Hum Reprod. 2017;23(4):257-268.
[8] WHEELER MB, RUBESSA M.Integration of microfluidics in animal in vitro embryo production.Mol Hum Reprod. 2017; 23(4):269.
[9] CHAVEZ SL. Microfluidics in reproductive biology: applying lab-on-a-chip technologies to assisted reproduction. Mol Hum Reprod. 2017;23(4):211-212.
[10] ROY TK, BRANDI S, TAPPE NM, et al. Embryo vitrification using a novel semi-automated closed system yields in vitro outcomes equivalent to the manual Cryotop method. Hum Reprod. 2014;29(11):2431-2438.
[11] LE GAC S, NORDHOFF V.Microfluidics for mammalian embryo culture and selection: where do we stand now? Mol Hum Reprod. 2017;23(4):213-226.
[12] KIMURA H, NAKAMURA H, AKAI T, et al. On-Chip Single Embryo Coculture With Microporous-Membrane-Supported Endometrial Cells. IEEE Trans Nanobioscience. 2009; 8(4): 318-324.
[13] HASHIMOTO S, KATO N, SAEKI K, et al.Selection of high-potential embryos by culture in poly(dimethylsiloxane) microwells and time-lapse imaging.Fertil Steril. 2012; 97(2): 332-337.
[14] GHERSEVICH S, MASSA E, ZUMOFFEN C. Oviductal secretion and gamete interaction. Reproduction. 2014;149(1): R1-R14.
[15] EZZATI M, DJAHANBAKHCH O, ARIAN S, et al.Tubal transport of gametes and embryos: a review of physiology and pathophysiology.J Assist Reprod Genet. 2014; 31(10): 1337-1347.
[16] MENEZO Y, GUERIN P.The mammalian oviduct: biochemistry and physiology.Eur J Obstet Gynecol Reprod Biol.1997;73(1):99-104.
[17] HUANG BK, CHOMA MA.Microscale imaging of cilia-driven fluid flow.Cell Mol Life Sci.2015;72(6):1095-1113.
[18] BUHI WC, ALVAREZ IM, KOUBA AJ. Secreted Proteins of the Oviduct. Cells Tissues Organs. 2000;166:165-179.
[19] BUHI WC. Characterization and biological roles of oviduct-specific, oestrogen-dependent glycoprotein. Reproduction.2002;123(3):355-362.
[20] MIKI K, CLAPHAM DE. Rheotaxis Guides Mammalian Sperm. Current Biology.2013;23(6):443-452.
[21] 赵晓娥,兰杰,王妍,等.小鼠输卵管上皮细胞的原代培养及纯化方法研究[J].动物医学进展,2009,30(2):30-34.
[22] 沈怡,毛跟红,高颖,等.改良人输卵管上皮细胞培养法及电镜观察[J]. 生殖医学杂志,2005,14(2):86-89.
[23] YEUNG WS, HO PC, LAU EY, et al.Improved development of human embryos in vitro by a human oviductal cell co-culture system.Hum Reprod. 1992; 7(8):1144-1149.
[24] JOSHI MS.Isolation, cell culture, and characterization of oviduct epithelial cells of the cow.Microsc Res Tech. 1995; 31(6):507-518.
[25] SUN TT, EICHNER R, NELSON WG, et al.Keratin classes: molecular markers for different types of epithelial differentiation.J Invest Dermatol.1983;81(1 Suppl): 109s-115s.
[26] STUMPTNER C, GOGG-KAMERER M, VIERTLER C, et al. Immunofluorescence and Immunohistochemical Detection of Keratins.Methods Enzymol. 2016;568(undefined):139-162.
[27] WHITESIDES GM, OSTUNI E, TAKAYAMA S, et al. Soft lithograpgy in biology and biochemistry. Annu Rev Biomed Eng.2001; 3: 335-373.
[28] ZHENG C, ZHAO L, CHEN GE, et al. Quantitative study of the dynamic tumor-endothelial cell interactions through an integrated microfluidic coculture system.Anal Chem. 2012; 84(4):2088-2093.
[29] ZHENG C, YU Z, ZHOU Y, et al. Live cell imaging analysis of the epigenetic regulation of the human endothelial cell migration at single-cell resolution. Lab Chip. 2012;12(17): 3063-3072.
[30] MESEGUER M, HERRERO J, TEJERA A, et al. The use of morphokinetics as a predictor of embryo implantation. Hum Reprod. 2011;10(26):2658-2671.
[31] LIU Y, CHAPPLE V, FEENAN K, et al. Time-lapse deselection model for human day 3 in vitro fertilization embryos: the combination of qualitative and quantitative measures of embryo growth. Fertil Steril. 2016;105(3):656-662.
[32] LONERGAN P, FAIR T.The ART of studying early embryo development: progress and challenges in ruminant embryo culture.Theriogenology. 2014;81(1):49-55.
[33] TEH WT, MCBAIN J, ROGERS P.What is the contribution of embryo-endometrial asynchrony to implantation failure? J Assist Reprod Genet. 2016;33(11):1419-1430.
[34] WHITE MD, ZENKER J, BISSIERE S, et al.How cells change shape and position in the early mammalian embryo.Curr Opin Cell Biol. 2017;44:7-13.
[35] MAILLO V, SÁNCHEZ-CALABUIG MJ, Lopera-Vasquez R, et al.Oviductal response to gametes and early embryos in mammals. Reproduction.2016;152(4):R127-R141.
[36] REFAAT B, LEDGER W. The expression of activins, their type II receptors and follistatin in human Fallopian tube during the menstrual cycle and in pseudo-pregnancy. Hum Reprod. 2011;26(12):3346-3354.
[37] ARGAÑARAZ ME, APICHELA SA, Miceli DC.LEFTY2 expression and localization in rat oviduct during early pregnancy. Zygote. 2012;20(1):53-60.
[38] SUAREZ SS.Mammalian sperm interactions with the female reproductive tract.Cell Tissue Res. 2016;363(1):185-194.
[39] LI S, Winuthayanon W.Oviduct: roles in fertilization and early embryo development.J Endocrinol. 2017;232(1):R1-R26.
[40] MÉNÉZO Y, GUÉRIN P, ELDER K.The oviduct: a neglected organ due for re-assessment in IVF.Reprod Biomed Online. 2015; 30(3):233-240.
[41] LIU J, PYNE DG, ABDELGAWAD M,et al.Appendix C: Automated Vitrification of Mammalian Embryos on a Digital Microfluidic Device.Methods Mol Biol. 2017;1568:309-316.
[42] HUANG HY, SHEN HH, TIEN CH, et al.Digital Microfluidic Dynamic Culture of Mammalian Embryos on an Electrowetting on Dielectric (EWOD) Chip.PLoS One. 2015; 10(5):e0124196.
[43] CORDOVA A, PERREAU C, UZBEKOVA S, et al. Development rate and gene expression of IVP bovine embryos cocultured with bovine oviduct epithelial cells at early or late stage of preimplantation development. Theriogenology. 2014;81(9):1163-1173.
[44] LEE SH, OH HJ, KIM MJ, et al.Effect of co-culture canine cumulus and oviduct cells with porcine oocytes during maturation and subsequent embryo development of parthenotes in vitro.Theriogenology. 2018;106:108-116.
[45] BONGSO A, NG SC, FONG CY, et al.Cocultures in human assisted reproduction. Support of embryos in vitro and their specificity.Ann N Y Acad Sci. 1991;626:438-444.
[46] HOSHI K, KANNO Y, KATAYOSE H, et al.Coculture of mouse embryos with cryopreserved human oviduct epithelial cells.J Assist Reprod Genet. 1994;11(7):367-372.
|