Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (28): 5941-5949.doi: 10.12307/2025.472
In vitro angiogenesis and osteogenesis properties of copper-doped mesoporous bioactive glass
Zeng Yu1, 2, Xie Chengwei3, Hong Yuanqi2, 4, Su Shenghui2, Dong Xieping2
- 1Jiangxi University of Chinese Medicine, Nanchang 330004, Jiangxi Province, China; 2Jiangxi Provincial People’s Hospital (First Affiliated Hospital of Nanchang Medical College), Key Laboratory of Digital Orthopedics of Jiangxi Provincial Health Commission, Nanchang 330006, Jiangxi Province, China; 3Department of Orthopedics II, Anxi County Hospital, Quanzhou 362400, Fujian Province, China; 4Shaanxi University of Science and Technology, Xi’an 710021, Shaanxi Province, China
-
Received:2024-05-29Accepted:2024-07-26Online:2025-10-08Published:2024-12-07 -
Contact:Dong Xieping, Doctoral supervisor, Chief physician, Professor, Jiangxi Provincial People’s Hospital (First Affiliated Hospital of Nanchang Medical College), Key Laboratory of Digital Orthopedics of Jiangxi Provincial Health Commission, Nanchang 330006, Jiangxi Province, China Su Shenghui, MD, Jiangxi Provincial People’s Hospital (First Affiliated Hospital of Nanchang Medical College), Key Laboratory of Digital Orthopedics of Jiangxi Provincial Health Commission, Nanchang 330006, Jiangxi Province, China -
About author:Zeng Yu, Master candidate, Jiangxi University of Chinese Medicine, Nanchang 330004, Jiangxi Province, China; Jiangxi Provincial People’s Hospital (First Affiliated Hospital of Nanchang Medical College), Key Laboratory of Digital Orthopedics of Jiangxi Provincial Health Commission, Nanchang 330006, Jiangxi Province, China Xie Chengwei, Associate chief physician, Department of Orthopedics II, Anxi County Hospital, Quanzhou 362400, Fujian Province, China
CLC Number:
Cite this article
Zeng Yu, Xie Chengwei, Hong Yuanqi, Su Shenghui, Dong Xieping. In vitro angiogenesis and osteogenesis properties of copper-doped mesoporous bioactive glass[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(28): 5941-5949.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
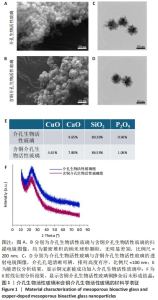
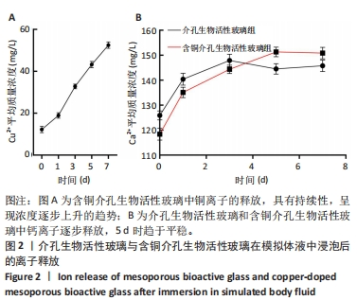
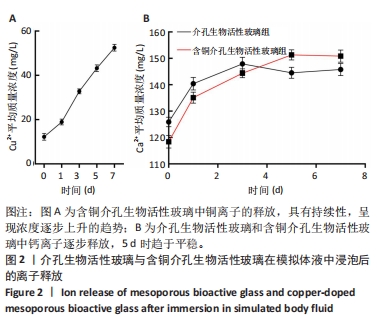
2.1 介孔生物活性玻璃材料学表征结果 扫描电镜下可见介孔生物活性玻璃和含铜介孔生物活性玻璃粉体均为紧密堆积的纳米球形颗粒,无明显差别,二者粒径为75 μm左右,粒径分布在50-100 μm范围内,见图1A,B;透射电镜下可见介孔生物活性玻璃和含铜介孔生物活性玻璃的介孔孔道清晰可辨,排列高度有序,孔道均匀一致,并且介孔生物活性玻璃中铜离子的掺杂对介孔的影响并不明显,见图1C,D。氮气吸附-脱附实验等温线计算介孔生物活性玻璃的比表面积为289.79 m2/g,含铜介孔生物活性玻璃的比表面积为307.65 m2/g。能谱仪检测结果显示含铜介孔生物活性玻璃粉末中成功加入铜元素,见图1E。X射线衍射分析结果显示,介孔生物活性玻璃和含铜介孔生物活性玻璃均无明显结晶衍射峰,见图1F。"

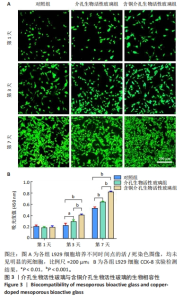
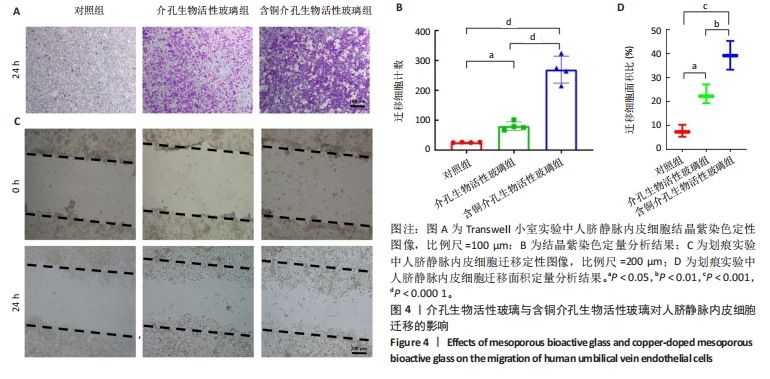
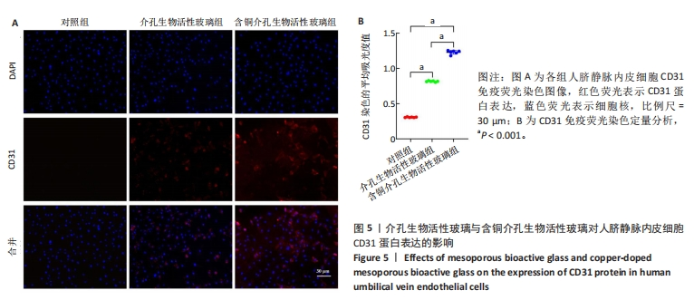
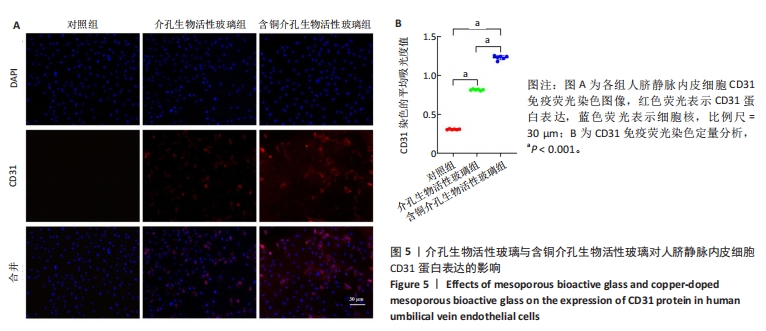
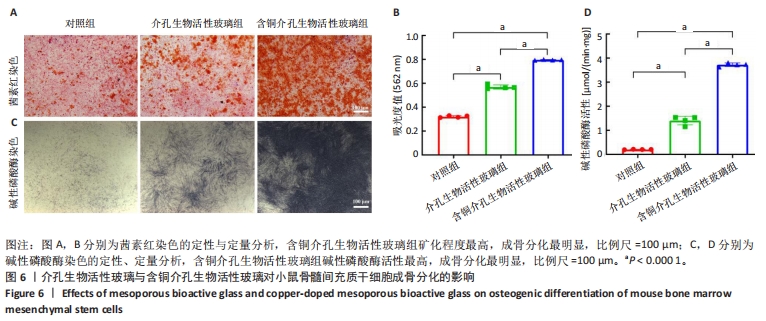
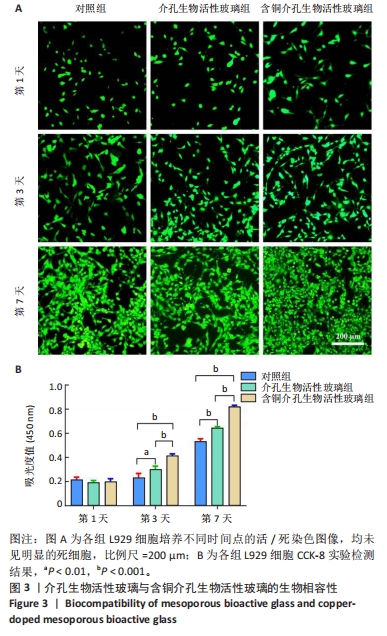
2.3 介孔生物活性玻璃的生物相容性评估结果 激光共聚焦显微镜下活死染色图像显示,随着培养时间的延长,3组细胞逐渐增多,培养第1天,3组细胞均存活,未见红色的死细胞;培养3 d后,3组细胞均存活,仍未见红色的死细胞,含铜介孔生物活性玻璃组细胞数量多于其他两组;培养第7天,3组仍未见红色的死细胞,均为绿色的活细胞,含铜介孔生物活性玻璃组细胞数量多于其他两组,见图3A。 CCK-8实验结果显示,随着培养时间的延长,3组细胞数量增多,培养3,7 d时,含铜介孔生物活性玻璃组细胞数多于介孔生物活性玻璃组(P < 0.001),介孔生物活性玻璃组细胞数多于对照组(P < 0.01,P < 0.001),见图3B。 以上结果提示,介孔生物活性玻璃与含铜介孔生物活性玻璃均具有良好的生物相容性。"
| [1] HENCH LL, THOMPSON I. Twenty-first century challenges for biomaterials. J R Soc Interface. 2010;7 Suppl 4(Suppl 4):S379-391. [2] ALDHAHER A, SHAHABIPOUR F, SHAITO A, et al. 3D hydrogel/bioactive glass scaffolds in bone tissue engineering: Status and future opportunities. Heliyon. 2023;9(7):e17050. [3] LI W, WU Y, ZHANG X, et al. Self-healing hydrogels for bone defect repair. RSC Adv. 2023;13(25):16773-16788. [4] ABODUNRIN OD, EL MABROUK K, BRICHA M. A review on borate bioactive glasses (BBG): effect of doping elements, degradation, and applications. J Mater Chem B. 2023;11(5):955-973. [5] O’KEEFE RJ, MAO J. Bone tissue engineering and regeneration: from discovery to the clinic--an overview. Tissue Eng Part B Rev. 2011; 17(6):389-392. [6] KOUSHIK TM, MILLER CM, ANTUNES E. Bone Tissue Engineering Scaffolds: Function of Multi-Material Hierarchically Structured Scaffolds. Adv Healthc Mater. 2023;12(9):e2202766. [7] HENG BC, BAI Y, LI X, et al. Electroactive Biomaterials for Facilitating Bone Defect Repair under Pathological Conditions. Adv Sci (Weinh). 2023;10(2):e2204502. [8] BARRETO MEV, MEDEIROS RP, SHEARER A, et al. Gelatin and Bioactive Glass Composites for Tissue Engineering: A Review. J Funct Biomater. 2022;14(1):23. [9] ERIKSSON E, BJÖRKENHEIM R, STRÖMBERG G, et al. S53P4 bioactive glass scaffolds induce BMP expression and integrative bone formation in a critical-sized diaphysis defect treated with a single-staged induced membrane technique. Acta Biomater. 2021;126:463-476. [10] HE L, YIN J, GAO X. Additive Manufacturing of Bioactive Glass and Its Polymer Composites as Bone Tissue Engineering Scaffolds: A Review. Bioengineering (Basel). 2023;10(6):672. [11] BIGHETTI-TREVISAN RL, SOUZA ATP, TOSIN IW, et al. Bioactive glass-ceramic for bone tissue engineering: an in vitro and in vivo study focusing on osteoclasts. Braz Oral Res. 2022;36:e022. [12] VALLET-REGÍ M. Nanostructured mesoporous silica matrices in nanomedicine. J Intern Med. 2010;267(1):22-43. [13] YAN X, YU C, ZHOU X, et al. Highly ordered mesoporous bioactive glasses with superior in vitro bone-forming bioactivities. Angew Chem Int Ed Engl. 2004;43(44):5980-5984. [14] YAN X, HUANG X, YU C, et al. The in-vitro bioactivity of mesoporous bioactive glasses. Biomaterials. 2006;27(18):3396-3403. [15] VALLET-REGÍ M, COLILLA M, IZQUIERDO-BARBA I, et al. Achievements in Mesoporous Bioactive Glasses for Biomedical Applications. Pharmaceutics. 2022;14(12):2636. [16] MENGER MM, LASCHKE MW, NUSSLER AK, et al. The vascularization paradox of non-union formation. Angiogenesis. 2022;25(3):279-290. [17] FANG TD, SALIM A, XIA W, et al. Angiogenesis is required for successful bone induction during distraction osteogenesis. J Bone Miner Res. 2005;20(7):1114-1124. [18] HUANG J, HAN Q, CAI M, et al. Effect of Angiogenesis in Bone Tissue Engineering. Ann Biomed Eng. 2022;50(8):898-913. [19] XU Z, KUSUMBE AP, CAI H, et al. Type H blood vessels in coupling angiogenesis-osteogenesis and its application in bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2023;111(7):1434-1446. [20] CHEN L, LIU J, GUAN M, et al. Growth Factor and Its Polymer Scaffold-Based Delivery System for Cartilage Tissue Engineering. Int J Nanomedicine. 2020;15:6097-6111. [21] XU HL, YU WZ, LU CT, et al. Delivery of growth factor-based therapeutics in vascular diseases: Challenges and strategies. Biotechnol J. 2017;12(5):1600243. [22] KARGOZAR S, MONTAZERIAN M, HAMZEHLOU S, et al. Mesoporous bioactive glasses: Promising platforms for antibacterial strategies.Acta Biomater. 2018;81:1-19. [23] JANA S, DATTA P, DAS H, et al. Copper and cobalt doped bioactive glass-fish dermal collagen electrospun mat triggers key events of diabetic wound healing in full-thickness skin defect model. J Mech Behav Biomed Mater. 2022;134:105414. [24] LI Y, LUO W, LIU Y, et al. Copper-containing titanium alloys promote the coupling of osteogenesis and angiogenesis by releasing copper ions. Biochem Biophys Res Commun. 2023;681:157-164. [25] LI S, ZHANG L, LIU C, et al. Spontaneous immunomodulation and regulation of angiogenesis and osteogenesis by Sr/Cu-borosilicate glass (BSG) bone cement to repair critical bone defects. Bioact Mater. 2023;23:101-117. [26] ZHAO Q, NI Y, WEI H, et al. Ion incorporation into bone grafting materials. Periodontol 2000. 2024;94(1):213-230. [27] SALVO J, SANDOVAL C. Role of copper nanoparticles in wound healing for chronic wounds: literature review. Burns Trauma. 2022;10:tkab047. [28] VERGNAUD F, MEKONNEN B, EL ABBASSI A, et al. Correlating the Effect of Composition and Textural Properties on Bioactivity for Pristine and Copper-Doped Binary Mesoporous Bioactive Glass Nanoparticles. Materials (Basel). 2023;16(20):6690. [29] HOSSEINI M, HASSANI BESHELI N, DENG D, et al. Facile post modification synthesis of copper-doped mesoporous bioactive glass with high antibacterial performance to fight bone infection. Biomater Adv. 2023;144:213198. [30] WESTHAUSER F, WILKESMANN S, NAWAZ Q, et al. Effect of manganese, zinc, and copper on the biological and osteogenic properties of mesoporous bioactive glass nanoparticles. J Biomed Mater Res A. 2021;109(8):1457-1467. [31] ANAND A, SENGUPTA S, KAŇKOVÁ H, et al. Influence of Copper-Strontium Co-Doping on Bioactivity, Cytotoxicity and Antibacterial Activity of Mesoporous Bioactive Glass. Gels. 2022;8(11):743. [32] ZHANG Y, ZHANG W, ZHANG X, et al. Erbium-ytterbium containing upconversion mesoporous bioactive glass microspheres for tissue engineering: luminescence monitoring of biomineralization and drug release. Acta Biomater. 2023;168:628-636. [33] JIMÉNEZ-HOLGUÍN J, ARCOS D, LOZANO D, et al. In Vitro and In Vivo Response of Zinc-Containing Mesoporous Bioactive Glasses in a Sheep Animal Model. Int J Mol Sci. 2022;23(22):13918. [34] ZARKOV A. Sol-Gel Technology Applied to Materials Science: Synthesis, Characterization and Applications. Materials (Basel). 2024;17(2):462. [35] HAN J, HASSANI BESHELI N, DENG D, et al. Tailoring Copper-Doped Bioactive Glass/Chitosan Coatings with Angiogenic and Antibacterial Properties. Tissue Eng Part C Methods. 2022;28(7):314-324. [36] SANCHEZ C, BOISSIèRE C, GROSSO D, et al. Design, Synthesis, and Properties of Inorganic and Hybrid Thin Films Having Periodically Organized Nanoporosity. Chem Mater. 2008;20(3):682-737. [37] DESHMUKH K, KOVÁŘÍK T, KŘENEK T, et al. Recent advances and future perspectives of sol–gel derived porous bioactive glasses: a revie. RSC Adv. 2020;10(56):33782-33835. [38] ZHU Y, WU C, RAMASWAMY Y, et al. Preparation, characterization and in vitro bioactivity of mesoporous bioactive glasses (MBGs) scaffolds for bone tissue engineering. Microporous Mesoporous Mater. 2008; 112(1-3):494-503. [39] ZHENG K, BIDER F, MONAVARI M, et al. Sol-gel derived B(2)O(3)-CaO borate bioactive glasses with hemostatic, antibacterial and pro-angiogenic activities. Regen Biomater. 2024;11:rbad105. [40] JONES JR, EHRENFRIED LM, HENCH LL. Optimising bioactive glass scaffolds for bone tissue engineering. Biomaterials. 2006;27(7):964-973. [41] SHEN Q, QI Y, KONG Y, et al. Advances in Copper-Based Biomaterials With Antibacterial and Osteogenic Properties for Bone Tissue Engineering. Front Bioeng Biotechnol. 2021;9:795425. [42] ZHANG Z, XUE H, XIONG Y, et al. Copper incorporated biomaterial-based technologies for multifunctional wound repair. Theranostics. 2024;14(2):547-570. [43] ALASVAND N, BEHNAMGHADER A, MILAN PB, et al. Tissue-engineered small-diameter vascular grafts containing novel copper-doped bioactive glass biomaterials to promote angiogenic activity and endothelial regeneration. Mater Today Bio. 2023;20:100647. [44] KANCZLER JM, OREFFO RO. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater. 2008;15:100-114. [45] TAO J, MIAO R, LIU G, et al. Spatiotemporal correlation between HIF-1α and bone regeneration. FASEB J. 2022;36(10):e22520. [46] VAN BRAKEL F, ZHAO Y, VAN DER EERDEN BCJ. Fueling recovery: The importance of energy coupling between angiogenesis and osteogenesis during fracture healing. Bone Rep. 2024;21:101757. [47] YANG Y, LI M, LUO H, et al. Surface-Decorated Graphene Oxide Sheets with Copper Nanoderivatives for Bone Regeneration: An In Vitro and In Vivo Study Regarding Molecular Mechanisms, Osteogenesis, and Anti-infection Potential. ACS Infect Dis. 2022;8(3):499-515. [48] KARKEHABADI H, RAHMATI A, ABBASI R, et al. Effect of copper oxide nanoparticles and light-emitting diode irradiation on the cell viability and osteogenic/odontogenic differentiation of human stem cells from the apical papilla. BMC Oral Health. 2023;23(1):249. [49] QIU X, FENG C, WANG W, et al. Copper-deposited diatom-biosilica enhanced osteogenic potential in periodontal ligament stem cells and rat cranium. J Biomed Mater Res B Appl Biomater. 2023;111(6): 1286-1298. [50] FIEHN LA, KUNISCH E, SAUR M, et al. A comparative in vitro and in vivo analysis of the impact of copper substitution on the cytocompatibility, osteogenic, and angiogenic properties of a borosilicate bioactive glass. J Biomed Mater Res A. 2024;112(10):1740-1759. [51] LIU J, XIAO Q, XIAO J, et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 2022;7(1):3. [52] TAN Z, ZHOU B, ZHENG J, et al. Lithium and Copper Induce the Osteogenesis-Angiogenesis Coupling of Bone Marrow Mesenchymal Stem Cells via Crosstalk between Canonical Wnt and HIF-1α Signaling Pathways. Stem Cells Int. 2021;2021:6662164. [53] ZHANG Y, HAO Z, WANG P, et al. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1α-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. 2019;52(2):e12570. |
| [1] | Li Zikai, Zhang Chengcheng, Xiong Jiaying, Yang Xirui, Yang Jing, Shi Haishan. Potential effects of ornidazole on intracanal vascularization in endodontic regeneration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-7. |
| [2] | Lai Pengyu, Liang Ran, Shen Shan. Tissue engineering technology for repairing temporomandibular joint: problems and challenges [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [3] | Lou Guo, Zhang Min, Fu Changxi. Exercise preconditioning for eight weeks enhances therapeutic effect of adipose-derived stem cells in rats with myocardial infarction [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1363-1370. |
| [4] | Cao Yue, Ye Xinjian, Li Biyao, Zhang Yining, Feng Jianying. Effect of extracellular vesicles for diagnosis and therapy of oral squamous cell carcinoma [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1523-1530. |
| [5] | Sun Yuting, Wu Jiayuan, Zhang Jian. Physical factors and action mechanisms affecting osteogenic/odontogenic differentiation of dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1531-1540. |
| [6] | Li Shuai, Liu Hua, Shang Yonghui, Liu Yicong, Zhao Qihang, Liu Wen. Stress distribution on the maxilla when wearing the Twin-block appliance for Class II malocclusion [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 881-887. |
| [7] | Han Haihui, Meng Xiaohu, Xu Bo, Ran Le, Shi Qi, Xiao Lianbo. Effect of fibroblast growth factor receptor 1 inhibitor on bone destruction in rats with collagen-induced arthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 968-977. |
| [8] | Ding Zhili, Huang Jie, Jiang Qiang, Li Tusheng, Liu Jiang, Ding Yu. Constructing rabbit intervertebral disc degeneration models by different methods under X-ray guidance: a comparative study [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 995-1002. |
| [9] | Xiao Fang, Huang Lei, Wang Lin. Magnetic nanomaterials and magnetic field effects accelerate bone injury repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 827-838. |
| [10] | Wang Sifan, He Huiyu, Yang Quan, Han Xiangzhen. miRNA-378a overexpression of macrophage cell line composite collagen sponge: anti-inflammation and tissue repair promotion [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 789-799. |
| [11] | Li Mingzhe, Ye Xiangling, Wang Bing, Yu Xiang. Preparation and osteogenic properties of liquid crystal display light-cured polylactic acid scaffold loaded with nano-tantalum [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 670-677. |
| [12] | Zhao Zengbo, Li Chenxi, Dou Chenlei, Ma Na, Zhou Guanjun. Anti-inflammatory and osteogenic effects of chitosan/sodium glycerophosphate/sodium alginate/leonurine hydrogel [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 678-685. |
| [13] | Yu Shuangqi, Ding Fan, Wan Song, Chen Wei, Zhang Xuejun, Chen Dong, Li Qiang, Lin Zuoli. Effects of polylactic acid-glycolic acid copolymer/lysine-grafted graphene oxide nanoparticle composite scaffolds on osteogenic differentiation of MC3T3 cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 707-712. |
| [14] | Dang Xiaowen, Huang Hailiang, Huang Lei, Wang Yajie . Research frontiers and hotspots of carbon nanomaterials in biomedical field over the past 10 years [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 752-760. |
| [15] | Ren Bo, Tang Yongliang, Li Ni, Liu Bangding. Thermosensitive antibacterial hydrogel for treatment of infected bone defects [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7269-7277. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||