Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (14): 2979-2988.doi: 10.12307/2025.396
Previous Articles Next Articles
Hyperbaric oxygen intervention eliminates exercise-induced fatigue in a high-intensity interval training shock microcycle
Pei Yunxiang, Wu Hao
- Capital University of Physical Education and Sports, Beijing 100191, China
-
Received:2024-04-20Accepted:2024-06-13Online:2025-05-18Published:2024-09-28 -
Contact:Wu Hao, PhD, Professor, Capital University of Physical Education and Sports, Beijing 100191, China -
About author:Pei Yunxiang, PhD candidate, Lecturer, Capital University of Physical Education and Sports, Capital University of Physical Education and Sports, Beijing 100191, China -
Supported by:National Key Research and Development Program “Science and Technology for Olympic Winter Games,” Nos. 2018YFF0300603 and 2018YFF0300902 (to WH)
CLC Number:
Cite this article
Pei Yunxiang, Wu Hao. Hyperbaric oxygen intervention eliminates exercise-induced fatigue in a high-intensity interval training shock microcycle[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(14): 2979-2988.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
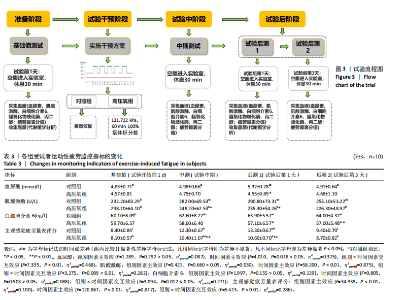
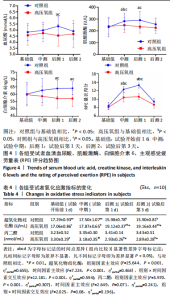
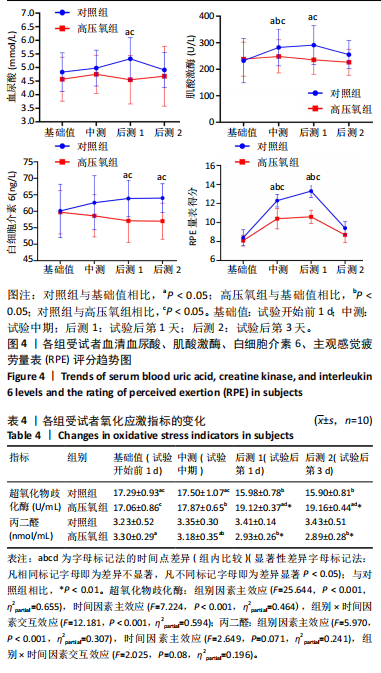
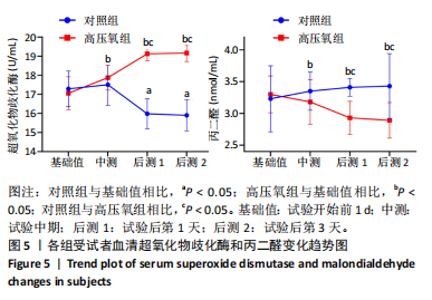
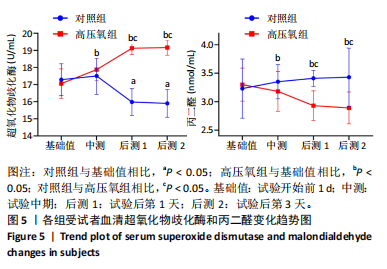
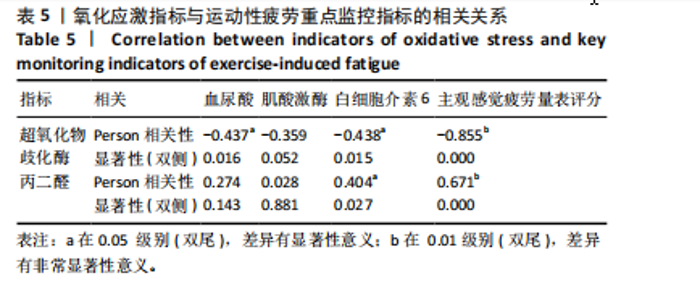
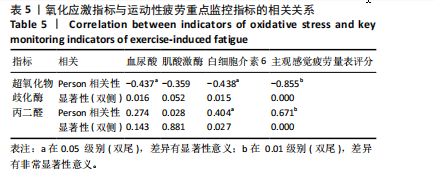
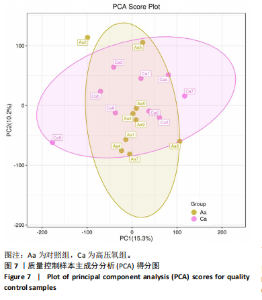
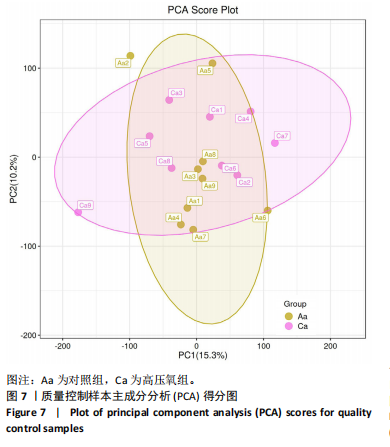
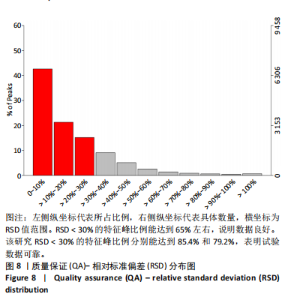
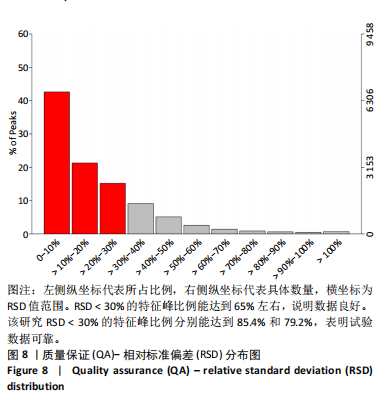
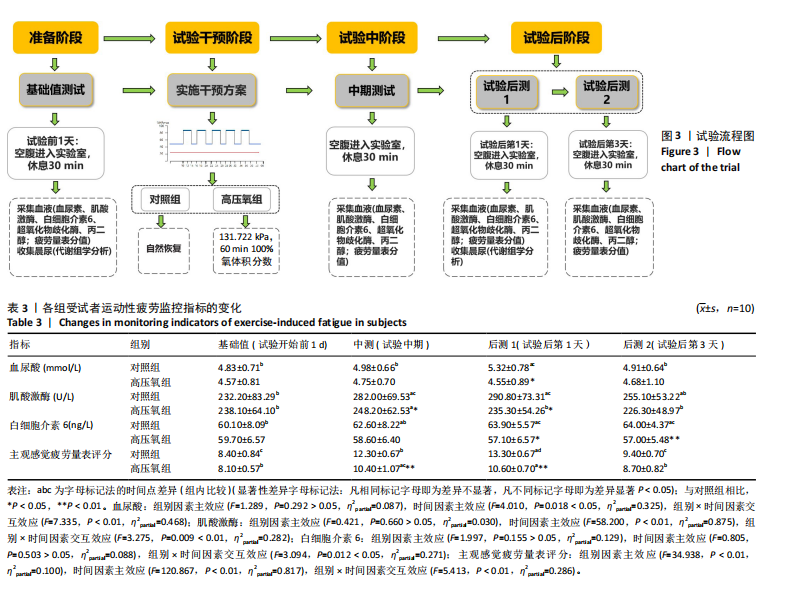
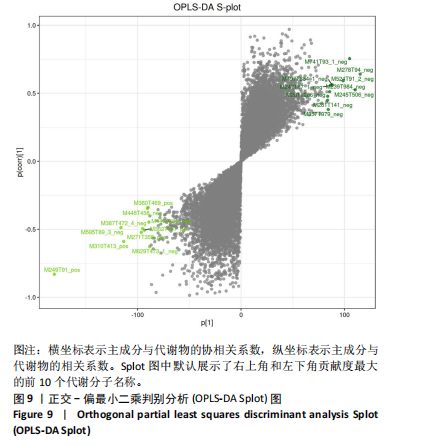
2.1 参与者数量分析 共招募20名受试者,均为首都体育学院有运动爱好的男性大学生,对照组10名,高压氧组10名。试验开始前,根据每人的空余时间,制定详细的训练课程计划表,严格按照训练计划表进行训练。有特殊情况时,提前沟通,协调训练时间,这也保证受试者人员全程参与。整个试验全程受试者依从性良好,训练过程中均未出现不良事件,没有出现受试者脱落情况。 2.2 试验流程图 见图3。 2.3 运动性疲劳监控指标的变化 重复测量方差分析结果显示:①血尿酸的变化:时间、组别×时间因素的效应均有统计学意义(P < 0.01),组别效应没有统计学意义(P > 0.05)。对照组后测1值显著高于基础值(P < 0.05),且显著高于高压氧组(P < 0.05),而高压氧组在试验前后无显著性差异(P > 0.05);②肌酸激酶的变化:时间、组别×时间因素的效应均有统计学意义(P < 0.001),组别效应没有统计学意义(P > 0.05)。对照组中测和后测1值均显著高于基础值(P < 0.05),而高压氧组仅中测值显著高于基础值(P < 0.05),且对照组中测和后测1值显著高于高压氧组(P < 0.05);③白细胞介素6的变化:组别×时间因素的效应有统计学意义(P < 0.05),对照组后测2个时间点均显著高于基础值(P < 0.05),且后测1值和后测2值显著高于高压氧组;④主观感觉疲劳量表评分的变化:时间、组别×时间因素的效应均有统计学意义(P < 0.01)。两组中测和后测1值均显著高于基础值(P < 0.05),对照组显著大于高压氧组(P < 0.05),见表3。为了让结果清晰明了,绘制图4。 2.4 氧化应激指标的变化 重复测量方差分析结果显示:①超氧化物歧化酶的变化:时间、组别、组别×时间因素的效应均有统计学意义(P < 0.001)。对照组后测1值和后测2值显著小于基础值和中测值(P < 0.05),高压氧组2个后测点值均显著大于基础值(P < 0.05),且后测1值和后测2值均显著大于中测值(P < 0.05)。高压氧组后测1值和后测2值均显著大于对照组(P < 0.05);②丙二醛的变化:时间效应有统计学意义(P < 0.001),组别、组别×时间因素的效应没有统计学意义(P > 0.05)。高压氧组后测1值和后测2值均显著小于基础值和中测值(P < 0.05),且显著小于对照组(P < 0.05),见表4。为了让结果清晰明了,绘制图5。 2.5 氧化应激指标与运动性疲劳重点监控指标的相关关系 由表5可知,受试者超氧化物歧化酶与血尿酸呈明显负相关(r=-0.437,P=0.016),与白细胞介素6呈明显负相关(r=-0.438,P=0.015),与主观感觉疲劳量表评分呈明显负相关(r=-0.855,P=0.000);受试者丙二醛与白细胞介素6呈明显正相关(r=0.404,P=0.027),与主观感觉疲劳量表评分呈明显正相关(r=0.671,P=0.000)。 2.6 代谢组学变化 2.6.1 基峰色谱图 经色谱分离流出的组分不断进入质谱,质谱连续扫描进行数据采集,得到的图谱即为基峰色谱图。该基峰质谱图各组别趋势高度相似,进一步证实数据集的高质量、可靠性和适用性,见图6。其中对照组标注Aa,高压氧组标注Ca。 2.6.2 多元统计分析 在基于质谱技术的代谢组学研究中,为了获得可靠且高质量的代谢组学数据,需进行质量控制(QC)[19]。该研究在LC-MS检测时利用质量控制样本进行质控,见图7;质量控制样本中,相对标准偏差(relative standard deviation,RSD) < 30%的特征峰比例能达到65%左右,说明数据良好[17]。该研究RSD < 30%的特征峰比例分别能达到85.4%和79.2%,表明试验数据可靠,见图8;OPLS-DA Splot图从另一个角度提供了有关代谢分子的重要性信息。Splot图主要用于识别与生物学过程中主要成分(也即Y矩阵)相关性强的代谢物。图中越靠近两个角(右上角和左下角)的代谢物其重要程度越高。该研究"
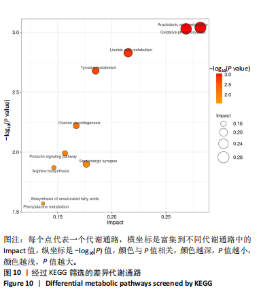
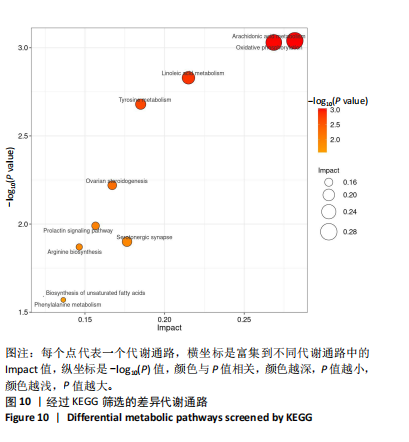
KEGG数据库进行功能注释,确定了与该研究密切相关的差异代谢通路,见图10。花生四烯酸代谢(arachidonic acid metabolism)和氧化磷酸化(oxidative phosphorylation)在试验干预后均发生显著变化。在筛选出来的差异代谢通路中存在多个密切相关的差异代谢物,进一步明确了几个重要的差异代谢物,如图11所示,主要是亚油酸(linoleic acid)、花生四烯酸(arachidonic acid,AA)、前列腺素 D2(prostaglandin D2)、白三烯 D4(leukotriene D4)、富马酸(fumaric acid)、泛醌1(ubiquinone-1)。 "
| [1] BUCHHEIT M, LAURSEN PB. High-intensity interval training, solutions to the programming puzzle. Part II: anaerobic energy, neuromuscular load and practical applications. Sports Med. 2013;43(10):927-954. [2] MARTINS C, STENSVOLD D, FINLAYSON G, et al. Effect of moderate- and high-intensity acute exercise on appetite in obese individuals. Med Sci Sports Exerc. 2015;47(1):40-48. [3] SU L, FU J, SUN S, et al. Effects of HIIT and MICT on cardiovascular risk factors in adults with overweight and/or obesity: A meta-analysis. PLoS One. 2019;14(1):e0210644. [4] STÖGGL T, SPERLICH B. Polarized training has greater impact on key endurance variables than threshold, high intensity, or high volume training. Front Physiol. 2014;5:33. [5] WAHL P, GÜLDNER M, MESTER J. Effects and sustainability of a 13-day high-intensity shock microcycle in soccer. J Sports Sci Med. 2014; 13(2):259-265. [6] BREIL FA, WEBER SN, KOLLER S, et al. Block training periodization in alpine skiing: effects of 11-day HIT on VO2max and performance. Eur J Appl Physiol. 2010;109(6):1077-1086. [7] ZINNER C, WAHL P, ACHTZEHN S, et al. Acute hormonal responses before and after 2 weeks of HIT in well trained junior triathletes. Int J Sports Med. 2014;35(4):316-322. [8] RØNNESTAD BR, HANSEN J, ELLEFSEN S. Block periodization of high-intensity aerobic intervals provides superior training effects in trained cyclists. Scand J Med Sci Sports. 2014;24(1):34-42. [9] RØNNESTAD BR, HANSEN J, THYLI V, et al. 5-week block periodization increases aerobic power in elite cross-country skiers. Scand J Med Sci Sports. 2016;26(2):140-146. [10] MCGAWLEY K, JUUDAS E, KAZIOR Z, et al. No Additional Benefits of Block- Over Evenly-Distributed High-Intensity Interval Training within a Polarized Microcycle. Front Physiol. 2017;8:413. [11] WIEWELHOVE T, FERNANDEZ-FERNANDEZ J, RAEDER C, et al. Acute responses and muscle damage in different high-intensity interval running protocols. J Sports Med Phys Fitness. 2016;56(5):606-615. [12] WIEWELHOVE T, RAEDER C, MEYER T, et al. Effect of Repeated Active Recovery During a High-Intensity Interval-Training Shock Microcycle on Markers of Fatigue. Int J Sports Physiol Perform. 2016;11(8):1060-1066. [13] TAKEMURA A, EDA N, SAITO T, et al. Mild hyperbaric oxygen for the early improvement of mood disturbance induced by high-intensity exercise. J Sports Med Phys Fitness. 2022;62(2):250-257. [14] KHAMIS MM, ADAMKO DJ, EL-ANEED A. Mass spectrometric based approaches in urine metabolomics and biomarker discovery. Mass Spectrom Rev. 2017;36(2):115-134. [15] ZELENA E, DUNN WB, BROADHURST D, et al. Development of a robust and repeatable UPLC-MS method for the long-term metabolomic study of human serum. Anal Chem. 2009;81(4):1357-1364. [16] WANT EJ, MASSON P, MICHOPOULOS F, et al. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat Protoc. 2013;8(1):17-32. [17] THÉVENOT EA, ROUX A, XU Y, et al. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses. J Proteome Res. 2015;14(8):3322-3335. [18] XIA J, WISHART DS. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat Protoc. 2011;6(6):743-760. [19] DUNN WB, BROADHURST D, BEGLEY P, et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat Protoc. 2011;6(7):1060-1083. [20] KO SF, CHEN KH, WALLACE CG, et al. Protective effect of combined therapy with hyperbaric oxygen and autologous adipose-derived mesenchymal stem cells on renal function in rodent after acute ischemia-reperfusion injury. Am J Transl Res. 2020;12(7):3272-3287. [21] SANTOS SILVA LOPES J, MONTEIRO DE MAGALHÃES NETO A, OLIVEIRA GONÇALVES LC, et al. Kinetics of Muscle Damage Biomarkers at Moments Subsequent to a Fight in Brazilian Jiu-Jitsu Practice by Disabled Athletes. Front Physiol. 2019;10:1055. [22] BARRANCO T, TVARIJONAVICIUTE A, TECLES F, et al. Changes in creatine kinase, lactate dehydrogenase and aspartate aminotransferase in saliva samples after an intense exercise: a pilot study. J Sports Med Phys Fitness. 2018;58(6):910-916. [23] TESEMA G, GEORGE M, MONDAL S, et al. Effects of one week different intensity endurance exercise on cardiorespiratory and cardiometabolic markers in junior young athletes. BMJ Open Sport Exerc Med. 2019; 5(1):e000644. [24] 李国印.高压氧对运动性疲劳大鼠血液生化指标的影响研究[J].江西科技师范大学学报,2019(6): 101-105. [25] CHEN CY, CHOU WY, KO JY, et al. Early Recovery of Exercise-Related Muscular Injury by HBOT. Biomed Res Int. 2019;2019:6289380. [26] BOUKHRIS O, TRABELSI K, ABDESSALEM R, et al. Effects of the 5-m Shuttle Run Test on Markers of Muscle Damage, Inflammation, and Fatigue in Healthy Male Athletes. Int J Environ Res Public Health. 2020; 17(12):4375. [27] DUPUY O, DOUZI W, THEUROT D, et al. An Evidence-Based Approach for Choosing Post-exercise Recovery Techniques to Reduce Markers of Muscle Damage, Soreness, Fatigue, and Inflammation: A Systematic Review With Meta-Analysis. Front Physiol. 2018;9:403. [28] LIAKOS CI, VYSSOULIS GP, MICHAELIDES AP, et al. The effects of angiotensin receptor blockers vs. calcium channel blockers on the acute exercise-induced inflammatory and thrombotic response. Hypertens Res. 2012;35(12):1193-1200. [29] KASAI N, KOJIMA C, SUMI D, et al. Inflammatory, Oxidative Stress, and Angiogenic Growth Factor Responses to Repeated-Sprint Exercise in Hypoxia. Front Physiol. 2019;10:844. [30] BARNETT A. Using recovery modalities between training sessions in elite athletes: does it help? Sports Med. 2006;36(9):781-796. [31] MOGHADAM N, HIEDA M, RAMEY L, et al. Hyperbaric Oxygen Therapy in Sports Musculoskeletal Injuries. Med Sci Sports Exerc. 2020;52(6): 1420-1426. [32] ESTON R. Use of ratings of perceived exertion in sports. Int J Sports Physiol Perform. 2012;7(2):175-182. [33] SHIMODA M, ENOMOTO M, HORIE M, et al. Effects of hyperbaric oxygen on muscle fatigue after maximal intermittent plantar flexion exercise. J Strength Cond Res. 2015;29(6):1648-1656. [34] POWERS SK, JACKSON MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88(4):1243-1276. [35] VESKOUKIS AS, NIKOLAIDIS MG, KYPAROS A, et al. Effects of xanthine oxidase inhibition on oxidative stress and swimming performance in rats. Appl Physiol Nutr Metab. 2008;33(6):1140-1154. [36] CHENG C, ZHANG J, LIU K, et al. Ginsenoside CK targeting KEAP1-DGR/Kelch domain disrupts the binding between KEAP1 and NRF2-DLG motif to ameliorate oxidative stress damage. Phytomedicine. 2023;119:154992. [37] HAMADA K, VANNIER E, SACHECK JM, et al. Senescence of human skeletal muscle impairs the local inflammatory cytokine response to acute eccentric exercise. FASEB J. 2005;19(2):264-266. [38] ROBINSON R, SRINIVASAN M, SHANMUGAM A, et al. Interleukin-6 trans-signaling inhibition prevents oxidative stress in a mouse model of early diabetic retinopathy. Redox Biol. 2020;34:101574. [39] PENG Y, YANG Q, GAO S, et al. IL-6 protects cardiomyocytes from oxidative stress at the early stage of LPS-induced sepsis. Biochem Biophys Res Commun. 2022;603:144-152. [40] HELMERSSON-KARLQVIST J, BJÖRKLUND-BODEGÅRD K, LARSSON A, et al. 24-Hour ambulatory blood pressure associates inversely with prostaglandin F(2α), interleukin-6 and F(2)-isoprostane formation in a Swedish population of older men. Int J Clin Exp Med. 2012;5(2):145-153. [41] XIE F, XU L, ZHU H, et al. Serum Metabolomics Based on GC-MS Reveals the Antipyretic Mechanism of Ellagic Acid in a Rat Model. Metabolites. 2022;12(6):479. [42] FINAUD J, LAC G, FILAIRE E. Oxidative stress : relationship with exercise and training. Sports Med. 2006;36(4):327-358. [43] SACHECK JM, MILBURY PE, CANNON JG, et al. Effect of vitamin E and eccentric exercise on selected biomarkers of oxidative stress in young and elderly men. Free Radic Biol Med. 2003;34(12):1575-1588. [44] YAGAMI T, YAMAMOTO Y, KOMA H. Physiological and Pathological Roles of 15-Deoxy-Δ12,14-Prostaglandin J2 in the Central Nervous System and Neurological Diseases. Mol Neurobiol. 2018;55(3):2227-2248. [45] 薛珊珊.用代谢组学方法研究DNA甲基化对花生四烯酸代谢的影响及血管内皮激活的机制[D].天津:天津医科大学,2015. [46] MARTINELLI N, GIRELLI D, MALERBA G, et al. FADS genotypes and desaturase activity estimated by the ratio of arachidonic acid to linoleic acid are associated with inflammation and coronary artery disease. Am J Clin Nutr. 2008;88(4):941-949. |
| [1] | Zhao Jiyu, Wang Shaowei. Forkhead box transcription factor O1 signaling pathway in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1923-1930. |
| [2] | He Guanghui, Yuan Jie, Ke Yanqin, Qiu Xiaoting, Zhang Xiaoling. Hemin regulates mitochondrial pathway of oxidative stress in mouse chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1183-1191. |
| [3] | He Bo, Chen Wen, Ma Suilu, He Zhijun, Song Yuan, Li Jinpeng, Liu Tao, Wei Xiaotao, Wang Weiwei, Xie Jing . Pathogenesis and treatment progress of flap ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1230-1238. |
| [4] | Lu Ranran, Zhou Xu, Zhang Lijie, Yang Xinling. Dimethyl fumarate alleviates nerve damage in a mouse model of Parkinson’s disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 989-994. |
| [5] | Sima Xinli, Liu Danping, Qi Hui. Effect and mechanism of metformin-modified bone marrow mesenchymal stem cell exosomes on regulating chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7728-7734. |
| [6] | Liu Xuan, Ding Yuqing, Xia Ruohan, Wang Xianwang, Hu Shujuan. Exercise prevention and treatment of insulin resistance: role and molecular mechanism of Keap1/nuclear factor erythroid2-related factor 2 signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7578-7588. |
| [7] | Zhang Xiaoyu, Wei Shanwen, Fang Jiawei, Ni Li. Prussian blue nanoparticles restore mitochondrial function in nucleus pulposus cells through antioxidation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7318-7325. |
| [8] | Su Yongkun, Sun Hong, Liu Miao, Yang Hua, Li Qingsong. Development of novel antioxidants and antioxidant combination carried by nano-hydrogel systems in treatment of intervertebral disc degeneration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7376-7384. |
| [9] | Wu Qingyun, Su Qiang. Antioxidant nanomedicine-mediated targeted therapy for myocardial ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7431-7438. |
| [10] | Chen Chao, Hu Yaoquan, Lyu Zhengpin, He Qicong, Yangyang Zijiu, Luo Haoyan, Wu Guishuai, Zuo Qianlin, Wang Xuenan, Zhang Fan. tert-Butyl hydroperoxide can induce ferroptosis in nucleus pulposus cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6858-6865. |
| [11] | Wang Ziheng, Wu Shuang. Oxidative stress-related genes and molecular mechanisms after spinal cord injury: data analysis and verification based on GEO database [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6893-6904. |
| [12] | Tian Yushi, Fu Qiang, Li Ji . Bioinformatics identification and validation of mitochondrial genes related to acute myocardial infarction [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6697-6707. |
| [13] | Zhang Xin, Guo Baojuan, Xu Huixin, Shen Yuzhen, Yang Xiaofan, Yang Xufang, Chen Pei. Protective effects and mechanisms of 3-N-butylphthalide in Parkinson’s disease cell models [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6466-6473. |
| [14] | Zhang Songjiang, Li Longyang, Zhou Chunguang, Gao Jianfeng. Central anti-inflammatory effect and mechanism of tea polyphenols in exercise fatigue model mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6474-6481. |
| [15] | Wu Xiaochou, Wang Huiying, Wang Jie, Zhang Caifeng, Hou Yanyun, Jin Bo. Protective mechanism of tanshinone IIA in mouse ovarian cryopreservation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(29): 6198-6204. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||