Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (28): 4546-4553.doi: 10.12307/2023.151
Previous Articles Next Articles
Icariin improves pituitary development in hypothyroidism model rats
Bai Gaigai1, Zhao Yujuan2, Wei Jiakai2, Huang Wendi2, An Yao2, Wang Zhi2
- 1Department of Endocrine Genetics and Metabolism, 2Department of Neonatology, Xi’an Children’s Hospital, Xi’an, Shaanxi 710000, China
-
Received:2021-11-24Accepted:2022-02-25Online:2023-10-08Published:2023-01-29 -
Contact:Wang Zhi, Master, Attending physician, Department of Neonatology, Xi’an Children’s Hospital, Xi’an, Shaanxi 710000, China -
About author:Bai Gaigai, Master, Attending physician, Department of Endocrine Genetics and Metabolism, Xi’an Children’s Hospital, Xi’an, Shaanxi 710000, China
CLC Number:
Cite this article
Bai Gaigai, Zhao Yujuan, Wei Jiakai, Huang Wendi, An Yao, Wang Zhi. Icariin improves pituitary development in hypothyroidism model rats[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(28): 4546-4553.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
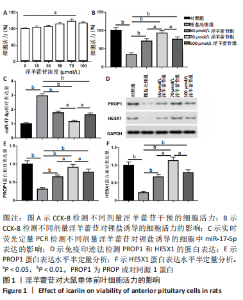
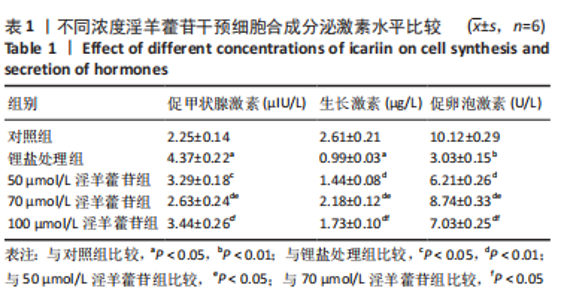
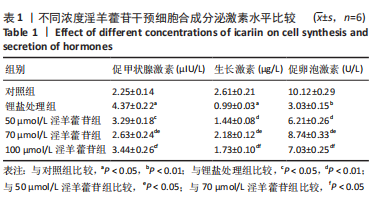
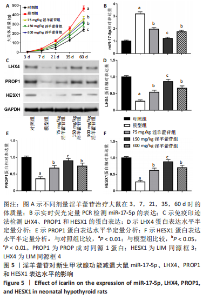
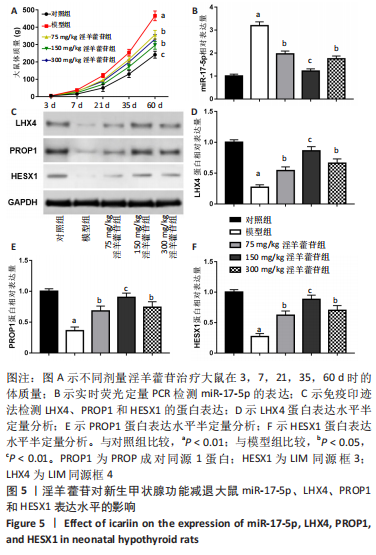
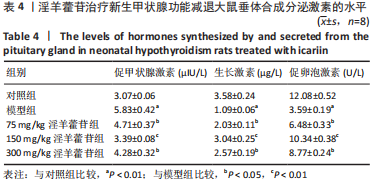
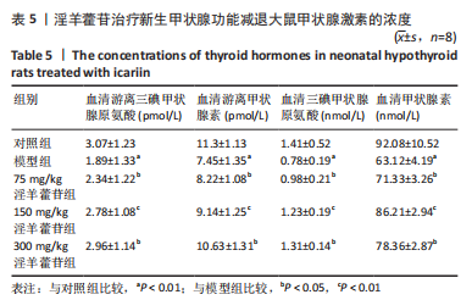
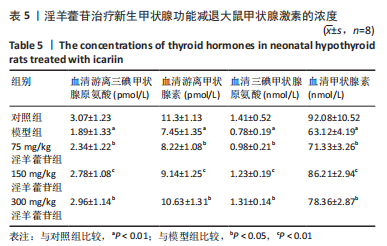
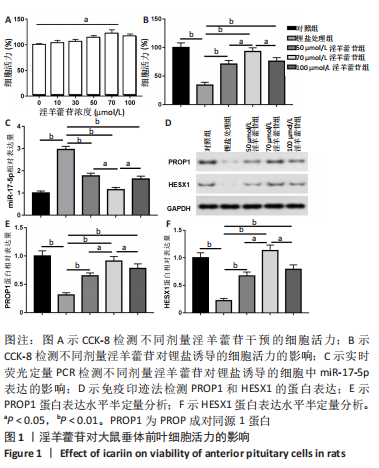
2.1 实验动物数量分析 共40只SD雌性大鼠纳入实验,其中模型组有1只小鼠手术过程中死亡,系实验人员操作失误,余无特殊,针对这只小鼠已进行后续实验补充。 2.2 淫羊藿苷对大鼠垂体前叶细胞合成分泌激素的影响 淫羊藿苷对大鼠垂体前叶细胞无明显毒副反应,当给予70 μmol/L 淫羊藿苷干预大鼠垂体前叶细胞时,细胞活力较未干预组显著升高(P < 0.05),见图1A。与对照组相比,锂盐处理组细胞活力明显降低,而给予50,70,100 μmol/L淫羊藿苷干预后细胞活力明显增加,其中70 μmol/L淫羊藿苷干预效果最佳,差异有显著性意义(P < 0.01),见图1B。故选择50,70,100 μmol/L剂量作为观察组进行后续实验。与对照组相比,锂盐处理组细胞中miR-17-5p的表达显著升高,而给予不同浓度淫羊藿苷处理后miR-17-5p的表达明显下调,其中70 μmol/L淫羊藿苷组最显著(P < 0.01),见图1C。Western印迹结果显示,当用锂盐处理细胞后,PROP1和HESX1的蛋白表达较对照组显著降低(P < 0.01),给予淫羊藿苷干预后表达上调,在浓度为70 μmol/L时达到峰值,该结果与锂盐处理组相比差异有显著性意义(P < 0.01),见图1D-F。"
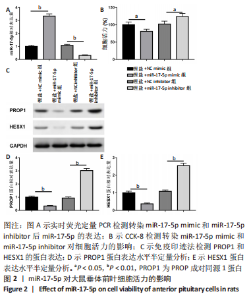
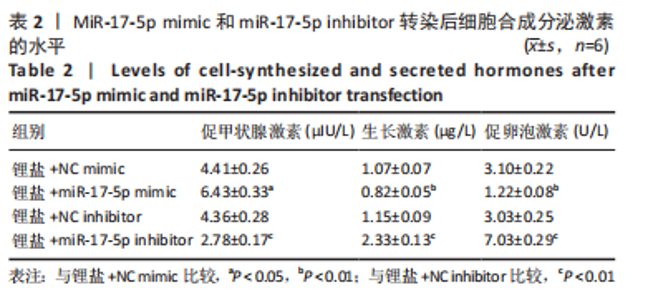
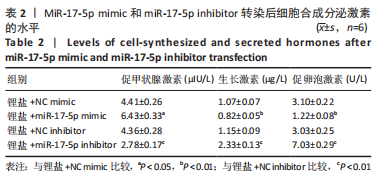
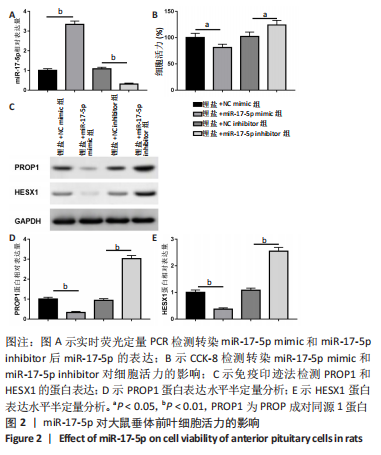
2.3 MiR-17-5p对大鼠垂体前叶细胞合成分泌激素的影响 miR-17-5p过表达效率和干扰效率见图2A。与锂盐+NC mimic组相比,锂盐+miR-17-5p mimic组细胞活力显著降低,锂盐+miR-17-5p inhibitor组细胞活力较锂盐+NC inhibitor组显著升高,差异有显著性意义(P < 0.05),见图2B。Western印迹结果显示,锂盐+miR-17-5p mimic组PROP1和HESX1的蛋白水平较锂盐+NC mimic组显著下调,而锂盐+miR-17-5p inhibitor组PROP1和HESX1蛋白水平较锂盐+NC inhibitor组明显升高,以上比较差异均有显著性意义(P < 0.01),见图2C-E。"
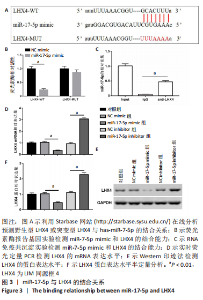
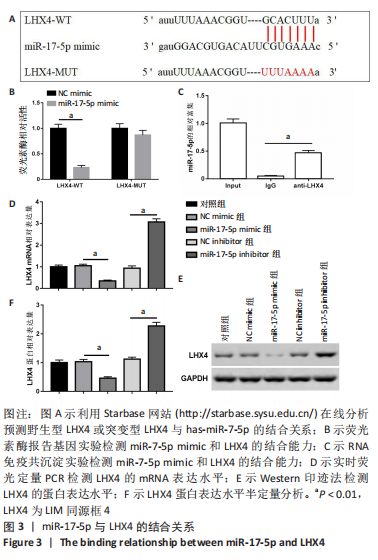
2.4 LHX4是miR-17-5p的靶基因 miR-17-5p和LHX4的结合序列见图3A。荧光素酶报告基因实验结果显示,与NC mimic组相比,miR-17-5p与野生型LHX4 3’UTR共转染后,检测到荧光素酶活性显著降低,差异有显著性意义(P < 0.01)。而与突变型LHX4 3’UTR共转染后,荧光素酶活性无明显变化,见图3B。RNA免疫共沉淀结果显示,miR-17-5p在LHX4抗体上的富集显著高于IgG组,差异有显著性意义(P < 0.01),见图3C。此外,miR-17-5p mimic组LHX4 mRNA和蛋白表达水平较NC mimic组显著降低,miR-17-5p inhibitor组LHX4 mRNA和蛋白表达水平较NC inhibitor明显上调,差异有显著性意义(P < 0.01),见图3D-F。"
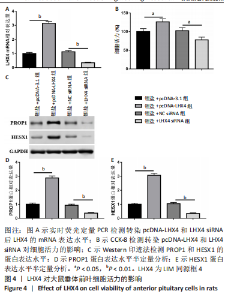
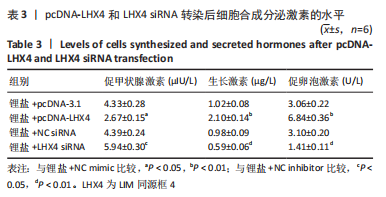
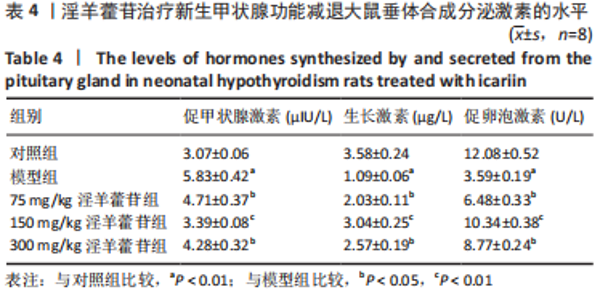
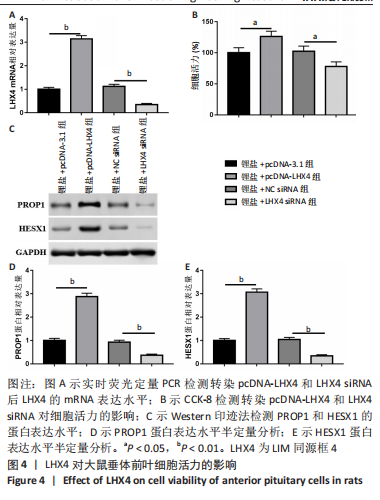
2.5 LHX4对大鼠垂体前叶细胞合成分泌激素的影响 此次实验测得转染pcDNA-LHX4过表达效率为pcDNA-3.1组的(3.14±0.18)倍,转染LHX4 siRNA干扰效率为NC siRNA组的(0.35±0.05)倍,差异有显著性意义(P < 0.01)。与锂盐+pcDNA-3.1组相比,锂盐+pcDNA-LHX4组细胞活力显著增强;与锂盐+NC siRNA组比较,锂盐+LHX4 siRNA组细胞活力降低,差异有显著性意义(P < 0.05)。Western印迹结果显示,锂盐 +pcDNA-LHX4组PROP1和HESX1的蛋白水平较锂盐+pcDNA-3.1组显著上调;锂盐+LHX4 siRNA组PROP1和HESX1蛋白水平较锂盐+NC siRNA组明显降低,以上比较差异均有显著性意义(P < 0.01),见图4。"
| [1] MAGRI F, CHIOVATO L, CROCE L, et al. Thyroid hormone therapy for subclinical hypothyroidism. Endocrine. 2019;66(1):27-34. [2] SUN J, HUI C, XIA T, et al. Effect of hypothyroidism on the hypothalamic-pituitary-ovarian axis and reproductive function of pregnant rats. BMC Endocr Disord. 2018;18(1):30. [3] BARGI-SOUZA P, PELICIARI-GARCIA RA, NUNES MT. Disruption of the Pituitary Circadian Clock Induced by Hypothyroidism and Hyperthyroidism: Consequences on Daily Pituitary Hormone Expression Profiles. Thyroid. 2019;29(4):502-512. [4] KUCHARSKA AM, WITKOWSKA-SĘDEK E, RUMIŃSKA M, et al. Thyroid Hormone Changes Related to Growth Hormone Therapy in Growth Hormone Deficient Patients. J Clin Med. 2021;10(22):5354. [5] CARVALHO CUNHA N, GOMES L, BASTOS M. Challenging diagnosis of resistance to thyroid hormone in a patient with pituitary adenoma. BMJ Case Rep. 2019;12(7):e229430. [6] BORTOLOTTO VC, PINHEIRO FC, ARAUJO SM, et al. Chrysin reverses the depressive-like behavior induced by hypothyroidism in female mice by regulating hippocampal serotonin and dopamine. Eur J Pharmacol. 2018;822:78-84. [7] BORTOLOTTO VC, ARAUJO SM, PINHEIRO FC, et al. Chrysin restores memory deficit in hypothyroidism mice: Behavioral, neurochemical and computational approaches involving the neurotrophinergic system. J Psychiatr Res. 2021;144:225-233. [8] AN R, LI B, YOU LS, et al. Improvement of kidney yang syndrome by icariin through regulating hypothalamus-pituitary-adrenal axis. Chin J Integr Med. 2015;21(10):765-771. [9] JIN J, WANG H, HUA X, et al. An outline for the pharmacological effect of icariin in the nervous system. Eur J Pharmacol. 2019;842:20-32. [10] EL-SHITANY NA, EID BG. Icariin modulates carrageenan-induced acute inflammation through HO-1/Nrf2 and NF-kB signaling pathways. Biomed Pharmacother. 2019;120:109567. [11] JING X, DU T, CHEN K, et al. Icariin protects against iron overload-induced bone loss via suppressing oxidative stress. J Cell Physiol. 2019; 234(7):10123-10137. [12] WANG YS, SHEN CY, JIANG JG. Antidepressant active ingredients from herbs and nutraceuticals used in TCM: pharmacological mechanisms and prospects for drug discovery. Pharmacol Res. 2019;150:104520. [13] KONG L, LIANG X, LIU A, et al. Icariin inhibits inflammation via immunomodulation of the cutaneous hypothalamus-pituitary-adrenal axis in vitro. Clin Exp Dermatol. 2019;44(2):144-152. [14] HU Y, WANG Q, WANG Z, et al. Circulating microRNA profiles and the identification of miR-593 and miR-511 which directly target the PROP1 gene in children with combined pituitary hormone deficiency. Int J Mol Med. 2015;35(2):358-366. [15] HA M, HUANG X, LI L, et al. PKCα mediated by the PI3K/Akt-FOXA1 cascade facilitates cypermethrin-induced hyperthyroidism. Sci Total Environ. 2021;757:143727. [16] BUDNY B, ZEMOJTEL T, KALUZNA M, et al. SEMA3A and IGSF10 Are Novel Contributors to Combined Pituitary Hormone Deficiency (CPHD). Front Endocrinol (Lausanne). 2020;11:368. [17] BUDNY B, KARMELITA-KATULSKA K, STAJGIS M, et al. Copy Number Variants Contributing to Combined Pituitary Hormone Deficiency. Int J Mol Sci. 2020;21(16):5757. [18] BIONDI B, COOPER DS. Thyroid hormone therapy for hypothyroidism. Endocrine. 2019;66(1):18-26. [19] PIJNACKER T, KOOISTRA HS, VERMEULEN CF, et al. Use of basal and TRH-stimulated plasma growth hormone concentrations to differentiate between primary hypothyroidism and nonthyroidal illness in dogs. J Vet Intern Med. 2018;32(4):1319-1324. [20] XU D, WU B, LI X, et al. Evaluation of the thyroid characteristics of patients with growth hormone-secreting adenomas. BMC Endocr Disord. 2019;19(1):94. [21] OHARA N, KOBAYASHI M, TUCHIDA M, et al. Isolated Adrenocorticotropic Hormone Deficiency and Primary Hypothyroidism in a Patient Undergoing Long-Term Hemodialysis: A Case Report and Literature Review. Am J Case Rep. 2020;21:e922376. [22] KHAN SH, IJAZ A. Subclinical-Hypothyroidism: A Pathology in Evolution. J Coll Physicians Surg Pak. 2019;29(2):150-158. [23] LOUTCHANWOOT P, VORTHERMS T, JARRY H. Evaluation of in vivo estrogenic potency of natural estrogen-active chemical, puerarin, on pituitary function in gonadectomized female rats. Life Sci. 2016;165: 75-82. [24] PENG T, SHANG H, YANG M, et al. Puerarin improved growth performance and postmortem meat quality by regulating lipid metabolism of cattle under hot environment. Anim Sci J. 2021;92(1): e13543. [25] AVBELJ STEFANIJA M, KOTNIK P, BRATANIČ N, et al. Novel Mutations in HESX1 and PROP1 Genes in Combined Pituitary Hormone Deficiency. Horm Res Paediatr. 2015;84(3):153-158. [26] BERTKO E, KLAMMT J, DUSATKOVA P, et al. Combined pituitary hormone deficiency due to gross deletions in the POU1F1 (PIT-1) and PROP1 genes. J Hum Genet. 2017;62(8):755-762. [27] CARRENO G, APPS JR, LODGE EJ, et al. Hypothalamic sonic hedgehog is required for cell specification and proliferation of LHX3/LHX4 pituitary embryonic precursors. Development. 2017;144(18):3289-3302. [28] WANG CZ, GUO LL, HAN BY, et al. Pituitary Stalk Interruption Syndrome: From Clinical Findings to Pathogenesis. J Neuroendocrinol. 2017;29(1):10.1111/jne.12451. [29] DEMIRAL M, DEMIRBILEK H, UNAL E, et al. Ectopic Posterior Pituitary, Polydactyly, Midfacial Hypoplasia and Multiple Pituitary Hormone Deficiency due to a Novel Heterozygous IVS11-2A>C(c.1957-2A>C) Mutation in the GLI2 Gene. J Clin Res Pediatr Endocrinol. 2020;12(3): 319-328. [30] GANGAT M, RADOVICK S. Pituitary Hypoplasia. Endocrinol Metab Clin North Am. 2017;46(2):247-257. |
| [1] | Qiao Luhui, Ma Ziyu, Guo Haoyu, Hou Yudong. Comparison of puerarin and icariin on the biological properties of mouse preosteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 872-877. |
| [2] | He Wenfeng, Xue Cheng, Zheng Jiankang, Shuai Zhuang, Yue Rongchuan. Effects of icariin combined with injectable chitosan/collagen composite hydrogel on angiogenesis in rats with myocardial infarction [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(25): 3992-3998. |
| [3] | Zhang Wenjie, Zhang Yong, Shi Ming, Tang Guangjun, Shi Pengzhi, Wang Junwu, Hu Man, Wang Pingchuan, Zhang Liang. Icariin regulates apoptosis of nucleus pulposus-derived mesenchymal stem cells to repair intervertebral disc degeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(24): 3803-3809. |
| [4] | Liu Yunqin, Lin Li, Xiao Wenhao, Ji Qiuming, Liu Yanqin. Effects of icariin on NRG1-ErbB signaling pathways in hippocampus of schizophrenia rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(20): 3236-3241. |
| [5] | Mei Jie, He Qiang, Sun Xin, Yin Hong, Qian Weiqing. Icariin promotes osteoblast proliferation and differentiation through a non-nuclear signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(20): 3129-3135. |
| [6] | Zhan Ying, Wu Xiaodan, Hao Shanhu. Establishment of an animal model of radioactive 131I-induced hypothyroidism in rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(17): 2631-2636. |
| [7] | Gao Yixuan, Wang Lingfeng, Ba Te, Li Fang, Cao Shengjun, Li Junliang, Zhou Biao, Chen Qiang. Adipose-derived stem cells overexpressing growth hormone promote proliferation and migration of fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(1): 8-14. |
| [8] | Li Jiajun, Xia Tian, Liu Jiamin, Chen Feng, Chen Haote, Zhuo Yinghong, Wu Weifeng. Molecular mechanism by which icariin regulates osteogenic signaling pathways in the treatment of steroid-induced avascular necrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 780-785. |
| [9] | Guo Xiaopeng, Liu Yingsong, Shang Hui. Silk fibroin/nano hydroxyapatite composite combined with icariin can promote the proliferation and differentiation of bone marrow mesenchymal stem cells into nucleus pulposus like cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(22): 3528-3534. |
| [10] | Zhang Jinming, Tian Yingzhou, Zhao Ling, Xiong Lihua, Wang Qiuping, Song Wei, Wen Jianxuan. Icariin alleviates osteoporosis by promoting osteogenic differentiation of bone marrow mesenchymal stem cells in mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(19): 2991-2996. |
| [11] | Dong Zhiheng, Gao Yan, Liu Zhen, Han Xiaoqian. Construction of icariin/polycaprolactone nanofiber membrane and its osteogenic ability in vitro [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(10): 1510-1514. |
| [12] | Yang Bingxuan, Jiang Tao, Jia Min, Li Peng, Liao Rongzhen, Li Zhao, Shao Min. Icariin promotes differentiation of mouse preosteoblasts via activating autophagy [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(1): 64-69. |
| [13] | Yang Junhui, Luo Jinli, Yuan Xiaoping. Effects of human growth hormone on proliferation and osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3956-3961. |
| [14] | Jiang Tao, Ling Cuimin, Chen Qingzhen, Yang Bingxuan, Lin Yanping, Shao Min. Icariin prevents osteoporosis by activating autophagy and promoting osteoblast differentiation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(17): 2643-2649. |
| [15] | Li Qi, Sun Guicai. Effect of icaritin on ferroptosis of bone marrow mesenchymal stem cells and their differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(13): 1988-1992. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||