Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (17): 2768-2774.doi: 10.12307/2022.549
Previous Articles Next Articles
Benefit and physiological mechanism of low-intensity resistance training with blood flow restriction intervention on muscle fitness
Yu Wei1, Song Gang2, Liu Yiwen3
- 1School of Physical Education, Leshan Normal University, Leshan 614000, Sichuan Province, China; 2School of Physical Education, Southwest University, Chongqing 400715, China; 3Department of Physical Education, North Sichuan Medical College, Nanchong 637100, Sichuan Province, China
-
Received:2021-04-19Revised:2021-05-21Accepted:2021-06-25Online:2022-06-18Published:2021-12-27 -
Contact:Liu Yiwen, MD candidate, Department of Physical Education, North Sichuan Medical College, Nanchong 637100, Sichuan Province, China -
About author:Yu Wei, Master, School of Physical Education, Leshan Normal University, Leshan 614000, Sichuan Province, China -
Supported by:the National Natural Science Foundation of China, No. 31360254 (to SG)
CLC Number:
Cite this article
Yu Wei, Song Gang, Liu Yiwen. Benefit and physiological mechanism of low-intensity resistance training with blood flow restriction intervention on muscle fitness[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(17): 2768-2774.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
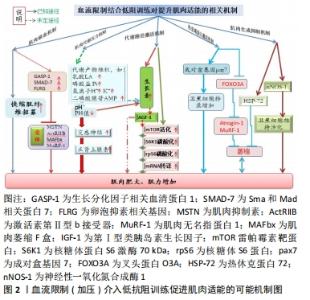
2.1.1 肌肉缺血机制 一般而言,运动开始先是招募慢缩肌(Ⅰ型肌纤维)参与工作,随着张力的增加再逐步招募快缩肌(Ⅱ型肌纤维)。然而,肌肉在局部缺血的情况下,则不会依照此顺序执行[11-12]。另一方面,Ⅱ型肌纤维的招募阈值会随着肌肉疲劳而下降,而血流限制训练因运动肢段血流量受限,从而增加了该活动肢段的代谢压力,并伴随更高的肌电活动,如此诱发肌肉疲劳进而达到招募更多快缩肌参与工作[13-15]。肌肉生成抑制素结合激活素Ⅱb受体会降低成肌细胞分化进入肌管,从而抑制肌细胞分化与卫星细胞增殖[16-18](如此达不到肌肉肥大的目标);若肌肉生成抑制素表达过度时,MuRF-1激活水平提升,则会降低肌肉分化[19],此时,卵泡抑制素相关基因(FLRG)及分化因子血清结合蛋白1(GASP-1)通过结合肌肉生成抑制素抢占了肌肉生成抑制素与激活素Ⅱb受体结合机会,故能抑制蛋白质水解[20-21],达到肌肉肥大之目的。还有就是Sma和Mad相关蛋白7(SMAD-7)也可对肌肉生成抑制素信号起抑制作用,提升MyoD活化水平,从而进一步降低肌肉生成抑制素对肌肉生长的抑制,增加肌肉生成及分化[22-23]。 2.1.2 肌肉代谢压力机制 血流限制训练能显著提升全血、血浆及肌肉内的血乳酸浓度[24-27],而血液酸性环境刺激有利于生长素的分泌。因为阻血带限制血流后,滞留在肌肉内的乳酸向外扩散的速度显著下降,而肌肉内酸性环境的提升会增强对第Ⅳ群痛觉神经的刺激,增强了下视丘-脑垂体的信号传递能力;也可能是血液中K+、H+、AMP(二磷酸腺苷)累积及组织缺氧提升了对第Ⅲ,Ⅳ群痛觉神经的刺激,这有利于生长素的合成与分泌[28]。类胰岛素生长因子1主要功能是刺激蛋白质合成与促进组织生长,而生长素浓度增加会刺激肝脏分泌类胰岛素生长因子1[29]。但血流限制后,类胰岛素生长因子1是否会增加?目前相关研究并未达成一致意见。有些研究表明血流限制训练(低强度结合加压训练)能显著提升类胰岛素生长因子1水平[30-31];也有研究认为血流限制训练(走路结合加压)并未发现类胰岛素生长因子1显著变化[6,32]。 2.1.3 代谢路径激活机制 哺乳动物雷帕霉素靶蛋白(mammalian target rapamycin,mTOR)被认为是调节骨骼肌生长的主要通路,它能整合细胞内外的合成性激素、细胞机械性刺激与营养物质刺激,通过改变其活性而间接调整核醣体与mRNA的结合率,进一步调节细胞内部蛋白质转译速率,在细胞蛋白合成上扮演非常重要的角色。血流限制训练有利于mTOR信号通路被上游分子磷酸化而激活,此时,蛋白激酶(Akt)被类胰岛素生长因子1激活进而诱导mTOR以刺激蛋白质翻译,并在促进肌肉生长中发挥重要作用。例如:血流限制训练中被其下游效应物核糖体S6激酶1磷酸化刺激,能提高核糖体蛋白与转译因子mRNA的蛋白质转译工作,促使蛋白质合成效率显著提高[33-34]。急性低强度阻力训练结合局部加压(200 mmHg)可显著提升核糖体S6激酶1的活性及肌肉蛋白合成表现[25];同样地,FRY等[35]以类似的方法同样发现:血流限制训练能显著提升mTOR、核糖体S6激酶1与rpS6的磷酸化过程,表明加压抗阻血训练的确有利于提升mTOR路径的磷酸作用。 2.1.4 热衰竭蛋白激活机制 叉头蛋白O3A(FOXO3A)为叉头转录因子(FOXO)的家族成员,FOXO3A一方面接受Akt的调节,同时又在胰岛素(或类胰岛素生长因子1)调节的下游信号通路中起关键作用,它受两种泛蛋白连接酶Atrogin-1及MuRF-1转录因子的共同控制[36-38]。Atrogin-1和MuRF-1皆参与肌细胞泛素蛋白水解路径,其表达水平提升则意味着骨骼肌蛋白质分解加强,即骨骼肌蛋白质的降解提速,故抑制FOXO3A蛋白的激活就可降低Atrogin-1与MuRF-1的表现,从而缓解肌肉萎缩[36,39-41]。研究表明,急性血流限制训练能显著降低泛素蛋白水解路径的mRNA表现[37],使FOXO3A下降原有水平的1.92倍、Atrogin-1下降2.1倍和MuRF-1下降2.44倍;促使卫星细胞转录,显著加快肌肉肥大信号[42-44]。还有研究发现,低强度抗阻结合局部加压(100 mmHg)训练2周后,加压组每条Ⅰ型与Ⅱ型肌纤维内的pax7含量及表现、最大收缩力及肌肉横断面积皆显著增加[44],说明该训练模式对提升转录速率及肌纤维蛋白质有益,见图2。"
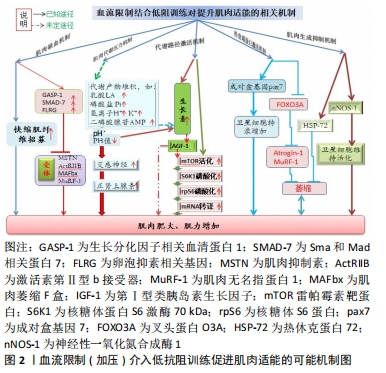

2.1.5 肌肉生成抑制机制 正常状况下,热休克蛋白的作用在于帮助蛋白质组装与转运及维持细胞稳态,它受缺氧、缺血再灌注及酸性环境所诱导,而血流限制训练恰好招致缺血、缺氧及代谢产物累积,因而能激发热休克蛋白活性。泛素蛋白水解能调节热休克蛋白72,从而阻止蛋白质降解。此外,神经性一氧化氮合成酶1是一种抗肌肉萎缩的复合蛋白,其主要作用是维持卫星细胞活化,从而对促进肌肉生长有益[45]。相关文献报道,经过2周加压阻血训练的大鼠,其肌肉肥大的效果显著,并伴随热休克蛋白72与神经性一氧化氮合成酶1活性显著增加(注类胰岛素生长因子1及一氧化氮浓度没改变),故推断肌肉肥大的现象很可能是由于热休克蛋白72与神经性一氧化氮合成酶1活性增加引起的[46-47]。 2.2 血流限制训练的生理效应"


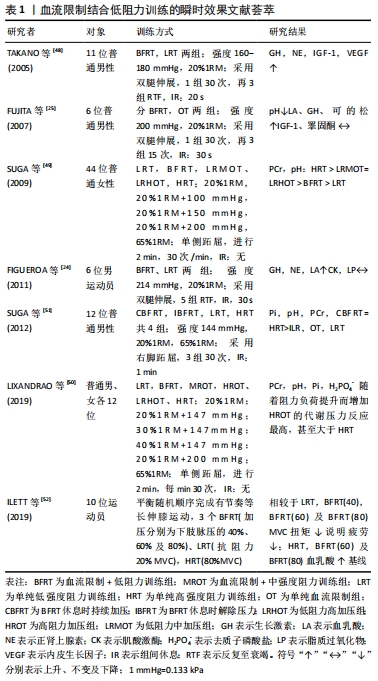
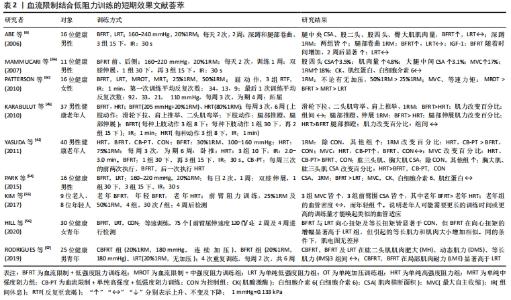
2.2.1 血流限制训练的瞬时性效应 表1结果显示:①急性(血流限制训练)中,低阻力设置一般采用20%1RM,训练后合成性激素反应与代谢压力显著增强,其中血流限制训练在提升合成性激素的效果方面显著优于单纯低强度阻力训练(LRT),几乎与单纯高强度阻力运动(HRT)具有同等效果类似[24-25,48]。多数血流限制训练后表明生长素皆显著增加[25, 48],但对类胰岛素生长因子1的影响报道并不一致,其原因有待于进一步阐明。②急性血流限制训练能显著造成代谢压力,但压力及阻力强度的提升所造成的代谢压力具有剂量反应特征,其中“低强度阻力+加压”方案设计中,阻力强度一般在20%-40%1RM,压力范围在144-230 mmHg、进行三四组(每组15-30次,组间休息30-60 s)或连续2 min,每分钟30次的频率[49-52]。③多数研究设计的组间休息,都是维持加压状态,也有部分学者并非如此[51],其组间休息时解除加压,结果发现组间休息持续加压所造成的代谢压力比解除加压来得高,造成更高的代谢产物堆积。④随着加压压力的增加,血流限制训练瞬时效果出现显著的级别特征,加压处于约60%的下肢脉压下,血流限制训练出现有效最小“阈值”,即最有效的训练条件[52-53]。尽管上述急性研究所获得的数据能为血流限制训练提供一些增加骨骼肌大小和力量的可能机制提供有益见解,但当训练计划转向中长期时,使用不同水平的血流限制效果仍有待后续深入研究。"
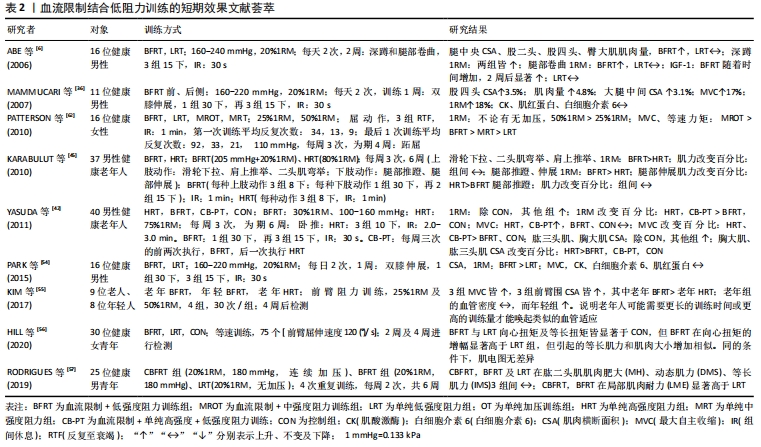
2.2.2 血流限制结合低阻训练的短期效应 表2显示:①从肌肉肥大效益看,血流限制训练进行到1-6周(两三次/周、加压160-240 mmHg、抗阻20%-50%1RM)时,健康成人及老人腿部肌肉横断面积及肌肉量皆有显著增加[6,32,54]。②当血流限制训练持续3-6周后,2次/d,抗阻强度介于20%-50%1RM,结果发现肱二头肌、腿伸肌、肱三头肌和胸大肌肌力百分比明显提升[55-57];而老年人经4周血流限制训练后,阻力设置25%-50%1RM,共4组,30次/组,结果表明血管密度无影响,表明老年人或许需要更长的训练时间或更高的训练量才能唤起类似的血管适应[55]。③血流限制训练持续一两周后,2次/周,低抗阻20%1RM,训练后白细胞介素6与肌红蛋白浓度无改变[32,54],说明未引起肌肉损伤及发炎反应。结果可见,要提升血流限制训练的短期效应,特别需要提升肌力或获得肌肉肥大,抗阻强度不能太低,一般要达到65%1RM,同时训练时间不能太短[3,53]。"
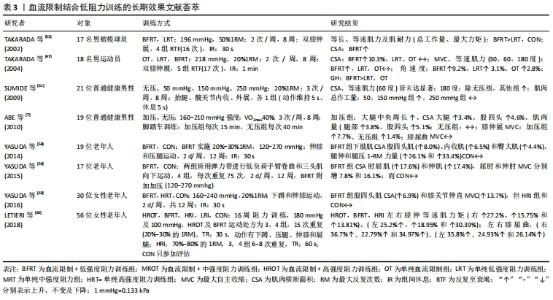
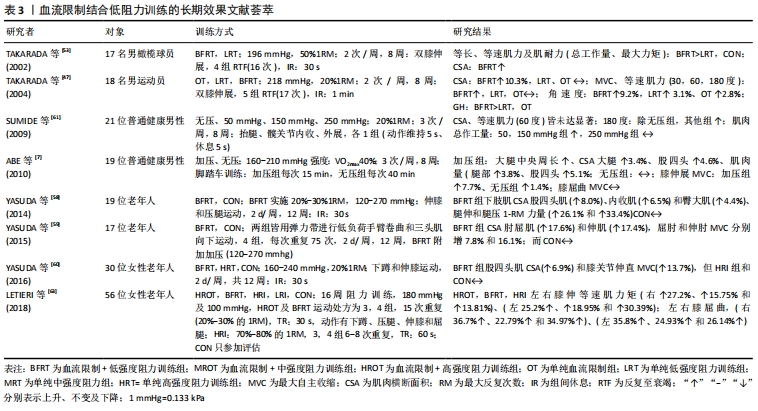
2.2.3 血流限制结合低阻训练的中长期效应 表3显示:①血流限制训练在竞技体育方面,运动选手经过8周及以上的血流限制训练后,受试者最大收缩力、等速肌力及肌肉横断面积显著增加,且不会因为自身训练量高而降低效果,甚至在一些专项力量训练方面效果明显[58-60]。②血流限制训练针对普通健身者,经过8-16周的血流限制训练后(两三次/周),皆能显著增加中、老年人的1RM、最大收缩力、等速肌力、肌肉横断面积及肌耐力[6-7]。③在阻力强度设置方面,长期的“高强度或中等强度阻力+有(无)加压”训练,结果发现在“中强度与高强度阻力训练+加压训练”并没有获得额外增加肌力和肌肉肥大的效果[61];但在短期效果表现上[62-63],“25%1RM+有(无)加压”训练和“50%1RM+有(无)加压训练”,训练时间4周、3次/周、每次3组,结果发现,有加压训练的脚跖屈动作,1RM及最大收缩力值显著高于无加压训练的脚,且“50%1RM+加压”训练脚的提升幅度显著高于“25%1RM+加压”训练的脚。④长期血流限制训练对增加老年(女性)人肌力与单纯高强度抗阻训练效果相似,但“加压+高强度抗阻”训练比纯粹高强度抗阻训练更有效,且停练6周后发现,肌肉力量保持效果更好。"
| [1] ROTA DS, THIAGO M, LIBARDI CA, et al. Concurrent training with blood flow restriction does not decrease inflammatory markers. Int J Sports Med. 2018;39(1):29-36. [2] PATON CD, ADDIS SM, TAYLOR LA. The effects of muscle blood flow restriction during running training on measures of aerobic capacity and run time to exhaustion. Eur J Appl Physiol. 2017;117(12):2579-2585. [3] KRAEMER WJ, RATAMESS NA. Fundamentals of resistance training: progression and exercise prescription. Med Sci Sports Exerc. 2004;36(4):674-688. [4] MANIMMANAKOM A, HAMLIN MJ, ROBB JJ, et al. Effects of low-load resistance training combined with blood flow restriction or hypoxia on muscle function and performance in netball athletes. J Sci Med Sport. 2013;16(4):337-342. [5] FROST DM, CRONIN J, NEWTON RU. A biomechanical evaluation of resistance fundamental concepts for training and sports performance. Sports Med. 2010;40(4):303-326. [6] ABE T, KEARNS CF, SATO Y. Muscle size and strength are increased following walk training with restricted venous blood flow from the leg muscle, Kaatsu-walk training. J Appl Physiol. 2006;100(5):1460-1466. [7] ABE T, SAKAMAKI M, FUJITA S, et al. Effects of low-intensity walk training with restricted leg blood flow on muscle strength and aerobic capacity in older adults. J Geriatr Phys Ther. 2010;33(1):34-40. [8] CLARKSON MJ, CONWAY L, WARMINGTON SA. Blood flow restriction walking and physical function in older adults: a randomized control trial.Journal of Science and Medicine in Sport, 2017;20(12):1041-1046. [9] TOTH MJ, CALLAHAN DM, MILLER MS, et al. Skeletal muscle fiber size and fiber type distribution in human cancer: effects of weight loss and relationship to physical function. Clin Nutr. 201635(6):1359-1365. [10] LU ZH, WOO J, KWOK T. The Effect of Physical Activity and Cardiorespiratory Fitness on All-Cause Mortality in Hong Kong Chinese Older Adults. J Gerontol A Biol Sci Med Sci. 2018;73(8):1132-1137. [11] AFSHARIPOUR B, MANZUR N, DUCHCHERER J, et al. Estimation of self-sustained activity produced by persistent inward currents using firing rate profiles of multiple motor units in humans. J Neurophysiol. 2020;124(1):63-85. [12] GOULD JR, CLELAND BT, MANI D, et al. Motor unit activity in biceps brachii of left-handed humans during sustained contractions with two load types. J Neurophysiol. 2016;116(3):1358-1365. [13] BERETTA-PICCOLI M, CESCON C, BARBERO M, et al. Reliability of surface electromyography in estimating muscle fiber conduction velocity: a systematic review. J Electromyogr Kinesiol. 2019;48:53-68. [14] DOWNS ME, HACKNEY KJ, MARTIN D, et al. Acute Vascular and Cardiovascular Responses to Blood Flow-Restricted Exercise. Med Sci Sports Exerc. 2014;46(8): 1489-1497. [15] ROSCHEL H, UGRINOWISTCH C, SANTOS AR, et al. Effect of eccentric action velocity on expression of genes related to myostatin signaling pathway in human skeletal muscle. Biol Sport. 2018;35(2):111-119. [16] LAURENTINO G, UGRINOWITSCH C, ROSCHEL H, et al. Strength training with blood flow restriction diminishes myostatin gene expression. Med Sci Sports Exerc, 2012,44(3):406-412. [17] GUO Y, WANG M, GE J, et al. Bioactive biodegradable polycitrate nanoclusters enhances the myoblast differentiation and in vivo skeletal muscle regeneration via p38 MAPK signaling pathway. Bioact Mater. 2020;5(3):486-495. [18] LOENNEKE JP, WILSON GJ, WILSON JM. A mechanistic approach to blood flow occlusion. Int J Sports Med. 2010;31(1):1-4. [19] MCFARLANE C, PLUMMER E, THOMAS M, et al. Myostatin induces cachexia by activating the ubiquitin proteolytic system through an NF-kappaB-independent, FoxO1-dependent mechanism. J Cell Physiol. 2006;209(2):501-514. [20] KAYA M, KAYA D, IDIMAN E, et al. A Novel Biomarker in diabetic macular edema with serous retinal detachment: serum chitinase-3-like protein 1. Ophthalmologic. 2019;241(2):90-97. [21] CARVALHO G, CARLA V, MANTOVANI CS, et al. CREB, NF-Y and MEIS1 conserved binding sites are essential to balance Myostatin promoter/enhancer activity during early myogenesis. Molecular Biology Reports. 2017;44(5):419-427. [22] JUANG J, YIN H, ZHANG C, et al. Effects of E. Coli Infection on the Expressions of TGF-β/Smads Signaling Pathway in Broiler Intestine. Poult Sci. 2020;22(1): eRBCA-2019-1101. [23] ZHANG ZP, JIANG HX, LI X, et al. MiR-92a regulates brown adipocytes differentiation, mitochondrial oxidative respiration, and heat generation by targeting SMAD7. J Cell Biochem. 2020;121(8-9):3825-3836. [24] FIGUEROA A, VICIL F. Post-exercise aortic hemodynamic responses to low-intensity resistance exercise with and without vascular occlusion. Scand J Med Sci Sports. 2011;21(3):431-436. [25] FUJITA S, ABE T, DRUMMOND MJ, et al. Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Appl Physiol. 2007;103(3):903-910. [26] REEVES GV, KRAEMER R, HOLLANDER DB, et al. Comparison of hormone responses following light resistance exercise with partial vascular occlusion and moderately difficult resistance exercise with occlusion. J Appl Physiol. 2006; 101(6):1616-1622. [27] WILK M, KRZYSZTOFIK M, GEPFERT M, et al. Technical and Training Related Aspects of Resistance Training Using Blood Flow Restriction in Competitive Sport - A Review. J Hum Kinet. 2018;65(1):249-260. [28] CHRISTIANSEN D, EIBYE KH, HOSTRUP M, et al. Blood flow-restricted training enhances thigh glucose uptake during exercise and muscle antioxidant function in humans. Metabolism. 2019;98:1-15. [29] YANAGISAWA O, SANOMURA M. Effects of low-load resistance exercise with blood flow restriction on high-energy phosphate metabolism and oxygenation level in skeletal muscle. Int Med Appl Sci. 2017;9(2):67-75. [30] HACKNEY KJ, DOWNS ME, PLOUTZ SL. Blood flow restricted exercise compared to high load resistance exercise during unloading. Aerosp Med Hum Perform. 2016;87(8):688-696. [31] LINERO C, CHOI SJ. Effect of blood flow restriction during low-intensity resistance training on bone markers and physical functions in postmenopausal women. J Exerc Sci Fit. 2021;19(1):57-65. [32] AAGAARD P, JACOBSEN M, JENSEN KY, et al. Effects of Chronic Blood-Flow Restriction Exercise on Skeletal Muscle Size and Myogenic Satellite Cell Expression. Med Sci Sports Exerc. 2016;48(5):1032-1033. [33] SOUZA EO, TRICOLI V, BUENO JC, et al. The acute effects of strength, endurance and concurrent exercises on the Akt/mTOR/p70S6K1 and AMPK signaling pathway responses in rat skeletal muscle. Braz J Med Biol Res. 2013;46(4):343-347. [34] JASTRZEBSKI K, HANNAN KM, TCHOUBRIEVA EB, et al. Coordinate regulation of ribosome biogenesis and function by the ribosomal protein S6 kinase, a key mediator of mTOR function. Growth Factors. 2007;25(4):209-226. [35] FRY CS, GLYNN EL, DRUMMOND MJ, et al. Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J Appl Physiol. 2010;108(5):1199-1209. [36] MAMMUCARI C, MILAN G, ROMANCELLO V, et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6(6):458-471. [37] MANINI TM, VINCENT KR, LEEUWENBURGH CL, et al. Myogenic and proteolytic mRNA expression following blood flow restricted exercise. Acta Physiologica. 2011;201(2):255-263. [38] O’NEILLl BT, BHARDWAJ G, PENNIMAN CM, et al. FoxO Transcription Factors Are Critical Regulators of Diabetes-Related Muscle Atrophy. Diabetes. 2019;68(3):556-570. [39] LOUIS E, RAUE U, YANG Y, et al. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol, 2007;103(5):1744-1751. [40] ISABEL MA, BELEN GSA, PRIEGO T, et al. Formoterol treatment prevents the effects of endotoxin on muscle TNF/NF-kappa B, Akt/mTOR, and proteolytic pathways in a rat model. Role of IGF-I and miRNA 29b. Am J Physiol Endocrinol Metab. 2018;315(4):E705-E714. [41] LIU, XY, YU RH, SUN LJ, et al. The nuclear phosphatase SCP4 regulates FoxO transcription factors during muscle wasting in chronic kidney disease. Kidney Int. 2017;92(2):336-348. [42] YASUDA T, OGASAWARA, R, SAKAMAKI, M, et al. Combined effects of low-intensity blood flow restriction training and high-intensity resistance training on muscle strength and size. Eur J Appl Physiol. 2011;111(10):2525-2533. [43] YANG QM, LI Y, ZHANG XL, et al. Zac1/GPR39 phosphorylating CaMK-II contributes to the distinct roles of Pax3 and Pax7 in myogenic progression. Biochim Biophys Acta Mol Basis Dis. 2018;1864(2):407-419. [44] NIELSEN JL, AAGAARD P, BECH RD, et al. Proliferation of myogenic stem cells in human skeletal muscle in response to low-load resistance training with blood flow restriction. J Physiol. 2012;590(17):4351-4361. [45] KARABULUT M, ABE T, SATO Y, et al. The effects of low-intensity resistance training with vascular restriction on leg muscle strength in older men. Eur J Appl Physiol. 2010;108(1):147-155. [46] GRGIC J, HOMOLAK J, MIKULIC P, et al. Inducing hypertrophic effects of type I skeletal muscle fibers: a hypothetical role of time under load in resistance training aimed at muscular hypertrophy. Med Hypotheses. 2018;112:40-42. [47] TAKARADA Y, TSURUTA T, ISHII N. Cooperative effects of exercise and occlusive stimuli on muscular function in low-intensity resistance exercise with moderate vascular occlusion. Japan J Physiol. 2004;4(6): 585-592. [48] TAKANO H, MORITA T, IIDA H, et al. Hemodynamic and hormonal responses to a short-term low-intensity resistance exercise with the reduction of muscle blood flow. Eur J Appl Physiol. 2005;95(1):65-73. [49] SUGA T, OKITA K, Morita N, et al. Intramuscular metabolism during low intensity resistance exercise with blood flow restriction .J Appl Physiol. 2009;106(4): 1119-1124. [50] LIXANDRAO ME, ROSCHEL H, UGRINOWITSCH C, et al. Blood-flow restriction resistance exercise promotes lower pain and ratings of perceived exertion compared with either high- or low-intensity resistance exercise performed to muscular failure. J Sport Rhabil. 2019;28(7):706-710. [51] SUGA T, OKITA K, TAKADA S, et al. Effect of multiple set on intramuscular metabolic stress during low-intensity resistance exercise with blood flow restriction. Eur J Appl Physiol. 2012;112(11):3915-3920. [52] ILETT MJ, RANTALAINEN L, KESKE MA, et al. The effects of restriction pressures on the acute responses to blood flow restriction exercise. Front Physiol. 2019; 10:1018-1024. [53] TAKARADA Y, SATO Y, ISHII N. Effects of resistance exercise combined with vascular occlusion on muscle function in athletes. Eur J Appl Physiol. 2002;86(4):308-314. [54] PARK SY, KWAK YS, HARVESON A, et al. Low intensity resistance exercise training with blood flow restriction: insight into cardiovascular function, and skeletal muscle hypertrophy in humans. Korean J Physiol Pharmacol. 2015;19(3):191-196. [55] KIM J, LANG JA, PILANIA N, et al. Effects of blood flow restricted exercise training on muscular strength and blood flow in older adults. Exp Erontol. 2017;99:127-132. [56] HILL EC, HOUSH TJ, KELLER JL, et al. Low-load blood flow restriction elicits greater concentric strength than non-blood flow restriction resistance training but similar isometric strength and muscle size. Eur J Appl Physiol. 2020;120(2):425-441. [57] RODRIGUES NG, GOMES DS, FREITAS L, et al. Effects of strength training with continuous or intermittent blood flow restriction on the hypertrophy, muscular strength and endurance of men. Acta Scientiarum-Health Sci. 2019;41:e42273. [58] YASUDA T, FUKUMURA K, FUKUDA T, et al. Muscle size and arterial stiffness after blood flow-restricted low-intensity resistance training in older adults. Scand J Med Sci Sports. 2014;24(5):799-806. [59] YASUDA T, FUKUMURA K, UCHIDA Y, et al. Effects of low-load, elastic band resistance training combined with blood flow restriction on muscle size and arterial stiffness in older adults. J Gerontol A Biol Sci Med Sci. 2015;70(8):950-958. [60] YASUDA T, FUKUMURA K, TOMARU T, et al. Thigh muscle size and vascular function after blood flow-restricted elastic band training in older women. Oncotarget. 2016;7(23):33595-33607. [61] SUMIDE T, SAKURABA K, SAWAKI K, et al. Effect of resistance exercise training combined with relatively low vascular occlusion. J Sci Med Sport. 2009;12(1):107-112. [62] PATTERSON SD, FERGUSON RA. Increase in calf post-occlusive blood flow and strength following short-term resistance exercise training with blood flow restriction in young women. Eur J Appl Physiol. 2010;108(5):1025-1033. [63] LETIERI RV, TEIXEIRA AM, FURTADO GE, et al. Effect of 16 weeks of resistance exercise and detraining comparing two methods of blood flow restriction in muscle strength of healthy older women: a randomized controlled trial. Exp Gerontol. 2018;114:78-86. |
| [1] | Gu Zhengqiu, Xu Fei, Wei Jia, Zou Yongdi, Wang Xiaolu, Li Yongming. Exploratory study on talk test as a measure of intensity in blood flow restriction training [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1154-1159. |
| [2] | Wei Xing, Liu Shufang, Mao Ning. Roles and values of blood flow restriction training in the rehabilitation of knee joint diseases [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 774-779. |
| [3] | Wang Yongchao, Wang Baochen, Wei Zhuying, Bao Chunyu, Liu Shijun, Meng Qinghua. Blood flow restriction method for inducing cross-education phenomenon and muscle function reconstruction [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(17): 2762-2767. |
| [4] | Chen Rong, Zeng Qing, Gong Ze, Huang Guozhi, . Different modes of blood flow restriction in the treatment of senile sarcopenia: therapeutic effects and safety factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(32): 5215-5221. |
| [5] | Yang Jun, Zhang Shaosheng. Mechanism of ribosome biogenesis and exercise adaptation of skeletal muscle hypertrophy [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(27): 4383-4388. |
| [6] | Zhao Jing, Yin Lian, Lei Xuemei, Li Miaomiao, Wang Kun, Zhang Tingran, Luo Jiong. KAASTU training for muscle fitness in the middle-aged and elderly adults: effects and strengths [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(23): 3737-3743. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||