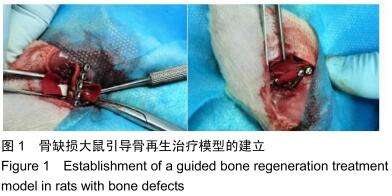
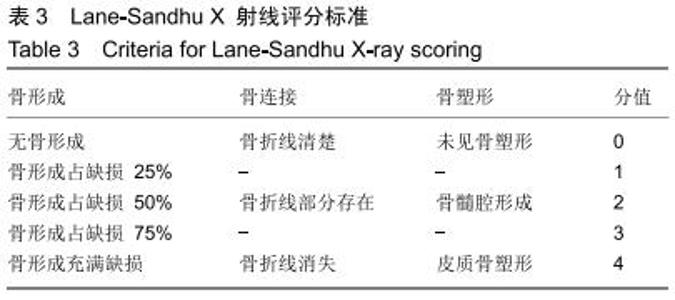
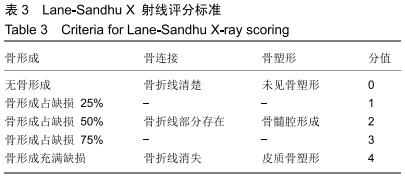
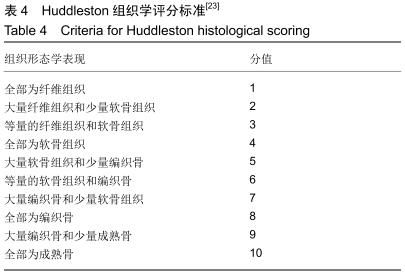
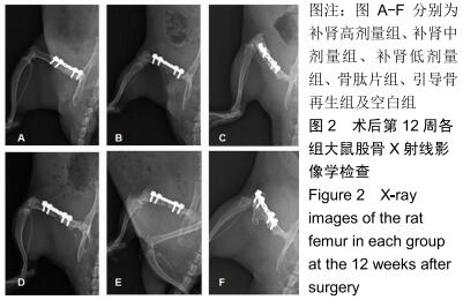
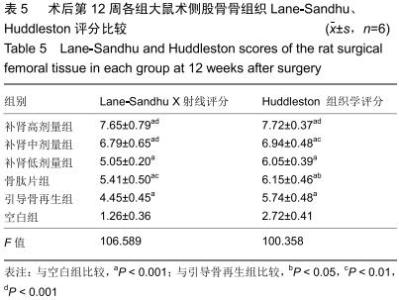
|
[1] 宁钰,赵红斌.骨缺损修复方法的研究进展[J].世界最新医学信息文摘, 2019,19(50):115-117.
[2] JORDANA F, LE VISAGE C, WEISS P. Bone substitutes. Med Sci. 2017;33(1):60-65.
[3] SPARKS DS, SALEH DB, ROZEN WM, et al. Vascularised bone transfer: History, blood supply and contemporary problems. J Plast Reconstr Aesthet Surg.2017; 70(1):1-11.
[4] TARCHALA M, HARVEY EJ, BARRALET J. Biomaterial-stabilized soft tissue healing for healing of critical-sized bonedefects: the masquelet technique. Adv Health Mater. 2016;5(6):630-640.
[5] GOURON R, PETIT L, BOUDOT C, et al. Osteoclasts and their precursors are present in the induced-membrane during bone reconstruction using the Masquelet technique. J Tissue Eng Reg Med. 2017;11(2):382-389.
[6] 臧志海. Masquelet技术临床应用进展[J].继续医学教育,2018,32(6): 78-80.
[7] ILIOPOULOS E, MORRISSEY N, CHO S, et al. Outcomes of the Ilizarov frame use in elderly patients.J Orthop Sci.2017;22(4): 783-786.
[8] 于福铎.Ilizarov技术在治疗下肢创伤后感染性骨缺损的研究[J].中国医药科学, 2019,9(16):218-220.
[9] BENIC GI, HAMMERLE CHF. Horizontal bone augmentation by means of guided bone regeneration. Periodontology. 2014;66(1): 13-40.
[10] GRUBER R, STADLINGER B, TERHEYDEN H. Cell-to-cell communication in guided bone regeneration: molecular and cellularmechanisms. Clin Oral Implants Res.2017;28(9):1139-1146.
[11] CHAPPUIS V, RAHMAN L, BUSER R, et al. Effectiveness of contour augmentation with guided bone regeneration: 10-year results. J Dent Res. 2018;97(3):266-274.
[12] MARDAS N, BUSETTI J, de FIGUEIREDO JAP, et al. Guided bone regeneration in osteoporotic conditions following treatment withzoledronic acid. Clin Oral Implants Res. 2017;28(3):362-371.
[13] KHOJASTEH A, KHEIRI L, MOTAMEDIAN SR, et al. Guided bone regeneration for the reconstruction of alveolar bone defects. Ann Maxillofac Surg. 2017;7(2):263-277.
[14] URBAN IA, MONJE A. Guided Bone Regeneration in Alveolar Bone Reconstruction. Oral Maxillofac Surg Clin North Am.2019; 31(2):331-338.
[15] TOLSTUNOV L, HAMRICK JFE, BROUMAND V, et al. Bone augmentation techniques for horizontal and vertical alveolar ridgedeficiency in oral implantology. Oral Maxillofac Surg Clin North Am. 2019;31(2):163-191.
[16] LOUIS PJ, SITTITAVORNWONG S. Managing bone grafts for the mandible. Oral Maxillofac Surg Clin North Am. 2019;31(2):317-330.
[17] 柳源,刁永帅,冯奇,等. “肾主骨”理论的研究进展[J].辽宁中医杂志, 2019,46(7):1558-1561.
[18] 蒋涛. 基于“肾主骨生髓”理论的补肾中药联合BMP-2诱导人脐血MSCs体外成骨分化的研究[D].南京:南京中医药大学,2012.
[19] NAU C, SIMON S, SCHAIBLE A, et al. Influence of the induced membrane filled with syngeneic bone and regenerativecells on bone healing in a critical size defect model of the rat's femur. Injury. 2018;49(10):1721-1731.
[20] NAU C, SEEBACH C, TRUMM A, et al. Alteration of Masquelet's induced membrane characteristics by different kinds of antibiotic enriched bone cement in a critical size defect model in the rat's femur. Injury. 2016;47(2):325-334.
[21] DAI Z, LI Y, YAN Y, et al. Evaluation of the internal fixation effect of nano-calcium-deficienthydroxyapatite/poly-amino acid composite screws for intraarticular fractures inrabbits. Int J Nanomed. 2018; 13:6625-6636.
[22] GUZEL Y, KARALEZLI N, BILGE O, et al. The biomechanical and histological effects of platelet-rich plasma on fracturehealing. Knee Surg Sports Traumatol Arthrosc. 2015;23(5):1378-1383.
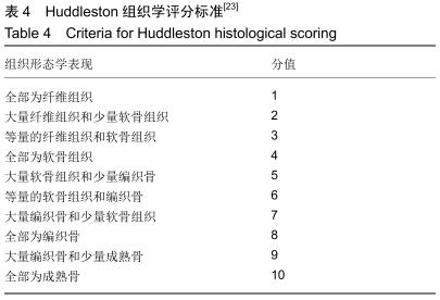
[23] HUDDLESTON PM, STECKELBERG JM, HANSSEN AD, et al. Ciprofloxacin inhibition of experimental fracture healing. J Bone Joint Surg Am. 2000;82(2):161-173.
[24] ELGALI I, OMAR O, DAHLIN C, et al. Guided bone regeneration: materials and biological mechanisms revisited. Eur J Oral Sci. 2017;125(5):315-337.
[25] KIM J, SCHWARZ F, SONG HY, et al. Ridge preservation of extraction sockets with chronic pathology using Bio-Oss((R)) Collagen with or without collagen membrane: an experimental study indogs. Clin Oral Implants Res. 2017;28(6):727-733.
[26] SMEETS R, KNABE C, KOLK A, et al. Novel silk protein barrier membranes for guided bone regeneration. J Biomed Materials Res. 2017;105(8):2603-2611.
[27] KINAIA BM, KAZERANI S, KORKIS S, et al. Effect of guided bone regeneration on immediately placed implants: Meta-analyses with at least 12 months follow up after functional loading. J Periodontol. 2019.
[28] 申震,姜自伟,李定,等.基于牵张成骨技术比较两种补肾法在成血管-成骨耦联机制中的作用差异[J].中华中医药杂志, 2019,34(5):2150-2155.
|