Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (18): 4782-4790.doi: 10.12307/2026.756
Previous Articles Next Articles
Relationship between inflammatory factors, white blood cells, and lumbar disc herniation
Gu Shan1, Zhang Long2, Li Zhigang3
- 1Research Center for Sports and Health Promotion, Southwest Medical University, Luzhou 646000, Sichuan Province, China; 2Department of Rehabilitation, Zigong First People’s Hospital, Zigong 643000, Sichuan Province, China; 3School of Physical Education, Southwest Medical University, Luzhou 646000, Sichuan Province, China
-
Received:2025-08-18Accepted:2025-10-30Online:2026-06-28Published:2025-12-12 -
Contact:Li Zhigang, PhD, Associate Professor, Master’s supervisor, School of Physical Education, Southwest Medical University, Luzhou 646000, Sichuan Province, China -
About author:Gu Shan, MS, Lecturer, Research Center for Sports and Health Promotion, Southwest Medical University, Luzhou 646000, Sichuan Province, China -
Supported by:Key Project of China Panxi Health Industry Research Center, No. PXKY-ZD-202403 (to GS); Higher Education Teaching Reform and Research Project of Southwest Medical University, No. JG2024154 (to GS); Postgraduate Education and Teaching Reform Project of Southwest Medical University, No. YJG202255 (to GS)
CLC Number:
Cite this article
Gu Shan, Zhang Long, Li Zhigang. Relationship between inflammatory factors, white blood cells, and lumbar disc herniation[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(18): 4782-4790.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
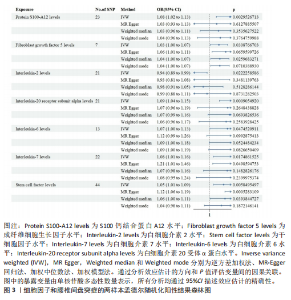
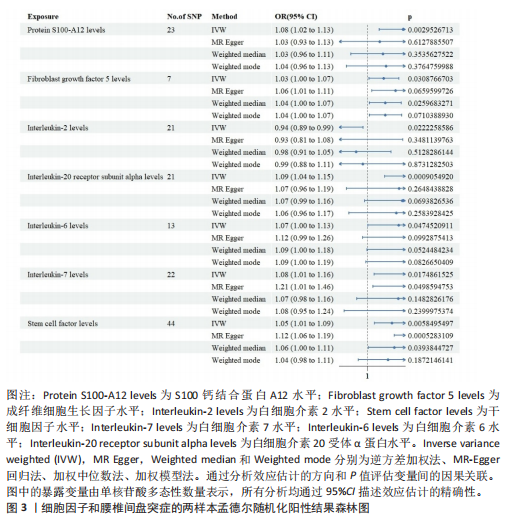
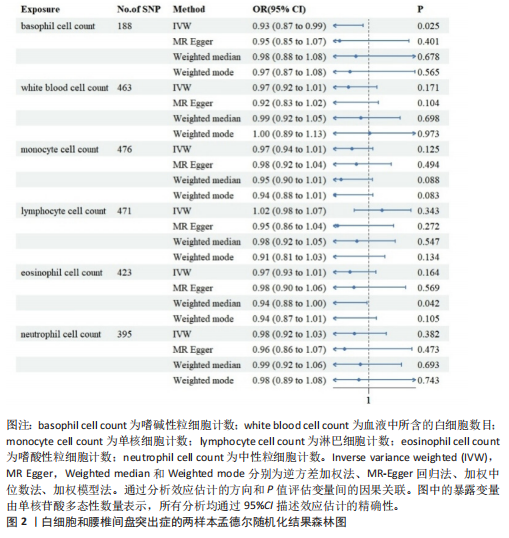
2.1 白细胞与腰椎间盘突出症的因果关系 在剔除连锁不平衡和弱工具变量后,将6种白细胞分别作为暴露,腰椎间盘突出症作为结局,共筛选出188个与嗜碱性粒细胞相关的单核苷酸多态性、463个与白细胞相关的单核苷酸多态性、476个与单核细胞相关的单核苷酸多态性、471个与淋巴细胞相关的单核苷酸多态性、423个与嗜酸性粒细胞相关的单核苷酸多态性、395个与绝对中性粒细胞相关的单核苷酸多态性。孟德尔随机化结果表明循环系统中的嗜碱性粒细胞和嗜酸性粒细胞与腰椎间盘突出症具有因果关系(逆方差加权法:P=0.025,OR=0.93,95%CI:0.87-0.99;加权中位数法:P=0.042,OR=0.94,95%CI:0.88-1.00),其余4种白细胞与腰椎间盘突出症风险均不具有显著因果关系(P > 0.05)(图2)。 2.2 细胞因子与腰椎间盘突出症的因果关系 在剔除连锁不平衡和弱工具变量后,将91种细胞因子分别作为暴露,腰椎间盘突出症作为结局,共筛选出6种在遗传学上可能与腰椎间盘突出症存在关系的蛋白。其中,S100钙结合蛋白A12水平(逆方差加权法:P < 0.05,OR=1.08,95%CI:1.02- 1.13)、成纤维细胞生长因子水平(逆方差加权法:P < 0.05,OR=1.03,95%CI:1.00-1.07)、白细胞介素20受体α蛋白水平(逆方差加权法:P < 0.05,OR=1.09,95%CI:1.04-1.15)、白细胞介素6水平(逆方差加权法:P < 0.05,OR=1.07,95%CI:1.00-1.13)、白细胞介素7水平(逆方差加权法:P < 0.05,OR=1.08,95%CI:1.01-1.16)、干细胞因子水平(逆方差加权法:P < 0.05,OR=1.05,95%CI:1.01-1.09)与腰椎间盘突出症呈正相关因果关系,而白细胞介素2水平(逆方差加权法:P < 0.05,OR=0.94,95%CI:0.89-0.99)与腰椎间盘突出症呈负相关因果关系(图3-5)。该研究同时采用MR?Egger"
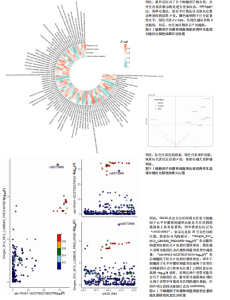
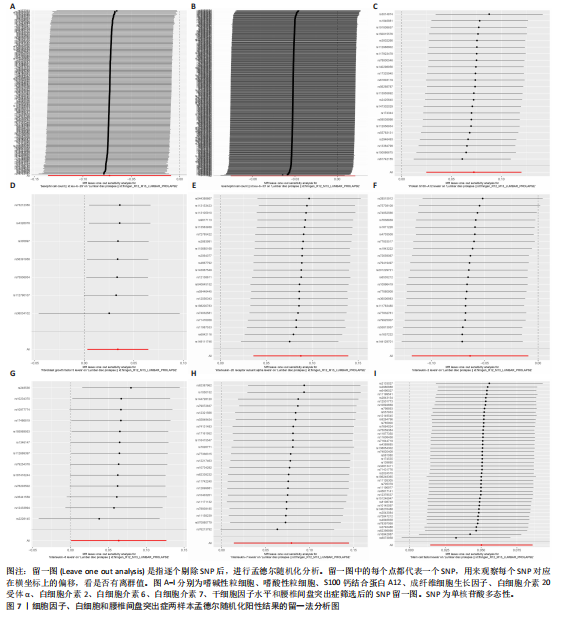
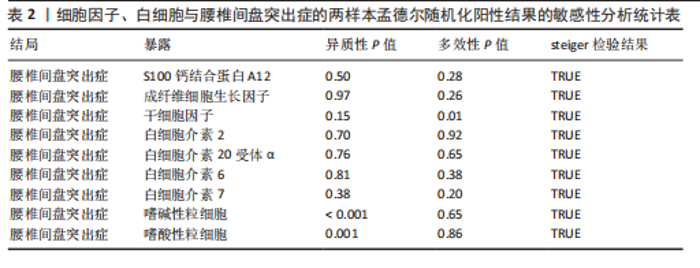
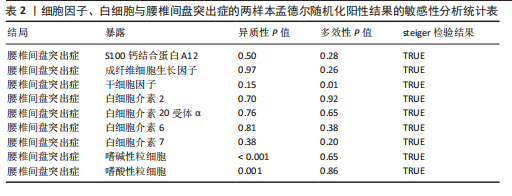
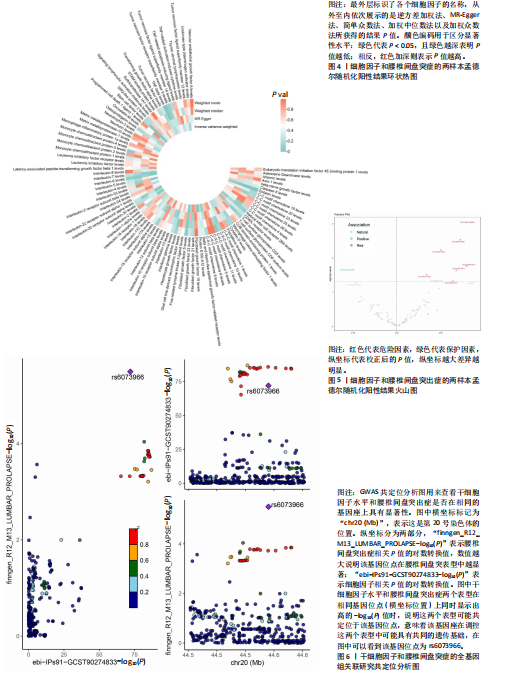
分析、加权中位数和加权众数法分析的结果均与逆方差加权法一致,表明因果关系可靠。 2.3 反向孟德尔随机化分析 在反向孟德尔随机化分析中,在腰椎间盘突出症作为暴露的情况下,并未发现任何阳性结局的细胞因子和白细胞。 2.4 共定位分析结果 全基因组关联研究共定位分析结果显示,干细胞因子水平H3+H4=0.80,其中H3=0.09,H4=0.71,S100钙结合蛋白A12水平H3+H4=0.02,成纤维细胞生长因子水平H3+H4=0.09,白细胞介素20受体α蛋白水平H3+H4=0.08,白细胞介素2水平H3+H4=0.02,白细胞介素6水平H3+H4=0.07,白细胞介素7水平H3+H4=0.02。以上几个阳性结果中,仅有干细胞因子水平在共定位中有显著意义(图6)。干细胞因子水平和腰椎间盘突出症的共定位结果中,H4=0.71,表明干细胞因子水平与腰椎间盘突出症在此区域内的单核苷酸多态性位点显著相关,并且是相同因果变异位点。共定位分析测得的最显著的单核苷酸多态性为rs6073966。 2.5 敏感性分析结果 为增强结果的稳定性,在此研究中还对结果进行了敏感性分析。MR-Egger截距检验表明除干细胞因子水平外的阳性结果不存在水平多效性(P > 0.05)(表2)。经Cochran’s Q检验,全部细胞因子阳性结果P值均≥0.05,说明无异质性。Steiger检验结果P值均 < 0.05为TRUE,说明阳性暴露对结果均为单向阳性作用,这一点和双向孟德尔随机化结果相互印证。留一法未显示异常,说明不存在偏移的单核苷酸多态性。敏感性分析肯定了结果的稳定性和可靠性(图7)。"
| [1] SALO V, MÄÄTTÄ J, SLIZ E, et al. Genome-wide meta-analysis conducted in three large biobanks expands the genetic landscape of lumbar disc herniations. Nat Commun. 2024;15(1):9424. [2] POJSKIC M, BISSON E, OERTEL J, et al. Lumbar disc herniation: Epidemiology, clinical and radiologic diagnosis WFNS spine committee recommendations. World Neurosurg X. 2024;22:100279. [3] WANG Z, LIU X, GAO K, et al. Clinical effects and biological mechanisms of exercise on lumbar disc herniation. Front Physiol. 2024;15:1309663. [4] JIN L, XIAO L, DING M, et al. Heterogeneous macrophages contribute to the pathology of disc herniation induced radiculopathy. Spine J. 2022;22(4):677-689. [5] FRANCISCO V, PINO J, GONZÁLEZ-GAY MÁ, et al. A new immunometabolic perspective of intervertebral disc degeneration. Nat Rev Rheumatol. 2022;18(1):47-60. [6] XUE P, WANG Y, LV L, et al. Roles of Chemokines in Intervertebral Disk Degeneration. Curr Pain Headache Rep. 2024;28(3):95-108. [7] RISBUD MV, SHAPIRO IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014; 10(1):44-56. [8] 高增杰,蒲翔,李来来,等.甾醇酯增加骨质疏松病理性骨折风险:来自IEU-GWAS与芬兰数据库的证据[J].中国组织工程研究,2025,29(15):2345-2353. [9] CHEN MH, RAFFIELD LM, MOUSAS A, et al. Trans-ethnic and Ancestry-Specific Blood-Cell Genetics in 746,667 Individuals from 5 Global Populations. Cell. 2020;182(5):1198-1213.e14. [10] JACOBSEN HE, KHAN AN, LEVINE ME, et al. Severity of intervertebral disc herniation regulates cytokine and chemokine levels in patients with chronic radicular back pain. Osteoarthritis Cartilage. 2020;28(10):1341-1350. [11] WERNERSSON S, PEJLER G. Mast cell secretory granules: armed for battle. Nat Rev Immunol. 2014;14(7):478-494. [12] FOWLKES V, WILSON CG, CARVER W, et al. Mechanical loading promotes mast cell degranulation via RGD-integrin dependent pathways. J Biomech. 2013;46(4):788-795. [13] KNEILLING M, HÜLTNER L, PICHLER BJ, et al. Targeted mast cell silencing protects against joint destruction and angiogenesis in experimental arthritis in mice. Arthritis Rheum. 2007;56(6):1806-1816. [14] STEVENS RL, SOMERVILLE LL, SEWELL D, et al. Serosal mast cells maintain their viability and promote the metabolism of cartilage proteoglycans when cocultured with chondrocytes. Arthritis Rheum. 1992; 35(3):325-335. [15] WIET MG, PISCIONERI A, KHAN SN, et al. Mast Cell-Intervertebral disc cell interactions regulate inflammation, catabolism and angiogenesis in Discogenic Back Pain. Sci Rep. 2017;7(1):12492. [16] LIAO Z, LIU H, MA L, et al. Engineering Extracellular Vesicles Restore the Impaired Cellular Uptake and Attenuate Intervertebral Disc Degeneration. ACS Nano. 2021;15(9):14709-14724. [17] HE J, ZHANG X, WEI Y, et al. Low-dose interleukin-2 treatment selectively modulates CD4(+) T cell subsets in patients with systemic lupus erythematosus. Nat Med. 2016;22(9):991-993. [18] WU PH, KIM HS, JANG IT. Intervertebral Disc Diseases PART 2: A Review of the Current Diagnostic and Treatment Strategies for Intervertebral Disc Disease. Int J Mol Sci. 2020;21(6):2135. [19] JORSSEN J, VAN HULST G, MOLLERS K, et al. Single-cell proteomics and transcriptomics capture eosinophil development and identify the role of IL-5 in their lineage transit amplification. Immunity. 2024;57(7): 1549-1566.e8. [20] YANG BG, SEOH JY, JANG MH. Regulatory Eosinophils in Inflammation and Metabolic Disorders. Immune Netw. 2017;17(1):41-47. [21] EBO DG, BAHRI R, TONTINI C, et al. Mast cell versus basophil activation test in allergy: Current status. Clin Exp Allergy. 2024;54(6):378-387. [22] MIYAKE K, KARASUYAMA H. Emerging roles of basophils in allergic inflammation. Allergol Int. 2017;66(3):382-391. [23] VOGL T, PRÖPPER C, HARTMANN M, et al. S100A12 is expressed exclusively by granulocytes and acts independently from MRP8 and MRP14. J Biol Chem. 1999;274(36):25291-25296. [24] LIRA-JUNIOR R, HOLMSTRÖM SB, CLARK R, et al. S100A12 Expression Is Modulated During Monocyte Differentiation and Reflects Periodontitis Severity. Front Immunol. 2020;11:86. [25] FOELL D, KANE D, BRESNIHAN B, et al. Expression of the pro-inflammatory protein S100A12 (EN-RAGE) in rheumatoid and psoriatic arthritis. Rheumatology (Oxford). 2003;42(11):1383-1389. [26] FOELL D, WITTKOWSKI H, HAMMERSCHMIDT I, et al. Monitoring neutrophil activation in juvenile rheumatoid arthritis by S100A12 serum concentrations. Arthritis Rheum. 2004;50(4):1286-1295. [27] ORCZYK K, SMOLEWSKA E. A Granulocyte-Specific Protein S100A12 as a Potential Prognostic Factor Affecting Aggressiveness of Therapy in Patients with Juvenile Idiopathic Arthritis. J Immunol Res. 2018; 2018:5349837. [28] FRIEDL A, CHANG Z, TIERNEY A, et al. Differential binding of fibroblast growth factor-2 and -7 to basement membrane heparan sulfate: comparison of normal and abnormal human tissues. Am J Pathol. 1997;150(4):1443-1455. [29] LI X, AN HS, ELLMAN M, et al. Action of fibroblast growth factor-2 on the intervertebral disc. Arthritis Res Ther. 2008; 10(2):R48. [30] PRATSINIS H, CONSTANTINOU V, PAVLAKIS K, et al. Exogenous and autocrine growth factors stimulate human intervertebral disc cell proliferation via the ERK and Akt pathways. J Orthop Res. 2012;30(6):958-964. [31] YANG J, XU W, CHEN D, et al. Evidence from Mendelian randomization analysis combined with meta-analysis for the causal validation of the relationship between 91 inflammatory factors and lumbar disc herniation. Medicine (Baltimore). 2024; 103(47):e40323. [32] LI J, YANG H, WANG T, et al. IL-20RA is Associated with the Risk of Diabetic Microangiopathy: A Bidirectional Mendelian Randomization Analysis and Clinical Validation. Diabetes Metab Syndr Obes. 2024;17:4803-4816. [33] SPOLSKI R, LI P, LEONARD WJ. Biology and regulation of IL-2: from molecular mechanisms to human therapy. Nat Rev Immunol. 2018;18(10):648-659. [34] POL JG, CAUDANA P, PAILLET J, et al. Effects of interleukin-2 in immunostimulation and immunosuppression. J Exp Med. 2020; 217(1):e20191247. [35] ZHANG F, ZHAO X, SHEN H, et al. Molecular mechanisms of cell death in intervertebral disc degeneration (Review). Int J Mol Med. 2016;37(6):1439-1448. [36] OROZCO VALENCIA A, CAMARGO KNIRSCH M, et al. Interleukin-2 as immunotherapeutic in the autoimmune diseases. Int Immunopharmacol. 2020;81:106296. [37] HUMRICH JY, VON SPEE-MAYER C, SIEGERT E, et al. Low-dose interleukin-2 therapy in refractory systemic lupus erythematosus: an investigator-initiated, single-centre phase 1 and 2a clinical trial. Lancet Rheumatol. 2019;1(1):e44-e54. [38] ZHANG R, ZHAO Y, CHEN X, et al. Low-dose IL-2 therapy in autoimmune diseases: An update review. Int Rev Immunol. 2024; 43(3):113-137. |
| [1] | Cai Ziming, Yu Qinghe, Ma Pengfei, Zhang Xin, Zhou Longqian, Zhang Chongyang, Lin Wenping. Heme oxygenase-1 alleviates lipopolysaccharide-induced inflammatory response in nucleus pulposus mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1624-1631. |
| [2] | He Jiale, Huang Xi, Dong Hongfei, Chen Lang, Zhong Fangyu, Li Xianhui. Acellular dermal matrix combined with adipose-derived stem cell exosomes promotes burn wound healing [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1699-1710. |
| [3] | Xia Linfeng, Wang Lu, Long Qianfa, Tang Rongwu, Luo Haodong, Tang Yi, Zhong Jun, Liu Yang. Human umbilical cord mesenchymal stem cell-derived exosomes alleviate blood-brain barrier damage in mice with septic encephalopathy [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1711-1719. |
| [4] | Cui Lianxu, Li Haomin, Xu Junrong, Tan Baodong, Lu Dahong, Peng Siwei, Wang Jinhui. Effect of umbilical cord mesenchymal stem cell conditioned medium on tissue repair after traumatic craniocerebral injury in miniature pigs [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1730-1735. |
| [5] | Cao Yong, Teng Hongliang, Tai Pengfei, Li Junda, Zhu Tengqi, Li Zhaojin. Interactions between cytokines and satellite cells in muscle regeneration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1808-1817. |
| [6] | Hou Chaowen, Li Zhaojin, Kong Jianda, Zhang Shuli. Main physiological changes in skeletal muscle aging and the multimechanism regulatory role of exercise [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1464-1475. |
| [7] | You Huijuan, Wu Shuzhen, Rong Rong, Chen Liyuan, Zhao Yuqing, Wang Qinglu, Ou Xiaowei, Yang Fengying. Macrophage autophagy in lung diseases: two-sided effects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1516-1526. |
| [8] | Guo Ying, Tian Feng, Wang Chunfang. Potential drug targets for the treatment of rheumatoid arthritis: large sample analysis from European databases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1549-1557. |
| [9] | Yin Yongcheng, Zhao Xiangrui, Yang Zhijie, Li Zheng, Li Fang, Ning Bin. Effect and mechanism of peroxiredoxin 1 in microglial inflammation after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1106-1113. |
| [10] | Zhang Di, Zhao Jun, Ma Guangyue, Sun Hui, Jiang Rong. Mechanism of depression-like behavior in chronic social defeat stress mice based on high-throughput sequencing [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1139-1146. |
| [11] | Li Haojing, Wang Xin, Song Chenglin, Zhang Shengnan, Chen Yunxin. Therapeutic efficacy of extracorporeal shock wave therapy in the upper trapezius muscle area combined with exercise control training in patients with chronic non-specific neck pain [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1162-1170. |
| [12] | Liu Yu, Lei Senlin, Zhou Jintao, Liu Hui, Li Xianhui. Mechanisms by which aerobic and resistance exercises improve obesity-related cognitive impairment [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1171-1183. |
| [13] | Yu Huifen, Mo Licun, Cheng Leping. The position and role of 5-hydroxytryptamine in the repair of tissue injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1196-1206. |
| [14] | Wen Xiaolong, Weng Xiquan, Feng Yao, Cao Wenyan, Liu Yuqian, Wang Haitao. Effects of inflammation on serum hepcidin and iron metabolism related parameters in patients with type 2 diabetes mellitus: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1294-1301. |
| [15] | Chen Yixian, Chen Chen, Lu Liheng, Tang Jinpeng, Yu Xiaowei. Triptolide in the treatment of osteoarthritis: network pharmacology analysis and animal model validation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 805-815. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||