Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (10): 2671-2680.doi: 10.12307/2026.648
Immune cells synergistically regulate inflammatory response, muscle regeneration and metabolic homeostasis in training-induced stress responses
Mao Sujie1, Gao Jie1, Pan Zhuangli2
- 1Graduate School of Harbin Sport University, Harbin 150000, Heilongjiang Province, China; 2Guizhou Institute of Sports Science (Guizhou Anti-Doping Center), Guiyang 550002, Guizhou Province, China
-
Received:2025-06-06Accepted:2025-06-28Online:2026-04-08Published:2025-09-01 -
Contact:Pan Zhuangli, PhD candidate, Assistant researcher, Guizhou Institute of Sports Science (Guizhou Anti-Doping Center), Guiyang 550002, Guizhou Province, China -
About author:Mao Sujie, PhD candidate, Graduate School of Harbin Sport University, Harbin 150000, Heilongjiang Province, China -
Supported by:Self-financed Project of Guizhou Institute of Sports Science, No. TKSKY2024004 (to PZL)
CLC Number:
Cite this article
Mao Sujie, Gao Jie, Pan Zhuangli. Immune cells synergistically regulate inflammatory response, muscle regeneration and metabolic homeostasis in training-induced stress responses[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(10): 2671-2680.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
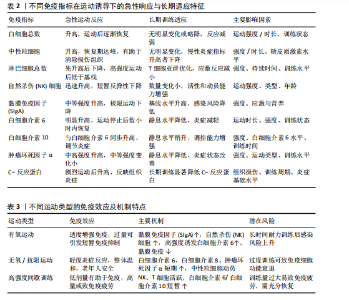
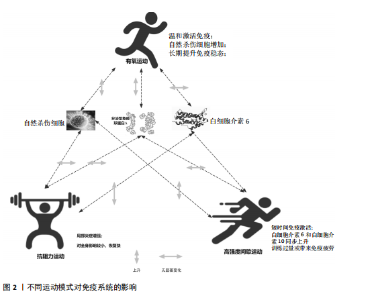
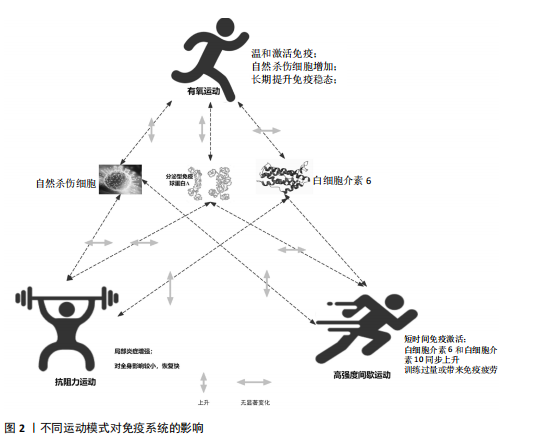
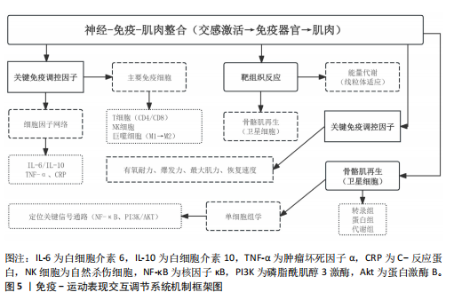
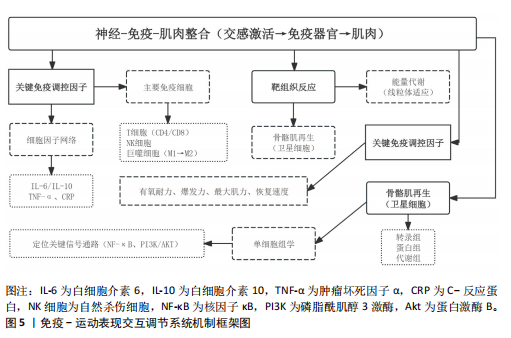
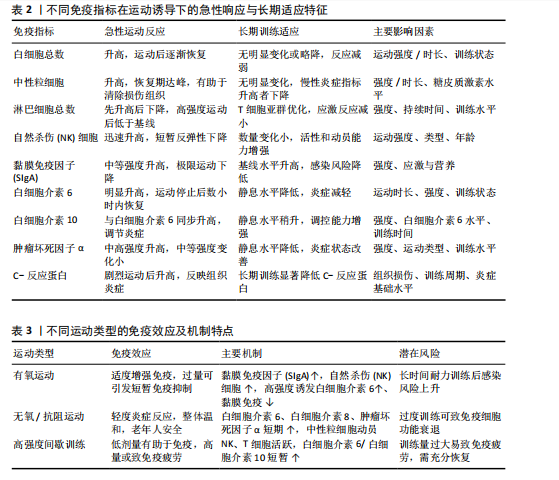
2.1 运动训练对免疫功能的影响 2.1.1 急性运动的免疫反应 (1)免疫系统的短期波动:急性运动会引起白细胞数量短暂升高,NK细胞和中性粒细胞在此阶段快速聚集,提示机体对运动刺激产生了快速的免疫应答[13]。运动过程中,交感神经系统被激活,大量释放儿茶酚胺(肾上腺素),促使NK细胞通过β肾上腺素受体进入血流;NK细胞作为对运动最敏感的免疫细胞,在运动开始几分钟内即迅速增多,增强机体的免疫监视能力[14]。中性粒细胞的变化则主要受血流切应力和应激激素的影响,在运动后期及恢复早期持续升高,其主要功能是清除运动过程中产生的组织损伤碎片,并通过释放细胞因子(白细胞介素8)调控局部炎症反应。这些免疫细胞的动员是机体对运动应激的快速应答,短时间内可增强免疫防御能力,但在高强度或长时间运动后,可能伴随免疫抑制的发生[15]。 运动过程中,骨骼肌大量合成和释放白细胞介素6[16],使其浓度在耐力运动后可升高至静息水平的数倍甚至数十倍。白细胞介素6既具有促炎作用,可反映组织代谢和炎症应激,也可通过诱导白细胞介素10等抗炎细胞因子发挥炎症抑制作用[16]。白细胞介素10水平通常在运动后同步升高,并在短时间内回归基线,其主要功能是限制促炎反应过度扩展,促进炎症消退[17-18]。而肿瘤坏死因子α的变化则因运动模式不同而有所差异,即使耐力运动强度较高,肿瘤坏死因子α的升高仍较为有限,甚至可能略有下降,这可能与白细胞介素6和白细胞介素10的协同调节有关;而高强度的抗阻训练或间歇训练可能会导致肿瘤坏死因子α短暂升高,反映肌肉组织的局部炎症反应[19]。这种促炎和抗炎因子的动态平衡决定了运动对免疫系统的整体影响,适量运动可促进免疫稳态调节,而超负荷运动则可能引发免疫抑制,使机体在运动后的“开放窗”期易受病原体侵袭(表2)。 (2)不同运动模式的免疫影响:不同形式的运动对免疫系统的影响有显著差异(表3),在有氧运动时,其免疫效应受运动负荷及时长的影响,中等强度耐力运动能动员NK细胞和吞噬细胞,提升机体的免疫监测水平,同时增进黏膜免疫效能,使SIgA水平升高[19]。长时间或高强度耐力训练会对免疫功能形成抑制作用,既往研究表明,完成马拉松比赛后,运动员体内白细胞介素6大量释放,同时伴随血管内皮黏附分子的增加和SIgA水平下降,该状态与“J型曲线”理论相符合,表明中等强度有氧运动能提升免疫力,而高强度运动或过度训练会损害免疫系统,因此,适当的有氧运动强度与持续时长,对维持免疫稳态有着重要意义。 (3)抗阻训练以高强度、短间歇的肌肉收缩为特征,通常会引起局部肌肉组织损伤和炎症递质(白细胞介素6、白细胞介素8、肿瘤坏死因子α)短暂升高,但总体上对全身免疫抑制较少[19]。抗阻训练后的免疫扰动时间持续较短,通常在数小时内恢复,且不会显著降低免疫功能。在老年受试者中,80% 1RM强度的抗阻训练并未导致免疫功能下降,说明合理的力量训练是安全的[20]。另一方面,高强度间歇训练结合了短暂的剧烈运动冲刺和间歇休息,能迅速激活NK细胞和效应T细胞,并引起白细胞介素6和白细胞介素10等促炎与抗炎因子的同步升高,形成复杂的免疫调节环境[21]。以往研究显示,在相同运动量下,高强度间歇训练诱导的白细胞介素6和白细胞介素10水平变化,与中等强度有氧运动相当,说明短时高强度训练对免疫系统是安全且有益的[22-23]。当高强度间歇训练冲刺次数过多或总训练时间过长时,对免疫系统的刺激可能接近高强度耐力运动,甚至诱发免疫疲劳[24-25]。因此,适度的抗阻训练和合理的高强度间歇训练可在避免免疫抑制的同时,实现对免疫功能的良性调节(图2)。 2.1.2 长期运动训练的适应性调整 (1)降低慢性炎症,提高免疫稳态:近期研究表明,持续开展中等强度运动可减少促炎细胞因子,降低全身炎症所带来的负荷[26],一项纳入2 557名健康受试者的Meta分析显示,规律运动持续超12周,可让血浆白细胞介素6、肿瘤坏死因子α和C-反应蛋白浓度显著降低[26]。合理运动可促进白细胞介素10等抗炎细胞因子表达,从而调节炎症平衡,与久坐的人群相比,从事运动的人在静息状态下炎症水平更低;运动不仅可以削减炎症应答程度,还能进一步降低机体的慢性炎症状态,让免疫系统更趋向于稳定状态[17]。 长期运动可优化T细胞亚群构成,降低CD4+/CD8+比值,提高效应T细胞的功能[27]。规律运动可减少衰老相关的炎症,使免疫系统处于更为稳健的适应状态[28]。训练周期的延长也影响抗炎适应的程度,研究发现,12周以上的中等强度运动可显著提高抗炎因子水平,而长期规律训练的个体,其免疫系统对运动诱导的炎症应答更加温和,恢复速度更快[22,29]。因此合理安排运动周期,保持适度训练负荷,有助于促进免疫稳态,降低慢性炎症,提高机体的健康水平。"
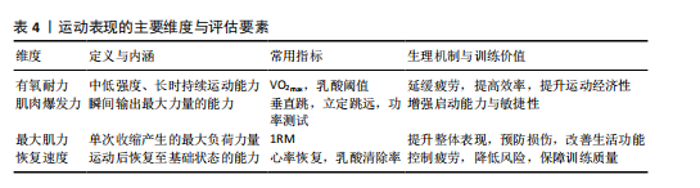
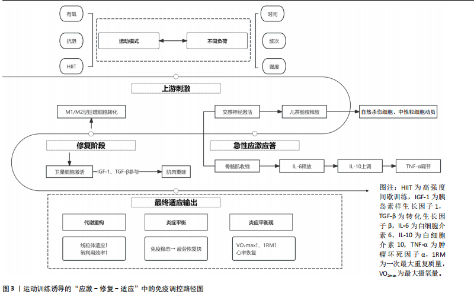
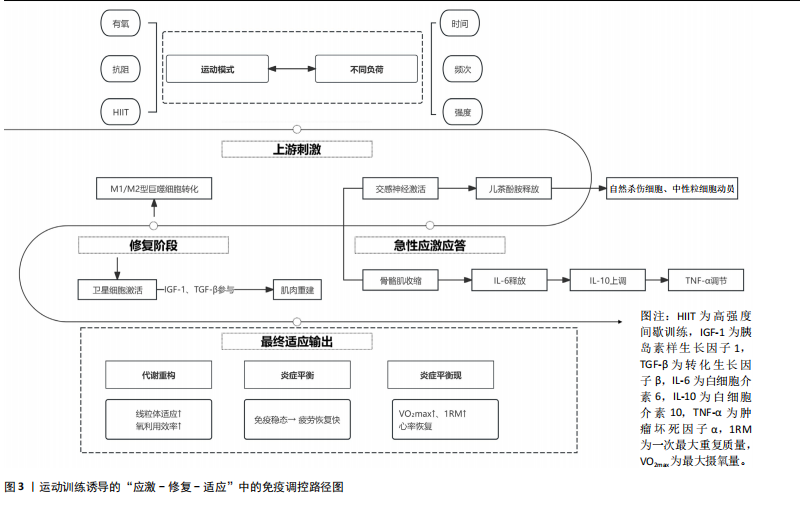
(2)增强免疫防御,提高应激适应:NK细胞作为先天免疫系统的重要组成部分,在长期训练后表现出更强的活性[30]。规律运动可以提高NK细胞的增殖能力和细胞毒活性,使其更有效地识别并杀伤病毒感染细胞及异常增生的肿瘤细胞[7]。虽然静息状态下训练者和非训练者的NK细胞数量差异不大,但训练者的NK细胞在运动应激后的动员速度更快,峰值更高,并且恢复能力更强[31]。长期运动有助于增强巨噬细胞和树突状细胞识别和处理病原的能力[7,11],提高免疫监视水平,使机体在面对感染时反应更快、更有效[32]。 适当运动可促进黏膜免疫增强,减少感染概率[6],能增加唾液中分泌型IgA水平,进而更有力地防御病原体入侵[7]。与久坐的人群相比较,频繁从事体力活动者的分泌型IgA水平更高[33],若训练强度超出限度,或打破这一免疫稳态,则引起慢性皮质醇水平上升、T细胞功能下降,进而让感染的概率有所增加[34],因此,开始制定运动方案时应恰当安排训练负荷,保障充分复原,结合营养补给,以发挥运动对免疫系统的调节作用,同时防止训练过度造成的负面效果。 (3)运动诱导的免疫适应:免疫系统在训练中也影响运动表现,KULLO等[35]研究表明,血清C-反应蛋白水平与VO2max呈中度负相关,控制体脂以及日常活动后,该关联依旧十分显著,显示出炎症负荷较低的个体一般会有更强的有氧能力。动物实验同样显示,肌源性白细胞介素6匮乏的小鼠,耐力水平与正常对照相比大概低20%,显示出白细胞介素6对于体能维持具有重要意义[36]。以往研究发现,调节性T细胞缺失会延长力量恢复时长,补充调节性T细胞或其分泌的Amphiregulin可促使肌肉功能恢复[37]。对运动员训练状态监测发现,连续2周的高强度集训致使赛艇选手调节性T细胞比例降低了约25%,2 km测试成绩停滞,甚至开始下滑,表明免疫耐受能力下降可能会影响训练效果,NK细胞活性也与运动表现关系密切,NK细胞活性越高,机体的乳酸清除速度往往会更快[38]。 高强度训练虽然能提升运动员体能水平,但也会打破免疫调节的稳定状态,剧烈运动结束之后的3-24 h,分泌型IgA下降的幅度可达到30%-50%,皮质醇升高至原先水平的1.5-2.0倍,证实机体处于免疫脆弱阶段[39-40],若恢复未达理想,这种状态可阻滞肌纤维修复,导致体能减退[2]。 2.2 运动训练对运动表现的影响 2.2.1 运动表现的内涵与评价指标 运动表现由有氧耐力、爆发力、最大力量和恢复能力等指标组成[41],这些维度既独立存在,又相互影响,决定运动员的训练成效和竞技表现(表4)。有氧耐力体现机体持续进行中低强度运动的能力,依靠心肺系统高效供氧和骨骼肌对氧气的有效利用。评估有氧耐力的核心指标为最大摄氧量(VO2max)和乳酸阈值:VO2max越高,个体所具有的耐力越好;乳酸阈值越高,表明运动员能够以较高的运动强度维持有氧能量供应[42]。提升有氧耐力可延缓疲劳发生、提高运动经济性,对耐力型运动项目尤为重要。 爆发力是肌肉在短时间内输出最大力量的能力,一般出现在起动、跳跃、冲刺等动作当中[43];最大肌力指肌肉做一次收缩动作时所能产生的最大力量,普遍用1RM评估。机体在剧烈运动完成后回归基础状态的能力,直接涉及训练连续性以及竞技状态稳定性,一般通过心率恢复和乳酸清除速率等指标评估[44],这些指标共同为运动表现的核心要素。 2.2.2 运动表现提升的生理机制 运动能力的增强依靠能量代谢系统和神经肌肉调节的适应性,训练能引起有氧和无氧代谢系统的结构与功能改变[45],有氧代谢为中低强度运动输送能量,训练可提升线粒体密度与氧化酶活性,提升氧气利用效率,由此延缓疲劳的产生,无氧代谢主要发生在高强度、短时间运动,依赖糖酵解迅速供给能源,伴随着乳酸产生;神经肌肉系统的适应性在增强运动能力时同样关键,力量训练可明显增强运动单位的招募效率,提高快缩肌纤维的动员和同步激活能力[46],规律训练能促进神经髓鞘化的发展,提升神经冲动传导速度,改善反应的及时性与动作的流畅表现,这些适应在速度、敏捷性以及协调性要求较高的项目中特别关键。 高强度训练会造成肌纤维轻微损伤,激活多条信号通路。其中,哺乳动物雷帕霉素靶蛋白通路促使蛋白质合成,加快修复过程[47],同时卫星细胞被激活,增殖并迁移至损伤部位,参与新肌纤维形成,该过程受胰岛素样生长因子1等因子调控。肌肉损伤还常伴随局部炎症反应[48]。早期炎症阶段,中性粒细胞和巨噬细胞清除坏死组织,随后巨噬细胞分泌信号分子,调控卫星细胞,促进肌肉再生。炎症反应适度有益,过强或持续过久则可能延缓修复,甚至加重损伤[49]。炎症调控对肌肉重塑至关重要。Emerson等[50]研究表明,冰敷及短期使用非类固醇抗炎药可抑制过强炎症,改善恢复效果,但过度干预可能干扰再生信号传导,影响组织修复。因此,合理控制炎症反应,优化训练与恢复安排,是实现肌肉适应的关键。蛋白质合成、卫星细胞活化与炎症调节的协同调控,是共同促进肌肉功能重塑的生理基础。 2.3 免疫功能与运动表现的交互调控机制 2.3.1 免疫介导的肌肉损伤与修复 高强度或长时间运动易引起肌纤维损伤,免疫系统在此过程中参与组织修复(图3)。中性粒细胞最早到达,清除残片并释放趋化因子,招募单核细胞;随后,巨噬细胞大量浸润,继续清除损伤组织,并通过分泌因子调节卫星细胞、成纤维细胞和内皮细胞,促进肌纤维再生与血管新生,维持局部稳态。研究发现,巨噬细胞缺失或功能异常会显著延缓肌纤维修复,表明其在训练后恢复中至关重要[51]。"
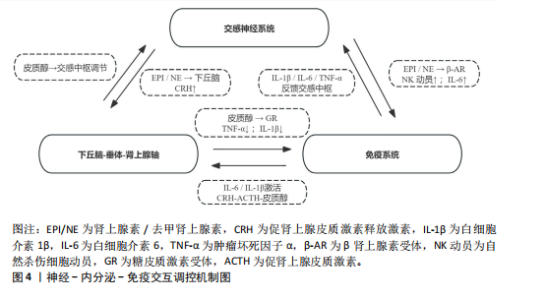
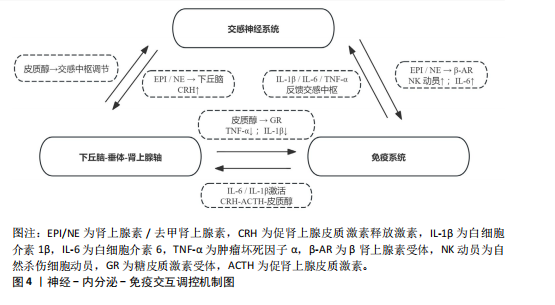
肌肉修复过程中,巨噬细胞由M1型转为M2型。M2型分泌转化生长因子β1、胰岛素样生长因子1和白细胞介素10,促进卫星细胞分化与肌纤维融合。M1主导炎症启动,M2负责组织重建,二者协同促进功能恢复?[52]。NK细胞也参与损伤后的免疫调节。运动后T细胞、B细胞和NK细胞短暂升高,NK细胞可能通过干扰素γ调控巨噬细胞,清除损伤组织,间接促进修复。尽管机制尚不完全清楚,但“清除-再生”过程中的免疫调控已被视为训练适应的关键一环[53]。 已有研究表明,骨骼肌在运动损伤修复过程中,获得性免疫细胞依时间顺序介入,从初期的清除反应过渡到组织重建[54],在损伤发生后6-12 h内,CD11c?树突状细胞首先到达受损区域,清除坏死组织,并通过转化生长因子β与白细胞介素33激活局部免疫;在24-72 h内,Foxp3?调节性T细胞聚集,分泌白细胞介素10与Amphiregulin,缓解炎症,诱导巨噬细胞M2型极化,激活卫星细胞,促进肌肉再生?[37];在72 h后,CD69?CD103?驻留记忆T细胞定植于损伤区域,在继发性损伤中可迅速释放干扰素γ和肿瘤坏死因子α,协同招募修复细胞,启动快速应答?[55]。 适量训练有助于维持调节性T细胞与驻留记忆T细胞活性,过度负荷则可能抑制其扩增,延长炎症过程[56-57]。 上述“树突状细胞-调节性T细胞-驻留记忆T细胞”通路在肌肉修复中具有关键调节作用,为调节性免疫干预提供了可能方向。靶向调节性T细胞-白细胞介素10轴或促进驻留记忆T细胞定植,有望提升组织恢复效率与运动表现[58]。 2.3.2 炎症与抗炎平衡对运动表现的影响 急性炎症反应在运动引起的肌肉微损伤修复过程中发挥着信号激活作用,骨骼肌在收缩过程中分泌的大量白细胞介素6,是一种典型的肌源性细胞因子。与免疫应答中由免疫细胞产生白细胞介素6不同,运动诱导的白细胞介素6主要来源于骨骼肌。运动中白细胞介素6的升高可上调白细胞介素1受体拮抗物和白细胞介素10的表达,同时抑制肿瘤坏死因子α的产生,从而形成先促炎、后抗炎的时序动态[53]。这一平衡机制不仅有助于肌肉的及时修复和炎症的自我限制,还通过免疫代谢轴影响能量分配与组织再生。白细胞介素10上调可促进巨噬细胞从M1型向M2型转化,进而增强肌肉修复进程并控制组织炎症的持续时间。 过度训练、恢复不足、持续应激状态一般会引起慢性炎症,促炎因子一直轻度升高,抗炎因子水平呈现相对下降,这种状态往往造成疲劳累积、恢复迟滞及肌肉的合成受限。临床研究表明,在风湿性关节炎与糖尿病患者中,白细胞介素6、肿瘤坏死因子α水平升高与肌力下降和疲劳感呈正相关,过度训练与慢性促炎状态具有密切联系,表现出持续的疲惫、训练无效、免疫抑制以及运动成绩下滑[59]。动物实验进一步验证了这一机制:在小鼠模型中,给予白细胞介素6长达2周,在90 s内骨骼肌力量下降了18.5%,而且抑制了线粒体的呼吸效能,说明炎症因子对肌肉代谢及耐力系统有显著影响[60]。 训练开始的数分钟后,交感神经大量释放肾上腺素和去甲肾上腺素,β?受体信号迅速动员?NK?细胞和效应?T?细胞,并促使骨骼肌分泌?白细胞介素6,产生促炎?促代谢效应;?30?60? min后,白细胞介素6 反向激活下丘脑-垂体-肾上腺轴,刺激皮质醇分泌,后者在?1?3 h达峰,通过糖皮质激素受体通路下调肿瘤坏死因子α、白细胞介素1β及?T?细胞活性,为早期交感?免疫兴奋“踩刹车”。当训练负荷适中时,交感?下丘脑-垂体-肾上腺轴?免疫循环可在?24? h内闭环;若负荷过大或恢复不足,皮质醇高位滞留且儿茶酚胺清除延缓,将延长炎症窗口期并抑制调节性T细胞/驻留记忆T细胞扩增。由此可见,神经-内分泌-免疫三方在时间序列上的精细协同(图4),是决定运动适应质量与感染风险的关键。正常情况下,交感神经介导的促炎反应在皮质醇开始抑制前及时减弱,以顺利完成损伤终止与修复启动。若交感亢进或皮质醇滞留打乱这一节奏,促炎信号将延宕,或抗炎抑制过度,均会拖慢肌肉再生并引发持续疲劳。因此,训练负荷须在“足够刺激”与“及时平息”之间精准取舍,使炎症窗口保持短暂可逆,保障修复效率与运动表现。 2.3.3 多组学视角下的交互调控 运用多组学技术(基因组学、转录组学、蛋白质组学和代谢组学),能够同步监测大量生物标志物变化,绘制从基因表达到代谢调控的整体图谱,揭示运动引发的免疫应答过程,反映免疫系统与能量代谢、组织修复和肌肉适应之间的复杂关系?[61]。 代谢组学研究表明,剧烈运动会引起乳酸、酮体和嘌呤类代谢物波动较大,这些代谢产物不仅影响能量供需,还调节免疫细胞极化,参与炎症调控。蛋白质组和转录组进一步探索了骨骼肌与免疫系统之间的信号传导机制。在运动过程中,骨骼肌可分泌"
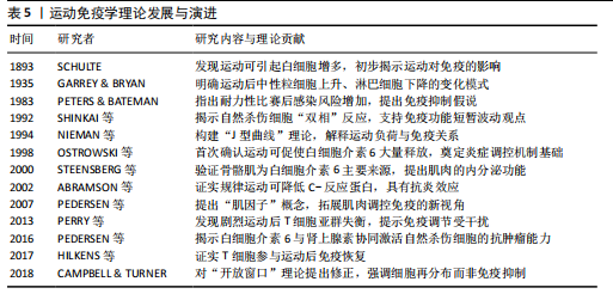
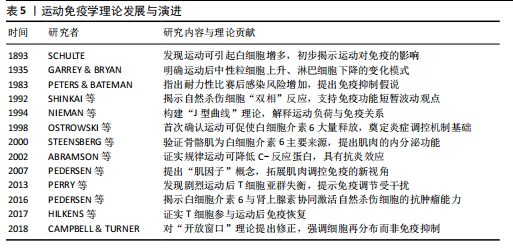
白细胞介素6、白细胞介素7、白细胞介素15等“肌因子”,从不同方式调控免疫细胞的募集、活化与炎症进程?[62]。蛋白质组学可以动态捕捉机体内分子的表达变化,转录组学则揭示了运动诱导的T细胞受体、B细胞受体和干扰素等免疫通路的激活情况,以及不同免疫细胞群的转录表达特征[63]。单细胞测序发现运动后骨骼肌中T细胞、B细胞、NK细胞和单核巨噬细胞数量上升,免疫活性增强。动物实验也证实了这一现象,不同组织对运动的免疫应答存在差异,提示这种调节具有明显的组织特异性[53]。 通过对基因表达谱、蛋白质组与代谢通量数据的整合,研究者得以系统筛选出稳定参与运动诱导免疫反应的关键因子。多种信号通路不仅影响免疫细胞功能,还参与调控肌肉修复、代谢适应和炎症终止过程[64-65]。白细胞介素6/白细胞介素10轴和肿瘤坏死因子α/核因子κB通路的双向调节作用也被认为是影响运动恢复质量的关键机制。整合分析还揭示了若干潜在“枢纽因子”:特定转录因子、代谢酶或表观调控因子,其在不同运动状态下具有较强的表达一致性和网络中心性,可作为未来靶向干预的候选分子。"
| [1] SIMPSON RJ, CAMPBELL JP, GLEESON M, et al. Can exercise affect immune function to increase susceptibility to infection? Exerc Immunol Rev. 2020;26:8-22. [2] SIMPSON RJ, KUNZ H, AGHA N, et al. Exercise and the Regulation of Immune Functions. Prog Mol Biol Transl Sci. 2015; 135:355-380. [3] NIEMAN DC, HOFFMAN-GOETZ L. Prolonged aerobic exercise, immune response, and risk of infection. Boca Raton: CRC Press, 1996. [4] CICCHELLA A, STEFANELLI C, MASSARO M. Upper Respiratory Tract Infections in Sport and the Immune System Response. A Review. Biology (Basel). 2021;10(5):362. [5] 张勉.赛前强化训练对我国田径短跨项目优秀运动员T淋巴细胞和唾液分泌型免疫球蛋白SIgA的影响[J].体育科学, 2002,22(4):91-94. [6] KUROWSKI M, SEYS S, BONINI M, et al. Physical exercise, immune response, and susceptibility to infections-current knowledge and growing research areas. Allergy. 2022;77(9):2653-2664. [7] SHAO T, VERMA HK, PANDE B, et al. Physical Activity and Nutritional Influence on Immune Function: An Important Strategy to Improve Immunity and Health Status. Front Physiol. 2021;12:751374. [8] PAŁKA T, AMBROŻY T, SADOWSKA-KRĘPA E, et al. The Levels of Markers of Muscle Damage, Inflammation, and Heat Shock Proteins in Judokas and the Extent of Their Changes during a Special Performance Test at Different Ambient Temperatures. Applied Sciences. 2023;13(16):9381. [9] HOOIJMANS MT, SCHLAFFKE L, BOLSTERLEE B, et al. Compositional and Functional MRI of Skeletal Muscle: A Review. J Magn Reson Imaging. 2024;60(3):860-877. [10] FORNAZIERO AM, NOVACK LF, NASCIMENTO VB, et al. Acute Responses of Youth Elite Players to a Football Match in Terms of Blood Markers. Sports (Basel). 2023;11(12): 242. [11] DOMÍNGUEZ-ANDRÉS J, DOS SANTOS JC, BEKKERING S, et al. Trained immunity: adaptation within innate immune mechanisms. Physiol Rev. 2023;103(1):313-346. [12] GRAY JI, FARBER DL. Tissue-Resident Immune Cells in Humans. Annu Rev Immunol. 2022;40:195-220. [13] CANNON JG. Exercise and the acute phase response. Exercise and Immune function. Boca Raton: CRC Press,2024. [14] IMAI J, KATAGIRI H. Regulation of systemic metabolism by the autonomic nervous system consisting of afferent and efferent innervation. Int Immunol. 2022;34(2):67-79. [15] QUINTANA-MENDIAS E, RODRÍGUEZ-VILLALOBOS JM, GASTELUM-ARELLANEZ A, et al. The Effect of Acute Physical Exercise on Natural Killer Cells Populations and Cytokine Levels in Healthy Women. Sports (Basel). 2023;11(10):189. [16] GŁÓWKA N, DURKALEC-MICHALSKI K, WOŹNIEWICZ M. Immunological Outcomes of Bovine Colostrum Supplementation in Trained and Physically Active People: A Systematic Review and Meta-Analysis. Nutrients. 2020;12(4):1023. [17] TEODORO TH, COSTA KPM, PRESTES J, et al. The effects of acute and chronic exercise on immune markers of TH1/TH2 cells in older adults: a systematic review. Front Physiol. 2025;16:1453747. [18] LIRA FS, DOS SANTOS T, CALDEIRA RS, et al. Short-Term High- and Moderate-Intensity Training Modifies Inflammatory and Metabolic Factors in Response to Acute Exercise. Front Physiol. 2017;8:856. [19] SELLAMI M, BRAGAZZI NL, ABOGHABA B, et al. The Impact of Acute and Chronic Exercise on Immunoglobulins and Cytokines in Elderly: Insights From a Critical Review of the Literature. Front Immunol. 2021;12: 631873. [20] FERREIRA-JÚNIOR JB, FREITAS EDS, CHAVES SFN. Exercise: A Protective Measure or an “Open Window” for COVID-19? A Mini Review. Front Sports Act Living. 2020;2:61. [21] PROSCHINGER S, SCHENK A, WEßELS I, et al. Intensity- and time-matched acute interval and continuous endurance exercise similarly induce an anti-inflammatory environment in recreationally active runners: focus on PD-1 expression in Tregs and the IL-6/IL-10 axis. Eur J Appl Physiol. 2023;123(11):2575-2584. [22] ROSE GL, SKINNER TL, MIELKE GI, et al. The effect of exercise intensity on chronic inflammation: A systematic review and meta-analysis. J Sci Med Sport. 2021;24(4): 345-351. [23] ZAID NSN, MUHAMAD AS, JAWIS MN, et al. High-Intensity Interval Training Protocols Variation Response to Immune Parameters and Cardiovascular Risk Factors: A Scoping Review. Springer: Proceedings of the 8th International Conference on Movement, Health and Exercise, 2003. [24] NOURPETELIAN R. Effect of high-intensity interval training on the central and peripheral nervous system in females. Kaunas: Lietuvos Sporto Universitetas, 2022. [25] MCDOUGLE JM, MANGINE GT, TOWNSEND JR, et al. Acute physiological outcomes of high-intensity functional training: a scoping review. PeerJ. 2023;11:e14493. [26] WANG YH, TAN J, ZHOU HH, et al. Long-term exercise training and inflammatory biomarkers in healthy subjects: a meta-analysis of randomized controlled trials. Front Psychol. 2023;14:1253329. [27] PADILHA CS, FIGUEIREDO C, MINUZZI LG, et al. Immunometabolic responses according to physical fitness status and lifelong exercise during aging: New roads for exercise immunology. Ageing Res Rev. 2021;68:101341. [28] PAN H, MENG R, JIA Z, et al. Exercise: a non-drug strategy of NK cell activation. Braz J Med Biol Res. 2024;57:e14144. [29] GOMARASCA M, MICIELSKA K, FARALDI M, et al. Impact of 12-Week Moderate-Intensity Aerobic Training on Inflammasome Complex Activation in Elderly Women. Front Physiol. 2022;13:792859. [30] 石海旺,李婕,吴冲云,等.运动改善药物依赖者免疫功能障碍的研究进展[J].中国体育科技,2021,57(6):38-45. [31] SIEDLIK J, DECKERT J, DUNBAR A, et al. Acute high-intensity exercise enhances T cell proliferation compared to moderate-intensity exercise. Appl Physiol Nutr Metab. 2025;50:1-12. [32] YU X, PEI W, LI B, et al. Immunosenescence, Physical Exercise, and their Implications in Tumor Immunity and Immunotherapy. Int J Biol Sci. 2025;21(3):910-939. [33] SÁNCHEZ MONTALVO A, GOHY S, ROMBAUX P, et al. The Role of IgA in Chronic Upper Airway Disease: Friend or Foe? Front Allergy. 2022;3:852546. [34] KEAST D. Immune responses to overtraining and fatigue. Boca Raton: CRC Press, 2024. [35] KULLO IJ, KHALEGHI M, HENSRUD DD. Markers of inflammation are inversely associated with VO2 max in asymptomatic men. J Appl Physiol (1985). 2007;102(4):1374-1379. [36] FÄLDT J, WERNSTEDT I, FITZGERALD SM, et al. Reduced exercise endurance in interleukin-6-deficient mice. Endocrinology. 2004;145(6):2680-2686. [37] BURZYN D, KUSWANTO W, KOLODIN D, et al. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155(6):1282-1295. [38] KRÜGER K, ALACK K, RINGSEIS R, et al. Exercise induced modulation of natural killer cell function and sprint performance in trained athletes. Eur J Appl Physiol. 2020;120:1767-1779. [39] NIEMAN DC. Exercise, infection, and immunity. Int J Sports Med. 1994;15 Suppl 3:S131-141. [40] WALSH NP, GLEESON M, PYNE DB, et al. Position statement. Part two: Maintaining immune health. Exerc Immunol Rev. 2011; 17:64-103. [41] KALÉN A, BISAGNO E, MUSCULUS L, et al. The role of domain-specific and domain-general cognitive functions and skills in sports performance: A meta-analysis. Psychol Bull. 2021;147(12):1290-1308. [42] MALONE D. Cardiovascular and Pulmonary System. London: Routledge, 2024. [43] JAMES LP, TALPEY SW, YOUNG WB, et al. Strength classification and diagnosis: not all strength is created equal. Strength Cond J. 2023;45(3):333-341. [44] SANDFORD GN, LAURSEN PB, BUCHHEIT M. Anaerobic Speed/Power Reserve and Sport Performance: Scientific Basis, Current Applications and Future Directions. Sports Med. 2021;51(10):2017-2028. [45] OLEKSANDRA B, YURIY F, SVITLANA S, et al. Adaptation of students with different body composition components to aerobic and anaerobic training. Sport Soc. 2021;21(1):1. [46] SPRIET LL. Anaerobic metabolism during exercise. Berlin: Springer, 2022. [47] ATTWATERS M, HUGHES SM. Cellular and molecular pathways controlling muscle size in response to exercise. FEBS J. 2022; 289(6):1428-1456. [48] LÄHTEENMÄKI EI, KOSKI M, KOSKELA I, et al. Resistance exercise with different workloads have distinct effects on cellular respiration of peripheral blood mononuclear cells. Physiol Rep. 2022;10(14):e15394. [49] WU J, REN B, WANG D, et al. Regulatory T cells in skeletal muscle repair and regeneration: recent insights. Cell Death Dis. 2022;13(8):680. [50] EMERSON DM, CHEN SC, KELLY MR, et al. Non-steroidal anti-inflammatory drugs on core body temperature during exercise: A systematic review. J Exerc Sci Fit. 2021; 19(2):127-133. [51] WANG X, ZHOU L. The Many Roles of Macrophages in Skeletal Muscle Injury and Repair. Front Cell Dev Biol. 2022;10:952249. [52] ARNOLD L, HENRY A, PORON F, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204(5):1057-1069. [53] WEI W, RAUN SH, LONG JZ. Molecular Insights From Multiomics Studies of Physical Activity. Diabetes. 2024;73(2):162-168. [54] KUSWANTO W, BURZYN D, PANDURO M, et al. Poor Repair of Skeletal Muscle in Aging Mice Reflects a Defect in Local, Interleukin-33-Dependent Accumulation of Regulatory T Cells. Immunity. 2016;44(2):355-367. [55] CEBRIÁN‑SERRANO A, KHOSRAVI‑ MAHARLOOEI M, BUCHHOLZ VR, et al. CD103⁺ tissue‑resident memory T cells expedite recall responses during skeletal‑muscle regeneration. Front Immunol. 2023;14:1183671. [56] SHAH F, GIRI PS, BHARTI AH, et al. Compromised melanocyte survival due to decreased suppression of CD4+ & CD8+ resident memory T cells by impaired TRM-regulatory T cells in generalized vitiligo patients. Exp Dermatol. 2024;33(1):e14982. [57] MAGNUSSON SP, LANGBERG H, KJAER M. The pathogenesis of tendinopathy: balancing the response to loading. Nat Rev Rheumatol. 2010;6(5):262-268. [58] SCHMITT EG, HARIBHAI D, WILLIAMS JB, et al. IL-10 produced by induced regulatory T cells (iTregs) controls colitis and pathogenic ex-iTregs during immunotherapy. J Immunol. 2012;189(12):5638-5648. [59] LOUATI K, BERENBAUM F. Fatigue in chronic inflammation - a link to pain pathways. Arthritis Res Ther. 2015;17:254. [60] VANDERVEEN BN, FIX DK, MONTALVO RN, et al. The regulation of skeletal muscle fatigability and mitochondrial function by chronically elevated interleukin-6. Exp Physiol. 2019;104(3):385-397. [61] NIEMAN DC, PENCE BD. Exercise immunology: Future directions. J Sport Health Sci. 2020;9(5):432-445. [62] DOCHERTY S, HARLEY R, MCAULEY JJ, et al. The effect of exercise on cytokines: implications for musculoskeletal health: a narrative review. BMC Sports Sci Med Rehabil. 2022;14(1):5. [63] YASEN A, SUN W, AINI A, et al. Single-Cell RNA Sequencing Reveals the Heterogeneity of Infiltrating Immune Cell Profiles in the Hepatic Cystic Echinococcosis Microenvironment. Infect Immun. 2021; 89(12):e0029721. [64] GUO Q, JIN Y, CHEN X, et al. NF-κB in biology and targeted therapy: new insights and translational implications. Signal Transduct Target Ther. 2024;9(1):53. [65] SU J, SONG Y, ZHU Z, et al. Cell-cell communication: new insights and clinical implications. Signal Transduct Target Ther. 2024;9(1):196. [66] MAUGHAN R. History of Exercise Physiology. J Sports Sci. 2015;33(15):1639-1640. [67] RAMESSUR R, DAND N, LANGAN SM, et al. Defining disease severity in atopic dermatitis and psoriasis for the application to biomarker research: an interdisciplinary perspective. Br J Dermatol. 2024;191(1):14-23. [68] NIEMAN DC. Risk of upper respiratory tract infection in athletes: an epidemiologic and immunologic perspective. J Athl Train. 1997;32(4):344-349. [69] SHINKAI S, SHORE S, SHEK PN, et al. Acute exercise and immune function. Relationship between lymphocyte activity and changes in subset counts. Int J Sports Med. 1992; 13(6):452-461. [70] OSTROWSKI K, ROHDE T, ASP S, et al. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J Physiol. 1999;515(Pt 1):287-291. [71] STEENSBERG A, VAN HALL G, OSADA T, et al. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol. 2000;529 Pt 1(Pt 1): 237-242. [72] ABRAMSON JL, VACCARINO V. Relationship between physical activity and inflammation among apparently healthy middle-aged and older US adults. Arch Intern Med. 2002; 162(11):1286-1292. [73] PEDERSEN BK, FISCHER CP. Beneficial health effects of exercise--the role of IL-6 as a myokine. Trends Pharmacol Sci. 2007; 28(4):152-156. [74] PERRY C, PICK M, BDOLACH N, et al. Endurance exercise diverts the balance between Th17 cells and regulatory T cells. PLoS One. 2013;8(10):e74722. [75] PEDERSEN L, IDORN M, OLOFSSON GH, et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab. 2016;23(3):554-562. [76] CLIFFORD T, WOOD MJ, STOCKS P, et al. T-regulatory cells exhibit a biphasic response to prolonged endurance exercise in humans. Eur J Appl Physiol. 2017;117(8):1727-1737. [77] CAMPBELL JP, TURNER JE. Debunking the Myth of Exercise-Induced Immune Suppression: Redefining the Impact of Exercise on Immunological Health Across the Lifespan. Front Immunol. 2018;9:648. [78] HU X, XU J, GU Y. Inverted U-Shaped Association of Soluble Transferrin Receptor Concentrations with Risks of Cardiovascular Diseases in Overweight Individuals: A Cross-Sectional Study. Rev Cardiovasc Med. 2024; 25(12):439. [79] NIE Y, MA Y, WU Y, et al. Association Between Physical Exercise and Mental Health During the COVID-19 Outbreak in China: A Nationwide Cross-Sectional Study. Front Psychiatry. 2021;12:722448. [80] PETERSEN AM, PEDERSEN BK. The anti-inflammatory effect of exercise. J Appl Physiol (1985). 2005;98(4):1154-1162. [81] CHEN Z, LAN H, LIAO Z, et al. Regulatory T cells-centered regulatory networks of skeletal muscle inflammation and regeneration. Cell Biosci. 2022;12(1):112. |
| [1] | Fu Lyupeng, Yu Peng, Liang Guoyan, Chang Yunbing. Electroactive materials applied in spinal surgery [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2113-2123. |
| [2] | Tan Jing, Li Li, Wang Liangliang, Qin Xiangyu. Bionic functional coating improves the integration of titanium implants and skin tissue interface [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2014-2022. |
| [3] | Peng Zhiwei, Chen Lei, Tong Lei. Luteolin promotes wound healing in diabetic mice: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1398-1406. |
| [4] | Yu Huifen, Mo Licun, Cheng Leping. The position and role of 5-hydroxytryptamine in the repair of tissue injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1196-1206. |
| [5] | Jin Zhiyong, Wang Yufeng, Zhao Binjie, Xiong Minquan, Yan Li. Effects of inter-limb asymmetry on athletic performance from the perspective of bilateral limb control strategy [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 949-963. |
| [6] | Zhang Zhaowei, Chen Ouzile, Bai Mingru, Wang Chenglin. Therapeutic potential of bioactive substances secreted by dental mesenchymal stem cells for bone repair [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 163-174. |
| [7] | Luo Wenbin, Li Ruoyun, Pan Chaofan, Luo Changjiang. Engineered exosomes for repairing tissue damage: application potential, excellent biological stability, and targeting specificity [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 204-217. |
| [8] | Chang Jinxia, Liu Yufei, Niu Shaohui, Wang Chang, Cao Jianchun. Visualization analysis of macrophage polarization in tissue repair process [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1486-1496. |
| [9] | Li Jialin, Zhang Yaodong, Lou Yanru, Yu Yang, Yang Rui. Molecular mechanisms underlying role of mesenchymal stem cell secretome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1512-1522. |
| [10] | Zhang Yu, Xu Ruian, Fang Lei, Li Longfei, Liu Shuyan, Ding Lingxue, Wang Yuexi, Guo Ziyan, Tian Feng, Xue Jiajia. Gradient artificial bone repair scaffold regulates skeletal system tissue repair and regeneration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 846-855. |
| [11] | Xiong Zhenghua, Zhou Jianghong, Shen Yi, Han Xuesong . Mesenchymal stem cells and extracellular vesicles in repair of endometrial injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6782-6791. |
| [12] | Zhang Tong, Wang Yan, Yang Chunjia, Yue Qingkun, Wu Qingtian. Role of functional hydrogels in tissue repair after traumatic brain injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(28): 6110-6117. |
| [13] | Ye Chao, Liu Xiaohong. Regulatory strategies for foreign body reactions in biomaterials [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3513-3520. |
| [14] | Jin Pengfei, Ren Jie. Biomechanical changes of the lower limbs in table tennis players with different foot contact patterns of table tennis forehand loop [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(14): 2995-3001. |
| [15] | Zhang Xinrui, Han Yue, Lei Lei, Liu Jianyu, Geng Chengkui. Comparative proteomic analysis of rat adipose-derived mesenchymal stem cells and their exosomes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(13): 2683-2689. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||