Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (31): 6708-6716.doi: 10.12307/2025.672
Previous Articles Next Articles
Biological mechanism of mitophagy in idiopathic pulmonary fibrosis
Xie Yizi1, 2, 3, 4, Lin Xueying1, 2, 4, Zhang Xinxin1, 2, 3, 4, Huang Xiufang1, 2, 3, 4, Zhan Shaofeng2, 4, Jiang Yong5, Cai Yan6
- 1The First Clinical Medical College of Guangzhou University of Chinese Medicine, Guangzhou 510000, Guangdong Province, China; 2The First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510000, Guangdong Province, China; 3Lingnan Medical Research Center of Guangzhou University of Chinese Medicine, Guangzhou 510000, Guangdong Province, China; 4Guangdong Clinical Research Academy of Chinese Medicine, Guangzhou 510000, Guangdong Province, China; 5Shenzhen Hospital of Integrated Traditional Chinese and Western Medicine, Shenzhen 518104, Guangdong Province, China; 6Guangdong Provincial Hospital of Chinese Medicine, Zhuhai, Zhuhai 519015, Guangdong Province, China
-
Received:2024-06-21Accepted:2024-08-12Online:2025-11-08Published:2025-02-25 -
Contact:Cai Yan, Master, Chief physician, Guangdong Provincial Hospital of Chinese Medicine, Zhuhai, Zhuhai 519015, Guangdong Province, China -
About author:Xie Yizi, Doctoral candidate, The First Clinical Medical College of Guangzhou University of Chinese Medicine, Guangzhou 510000, Guangdong Province, China; The First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510000, Guangdong Province, China; Lingnan Medical Research Center of Guangzhou University of Chinese Medicine, Guangzhou 510000, Guangdong Province, China; Guangdong Clinical Research Academy of Chinese Medicine, Guangzhou 510000, Guangdong Province, China -
Supported by:Cultivation and Construction Project of Respiratory Disease Regional Diagnosis and Treatment Center of Guangdong Provincial Hospital of Chinese Medicine, Zhuhai (to CY); Science and Technology Research Special Project of Guangdong Provincial Hospital of Chinese Medicine, No. YN2023MS09 (to CY); Excellent Clinical Talent Training Project of The Second Guangdong Provincial Hospital of Chinese Medicine, No. (2024)88 (to CY); Sanming Project of Medicine in Shenzhen, No. SZZYSM202206013; Guangdong Provincial Key Department (Traditional Chinese and Western Medicine Collaborative Department) Construction Project (to JY); National Traditional Chinese Medicine Advantage Specialty Construction Project (Department of Pulmonary Disease, The First Affiliated Hospital of Guangzhou University of Chinese Medicine) (to ZSF)
CLC Number:
Cite this article
Xie Yizi, Lin Xueying, Zhang Xinxin, Huang Xiufang, Zhan Shaofeng, Jiang Yong, Cai Yan. Biological mechanism of mitophagy in idiopathic pulmonary fibrosis[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6708-6716.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
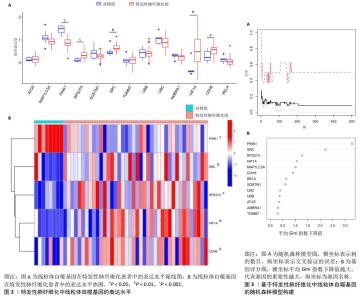
2.1 特发性肺纤维化与线粒体自噬相关基因的获取 通过GSE53845芯片,共获得10 564个特发性肺纤维化潜在基因。通过数据库与文献检索,共获得37个与线粒体自噬有关的基因。 2.2 特发性肺纤维化的线粒体自噬特征基因筛选 特发性肺纤维化中与线粒体自噬相关基因有13个,分别是ATG5、MAP1LC3A、PINK1、RPS27A、SQSTM1、SRC、TOMM7、UBB、UBC、AMBRA1、HIF1A、CDH6和RELA。 通过组间差异表达水平计算,发现PINK1、RPS27A、SRC、HIF1A和CDH6在特发性肺纤维化组和对照组之间的表达水平存在差异,且差异有显著性意义(P < 0.05)。其中,RPS27A、SRC、HIF1A和CDH6在特发性肺纤维化患者中呈高表达趋势,而PINK1呈低表达趋势,见图2。 通过构建机器学习方法中的随机森林模型,发现评分 > 0.5的基因有PINK1、SRC、RPS27A、HIF1A、MAP1LC3A、CDH6、RELA、SQSTM1,见图3。 对组间差异表达基因与随机森林模型所得的基因取交集,得到5个特发性肺纤维化的线粒体自噬特征基因,分别为PINK1、RPS27A、SRC、HIF1A与CDH6,见图4。 "


2.3 特发性肺纤维化中线粒体自噬生物过程的预测 对5个特发性肺纤维化的线粒体自噬特征基因进行富集分析,探索相关的生物过程。 如图5A,B所示,GO功能富集分析主要涉及泛素蛋白连接酶结合、对氧化应激诱导的神经元内在凋亡信号通路的负调控、细胞对缺氧的反应等。 如图5C所示,KEGG通路富集分析主要涉及动物的线粒体自噬和卡波西肉瘤相关疱疹病毒感染。 如图5D所示,Reactome通路富集分析主要涉及PINK1-PRKN介导的线粒体自噬、NOTCH1细胞内结构域调控转录、ERBB2的信号传递、EGFR的信号传递、非受体酪氨酸激酶的信号传导、调节RUNX3的表达和活性、ERBB4的信号传递。 如图5E所示,WIKI通路富集分析主要涉及血管生成与Notch信号通路。 通过富集分析认为,线粒体自噬调控特发性肺纤维化并非通过单一生物过程影响的,而是与其他生物过程相互交错、相互影响,共同发挥调节作用。 "


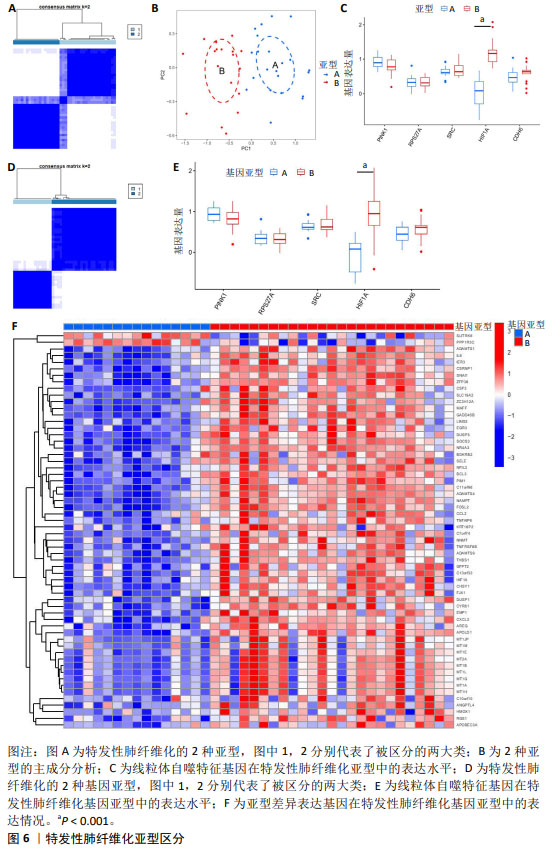
2.4 特发性肺纤维化的线粒体自噬亚型区分 亚型区分可从分子机制层面对特发性肺纤维化进行分类,有助于临床诊断分型以及靶向分子机制的个体化治疗。根据5个特发性肺纤维化的线粒体自噬特征基因区分疾病亚型,见图6A。主成分分析表明线粒体自噬特征基因可以有效区分2种特发性肺纤维化亚型,见图6B。PINK1在亚型B的表达水平偏低,而HIF1A和CDH6在亚型B的表达水平偏高,RPS27A、SRC在亚型A、B之间表达水平相似,但只有HIF1A的表达差异有显著性意义(P < 0.05),见图6C。此外,还计算出亚型A和亚型B之间的差异表达基因共60个。 为进一步探索线粒体自噬对特发性肺纤维化分型的影响,该研究基于60个亚型A与亚型B之间的差异表达基因,再次将特发性肺纤维化患者分成2个基因亚型,分别为基因亚型A与基因亚型B,见图6D。5个线粒体自噬特征基因在基因亚型A、B中的表达趋势与在亚型A、B中的表达趋势相似,见图6E。这提示根据线粒体自噬对特发性肺纤维化进行亚型区分具有较高的可信度。差异表达基因在基因亚型A中普遍为低表达,而在基因亚型B中普遍为高表达,见图6F。 值得注意的是,在两次分型中均只有HIF1A存在显著的表达差异(P < 0.05),且在亚型B与基因亚型B中均呈高表达水平,这提示HIF1A可能成为特发性肺纤维化线粒体自噬亚型区分的关键基因。 "
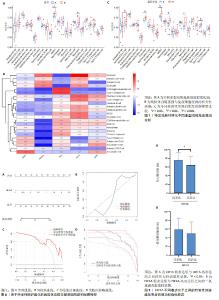
2.5 免疫浸润分析 在特发性肺纤维化亚型之间,骨髓来源抑制性细胞、中性粒细胞、1型辅助性T细胞存在浸润差异,且差异有显著性意义(P < 0.05),见图7A。分析5个线粒体自噬特征基因与免疫细胞之间的相关性,发现HIF1A与免疫细胞存在较强的正相关性,见图7B。在基因亚型之间,活化的CD4+ T细胞、嗜酸性粒细胞、骨髓来源抑制性细胞、肥大细胞、中性粒细胞与1型辅助性T细胞存在浸润差异,且差异有显著性意义(P < 0.05),见图7C。由此可见,特发性肺纤维化亚型与基因亚型之间的免疫浸润差异存在一定的相似性,再一次提示亚型分析的可信度。此外,骨髓来源抑制性细胞、中性粒细胞与1型辅助性T细胞在亚型B、基因亚型B中均呈上调状态,提示免疫状态较为活跃。 2.6 列线图模型构建 通过特发性肺纤维化亚型分析及单样本基因集富集分析,筛选出特发性肺纤维化的线粒体自噬关键基因HIF1A,构建列线图模型。根据患者HIF1A的表达水平可预测特发性肺纤维化的发病风险,见图8A。以Bootstrap法对列线图进行内部验证,对原始数据进行重复抽样1 000次,结果显示平均绝对误差为0.052。实际曲线和理想曲线拟合度一般,见图8B。临床决策曲线提示,当风险阈值为0.65-0.99时,其临床获益率良好,见图8C。临床影响曲线提示,当高风险阈值 > 0.7时,模型判定为特发性肺纤维化的高风险人群与实际发生特发性肺纤维化的人群高度重叠,见图8D。因此,检测HIF1A的表达水平可能预测临床特发性肺纤维化发生风险。 2.7 线粒体自噬关键基因与特发性肺纤维化临床特征关系的探索 HIF1A高表达组的用力肺活量显著低于HIF1A低表达组(P < 0.05),HIF1A高表达组的一氧化碳弥散量也低于HIF1A低表达组,但差异无显著性意义(P > 0.05),见图9。这提示HIF1A高表达的患者肺功能更差,纤维化程度更严重。 "

| [1] 于娜,周家为,李霞,等.成人特发性肺纤维化(更新)和进行性肺纤维化临床实践指南(2022版)解读[J].中国现代医学杂志,2023,33(14):1-8. [2] MARTINEZ FJ, COLLARD HR, PARDO A, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers. 2017;3:17074. [3] LUPPI F, KALLURI M, FAVERIO P, et al. Idiopathic pulmonary fibrosis beyond the lung: understanding disease mechanisms to improve diagnosis and management. Respir Res. 2021;22(1):109. [4] SPAGNOLO P, KROPSKI JA, JONES MG, et al. Idiopathic pulmonary fibrosis: Disease mechanisms and drug development. Pharmacol Ther. 2021;222:107798. [5] 姜秋燕,梁庆,高劭妍,等.特发性肺纤维化治疗药物研究进展[J].中国新药杂志,2021,30(14):1274-1281. [6] DIAMANTOPOULOS A, WRIGHT E, VLAHOPOULOU K, et al. The Burden of Illness of Idiopathic Pulmonary Fibrosis: A Comprehensive Evidence Review. Pharmacoeconomics. 2018;36(7):779-807. [7] ZANK DC, BUENO M, MORA AL, et al. Idiopathic Pulmonary Fibrosis: Aging, Mitochondrial Dysfunction, and Cellular Bioenergetics. Front Med (Lausanne). 2018;5:10. [8] KURITA Y, ARAYA J, MINAGAWA S, et al. Pirfenidone inhibits myofibroblast differentiation and lung fibrosis development during insufficient mitophagy. Respir Res. 2017;18(1):114. [9] BARRETT T, WILHITE SE, LEDOUX P, et al. NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Res. 2013;41(Database issue):D991-D995. [10] SUN K, JING X, GUO J, et al. Mitophagy in degenerative joint diseases. Autophagy. 2021;17(9):2082-2092. [11] 徐文飞,明春玉,段戡,等.WGCNA和机器学习识别骨关节炎铁死亡特征基因及实验验证[J].中国组织工程研究, 2024,28(30):4909-4914. [12] KOLBERG L, RAUDVERE U, KUZMIN I, et al. g:Profiler-interoperable web service for functional enrichment analysis and gene identifier mapping (2023 update). Nucleic Acids Res. 2023;51(W1):W207-W212. [13] LEE SY, AN HJ, KIM JM, et al. PINK1 deficiency impairs osteoblast differentiation through aberrant mitochondrial homeostasis. Stem Cell Res Ther. 2021;12(1):589. [14] GAN ZY, CALLEGARI S, COBBOLD SA, et al. Activation mechanism of PINK1. Nature. 2022;602(7896):328-335. [15] BUENO M, LAI YC, ROMERO Y, et al. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J Clin Invest. 2015;125(2):521-538. [16] WEI Y, SUN L, LIU C, et al. Naringin regulates endoplasmic reticulum stress and mitophagy through the ATF3/PINK1 signaling axis to alleviate pulmonary fibrosis. Naunyn Schmiedebergs Arch Pharmacol. 2023;396(6):1155-1169. [17] TWAMLEY-STEIN GM, PEPPERKOK R, Ansorge W, et al. The Src family tyrosine kinases are required for platelet-derived growth factor-mediated signal transduction in NIH 3T3 cells. Proc Natl Acad Sci U S A. 1993;90(16):7696-7700. [18] AMANCHY R, ZHONG J, HONG R, et al. Identification of c-Src tyrosine kinase substrates in platelet-derived growth factor receptor signaling. Mol Oncol. 2009;3(5-6): 439-450. [19] BARBAYIANNI I, KANELLOPOULOU P, FANIDIS D, et al. SRC and TKS5 mediated podosome formation in fibroblasts promotes extracellular matrix invasion and pulmonary fibrosis. Nat Commun. 2023;14(1):5882. [20] PARK SR, KIM HJ, YANG SR, et al. A novel endogenous damage signal, glycyl tRNA synthetase, activates multiple beneficial functions of mesenchymal stem cells. Cell Death Differ. 2018;25(11):2023-2036. [21] ZHOU MS, ZHENG SY, CHEN C, et al. Gene expression analysis to identify mechanisms underlying improvement of myocardial fibrosis by finerenone in SHR. Biochem Pharmacol. 2024;220:115975. [22] TSUBOUCHI K, ARAYA J, KUWANO K. PINK1-PARK2-mediated mitophagy in COPD and IPF pathogeneses. Inflamm Regen. 2018;38:18. [23] CHOURASIA AH, TRACY K, FRANKENBERGER C, et al. Mitophagy defects arising from BNip3 loss promote mammary tumor progression to metastasis. EMBO Rep. 2015;16(9):1145-1163. [24] KHAWAJA AA, CHONG DLW, SAHOTA J, et al. Identification of a Novel HIF-1α-αMβ2 Integrin-NET Axis in Fibrotic Interstitial Lung Disease. Front Immunol. 2020;11:2190. [25] PHILIP K, MILLS TW, DAVIES J, et al. HIF1A up-regulates the ADORA2B receptor on alternatively activated macrophages and contributes to pulmonary fibrosis. FASEB J. 2017;31(11):4745-4758. [26] KOLAHIAN S, ÖZ HH, ZHOU B, et al. The emerging role of myeloid-derived suppressor cells in lung diseases. Eur Respir J. 2016;47(3):967-977. [27] FERNANDEZ IE, GREIFFO FR, FRANKENBERGER M, et al. Peripheral blood myeloid-derived suppressor cells reflect disease status in idiopathic pulmonary fibrosis. Eur Respir J. 2016;48(4):1171-1183. [28] LIU T, GONZALEZ DE LOS SANTOS F, RINKE AE, et al. B7H3-dependent myeloid-derived suppressor cell recruitment and activation in pulmonary fibrosis. Front Immunol. 2022;13:901349. [29] XU Y, LAN P, WANG T. The Role of Immune Cells in the Pathogenesis of Idiopathic Pulmonary Fibrosis. Medicina (Kaunas). 2023;59(11):1984. [30] ACHAIAH A, RATHNAPALA A, PEREIRA A, et al. Neutrophil lymphocyte ratio as an indicator for disease progression in Idiopathic Pulmonary Fibrosis. BMJ Open Respir Res. 2022;9(1):e001202. [31] WILSON MS, MADALA SK, RAMALINGAM TR, et al. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med. 2010;207(3):535-552. [32] LI Y, LI S, GU M, et al. Application of network composite module analysis and verification to explore the bidirectional immunomodulatory effect of Zukamu granules on Th1 / Th2 cytokines in lung injury. J Ethnopharmacol. 2022;299:115674. [33] ANDERSSON CK, ANDERSSON-SJÖLAND A, MORI M, et al. Activated MCTC mast cells infiltrate diseased lung areas in cystic fibrosis and idiopathic pulmonary fibrosis. Respir Res. 2011;12(1):139. [34] OVERED-SAYER C, RAPLEY L, MUSTELIN T, et al. Are mast cells instrumental for fibrotic diseases? Front Pharmacol. 2014;4:174. [35] HIRAHARA K, AOKI A, MORIMOTO Y, et al. The immunopathology of lung fibrosis: amphiregulin-producing pathogenic memory T helper-2 cells control the airway fibrotic responses by inducing eosinophils to secrete osteopontin. Semin Immunopathol. 2019;41(3):339-348. [36] LIU J, WANG J, XIONG A, et al. Mitochondrial quality control in lung diseases: current research and future directions. Front Physiol. 2023;14:1236651. [37] WANG L, CHEN R, LI G, et al. FBW7 Mediates Senescence and Pulmonary Fibrosis through Telomere Uncapping. Cell Metab. 2020;32(5):860-877.e9. [38] ZHOU Y, HUANG X, HECKER L, et al. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest. 2013; 123(3):1096-1108. [39] BODEMPUDI V, HERGERT P, SMITH K, et al. miR-210 promotes IPF fibroblast proliferation in response to hypoxia. Am J Physiol Lung Cell Mol Physiol. 2014;307(4): L283-L294. [40] YANG L, GILBERTSEN A, XIA H, et al. Hypoxia enhances IPF mesenchymal progenitor cell fibrogenicity via the lactate/GPR81/HIF1α pathway. JCI Insight. 2023;8(4):e163820. [41] WANG Y, SUN Q, GENG R, et al. Notch intracellular domain regulates glioblastoma proliferation through the Notch1 signaling pathway. Oncol Lett. 2021;21(4):303. [42] WASNICK R, KORFEI M, PISKULAK K, et al. Notch1 Induces Defective Epithelial Surfactant Processing and Pulmonary Fibrosis. Am J Respir Crit Care Med. 2023; 207(3):283-299. [43] SHARMA B, SINGH VJ, CHAWLA PA. Epidermal growth factor receptor inhibitors as potential anticancer agents: An update of recent progress. Bioorg Chem. 2021;116: 105393. [44] PATNAIK SK, CHANDRASEKAR MJN, NAGARJUNA P, et al. Targeting of ErbB1, ErbB2, and their Dual Targeting Using Small Molecules and Natural Peptides: Blocking EGFR Cell Signaling Pathways in Cancer: A Mini-Review. Mini Rev Med Chem. 2022; 22(22):2831-2846. [45] LIU X, GENG Y, LIANG J, et al. HER2 drives lung fibrosis by activating a metastatic cancer signature in invasive lung fibroblasts. J Exp Med. 2022;219(10):e20220126. [46] JIANG Y, SHI J, ZHOU J, et al. ErbB4 promotes M2 activation of macrophages in idiopathic pulmonary fibrosis. Open Life Sci. 2023; 18(1):20220692. |
| [1] | Li Jiagen, Chen Yueping, Huang Keqi, Chen Shangtong, Huang Chuanhong. The construction and validation of a prediction model based on multiple machine learning algorithms and the immunomodulatory analysis of rheumatoid arthritis from the perspective of mitophagy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-15. |
| [2] | Lai Pengyu, Liang Ran, Shen Shan. Tissue engineering technology for repairing temporomandibular joint: problems and challenges [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [3] | Liu Lin, Liu Shixuan, Lu Xinyue, Wang Kan. Metabolomic analysis of urine in a rat model of chronic myofascial trigger points [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1585-1592. |
| [4] | Su Xiaoyang, Chen Wenting, Fu Yidan, Zhao Yan, Lan Danfeng, Yang Qiuping. Correlation between Mer receptor tyrosine kinase and diabetic peripheral neuropathy in Sprague-Dawley rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1593-1599. |
| [5] | Li Kaiying, Wei Xiaoge, Song Fei, Yang Nan, Zhao Zhenning, Wang Yan, Mu Jing, Ma Huisheng. Mechanism of Lijin manipulation regulating scar formation in skeletal muscle injury repair in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1600-1608. |
| [6] | Li Jun, Gong Jingjing, Sun Guobin, Guo Rui, Ding Yang, Qiang Lijuan, Zhang Xiaoli, Fang Zhanhai . miR-27a-3p promotes the proliferation of human hypertrophic scar fibroblasts by regulating mitogen-activated protein kinase signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1609-1617. |
| [7] | Li Huayuan, Li Chun, Liu Junwei, Wang Ting, Li Long, Wu Yongli. Effect of warm acupuncture on PINK1/Parkin pathway in the skeletal muscle of rats with chronic fatigue syndrome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1618-1625. |
| [8] | Jing Ruyi, Chen Yingxin, Cao Lei . Prognosis of deep lamellar keratoplasty versus penetrating keratoplasty in the treatment of stromal corneal dystrophy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1626-1633. |
| [9] | Wang Xuanqiang, Zhang Wenyang, Li Yang, Kong Weiqian, Li Wei, Wang Le, Li Zhongshan, Bai Shi. Effects of chronic exposure to low-frequency pulsed magnetic fields on contractility and morphology of the quadriceps muscle in healthy adults [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1634-1642. |
| [10] | Zhang Yuxin, Yu Cong, Zhang Cui, Ding Jianjun, Chen Yan. Differences in postural control ability between older adults with mild cognitive impairment and those with normal cognition under different single-task and dual-task conditions [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1643-1649. |
| [11] | Zhou Panpan, Cui Yinglin, Zhang Wentao, Wang Shurui, Chen Jiahui, Yang Tong . Role of cellular autophagy in cerebral ischemic injury and the regulatory mechanism of traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1650-1658. |
| [12] | Yu Jingbang, Wu Yayun. Regulatory effect of non-coding RNA in pulmonary fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1659-1666. |
| [13] | Wang Qiuyue, Jin Pan, Pu Rui . Exercise intervention and the role of pyroptosis in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1667-1675. |
| [14] | Zhu Hanmin, Wang Song, Xiao Wenlin, Zhang Wenjing, Zhou Xi, He Ye, Li Wei, . Mitophagy regulates bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1676-1683. |
| [15] | Yuan Weibo, Liu Chan, Yu Limei. Potential application of liver organoids in liver disease models and transplantation therapy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1684-1692. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||