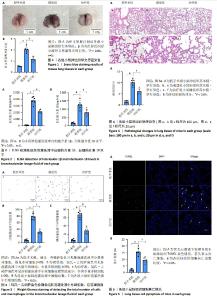
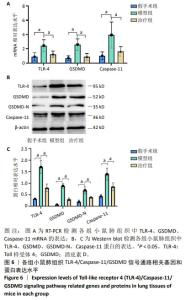
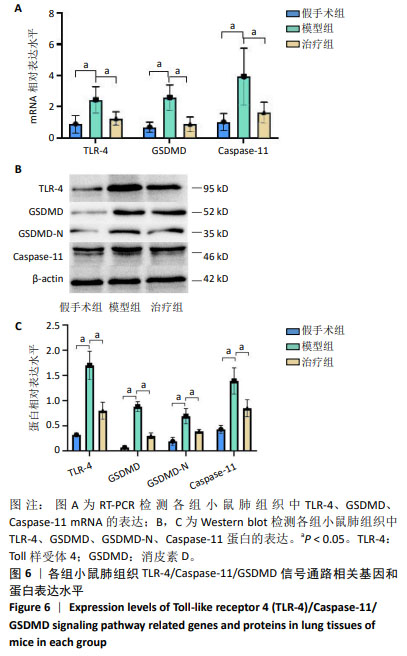
[1] GRASSELLI G, CALFEE CS, CAMPOROTA L, et al. ESICM guidelines on acute respiratory distress syndrome: definition, phenotyping and respiratory support strategies. Intensive Care Med. 2023;49(7): 727-759.
[2] MEYER NJ, GATTINONI L, CALFEE CS. Acute respiratory distress syndrome. Lancet. 2021;398(10300):622-637.
[3] MATTHAY MA, ARABI Y, ARROLIGA AC, et al. A New Global Definition of Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2024;209(1):37-47.
[4] HE X, LI C, YIN H, et al. Mesenchymal stem cells inhibited the apoptosis of alveolar epithelial cells caused by ARDS through CXCL12/CXCR4 axis. Bioengineered. 2022;13(4):9060-9070.
[5] VASUDEVAN SO, BEHL B, RATHINAM VA. Pyroptosis-induced inflammation and tissue damage. Semin Immunol. 2023;69:101781.
[6] 陆大浩,高巨.细胞焦亡在脓毒症急性肺损伤中作用的研究进展[J].临床麻醉学杂志,2024,40(4):412-415.
[7] WEI S, SHEN Z, YIN Y, et al. Advances of presepsin in sepsis-associated ARDS. Postgrad Med J. 2024;100(1182):209-218.
[8] DE BACKER D, DEUTSCHMAN CS, HELLMAN J, et al. Surviving Sepsis Campaign Research Priorities 2023. Crit Care Med. 2024;52(2): 268-296.
[9] LIU HH, WANG JS, LIN JZ, et al. LPS induced PCT production via TLR-4/NF-кB passway:it is the difference of G-/G+ bacteremia rats. Cytokine. 2021;137:155317.
[10] LIANG TY, LU LH, TANG SY, et al. Current status and prospects of basic research and clinical application of mesenchymal stem cells in acute respiratory distress syndrome. World J Stem Cells. 2023;15(4): 150-164.
[11] SU Y, SILVA JD, DOHERTY D, et al. Mesenchymal stromal cells-derived extracellular vesicles reprogramme macrophages in ARDS models through the miR-181a-5p-PTEN-pSTAT5-SOCS1 axis. Thorax. 2023;78(6):617-630.
[12] WAN Y, YU Y, YU C, et al. Human umbilical cord mesenchymal stem cell exosomes alleviate acute kidney injury by inhibiting pyroptosis in rats and NRK-52E cells. Ren Fail. 2023;45(1):2221138.
[13] YIN Y, TANG L, LIU K, et al. Attenuation of Lipopolysaccharide-induced Liver Injury by Bone Marrow Mesenchymal Stem Cells via Inhibiting the NLRP3 Inflammasome and Hepatocyte Pyroptosis. Curr Stem Cell Res Ther. 2022;17(4):361-369.
[14] 刘坚,龙婷婷,李晨彦,等.人脐带血间充质干细胞通过抑制MEK/ERK通路改善LPS诱导的AECⅡ凋亡机制的研究[J].中国医药导报, 2020,17(6):13-18.
[15] 陈刚,廖前德,胡优威,等.成骨诱导人脐带间充质干细胞与纳米羟基磷灰石/聚酰胺66支架材料的生物相容性[J].中国组织工程研究,2012,16(16):2931-2934.
[16] 刘坚,吕海金,安玉玲,等.间充质干细胞抑制MEK/ERK信号通路改善盲肠结扎穿孔所致急性肺损伤中肺泡Ⅱ型上皮细胞的凋亡[J].中山大学学报(医学科学版),2016,37(3):367-375.
[17] CHAUDHURI D, NEI AM, ROCHWERG B, et al. 2024 Focused Update: Guidelines on Use of Corticosteroids in Sepsis, Acute Respiratory Distress Syndrome, and Community-Acquired Pneumonia. Crit Care Med. 2024;52(5):e219-e233.
[18] BLACK LP, HOPSON C, BARKER G, et al. Trends in cholesterol and lipoproteins are associated with acute respiratory distress syndrome incidence and death among sepsis patients. Shock. 2024;61(2): 260-265.
[19] CHANG Y, YOO HJ, KIM SJ, et al. A targeted metabolomics approach for sepsis-induced ARDS and its subphenotypes. Crit Care. 2023; 27(1):263.
[20] SONG R, HE S, WU Y, et al. Pyroptosis in sepsis induced organ dysfunction. Curr Res Transl Med. 2024;72(2):103419.
[21] LIU X, LIEBERMAN J. Inflammasome-independent pyroptosis. Curr Opin Immunol. 2024;88:102432.
[22] ZHOU S, YANG X, MO K, et al. Pyroptosis and polarization of macrophages in septic acute lung injury induced by lipopolysaccharide in mice. Immun Inflamm Dis. 2024;12(3):e1197.
[23] SUN W, LU H, DONG S, et al. Beclin1 controls caspase-4 inflammsome activation and pyroptosis in mouse myocardial reperfusion-induced microvascular injury. Cell Commun Signal. 2021;19(1):107.
[24] 翟延评,何耀军,潘嘉宁,等.Casp-8通过调控NLRP3炎性小体介导的细胞焦亡减轻脓毒症引起的急性肺损伤[J].东南大学学报(医学版),2023,42(1):64-71.
[25] WANG YC, LIU QX, LIU T, et al. Caspase-1-dependent pyroptosis of peripheral blood mononuclear cells predicts the development of sepsis in severe trauma patients: A prospective observational study. Medicine (Baltimore). 2018;97(8):e9859.
[26] SUZUKI H, SOZEN T, HASEGAWA Y, et al. Caspase-1 inhibitor prevents neurogenic pulmonary edema after subarachnoid hemorrhage in mice. Stroke. 2009;40(12):3872-3875.
[27] COLL RC, SCHRODER K, PELEGRÍN P. NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol Sci. 2022;43(8): 653-668.
[28] WU YT, XU WT, ZHENG L, et al. 4-octyl itaconate ameliorates alveolar macrophage pyroptosis against ARDS via rescuing mitochondrial dysfunction and suppressing the cGAS/STING pathway. Int Immunopharmacol. 2023;118:110104.
[29] ZHANG ZT, ZHANG DY, XIE K, et al. Luteolin activates Tregs to promote IL-10 expression and alleviating caspase-11-dependent pyroptosis in sepsis-induced lung injury. Int Immunopharmacol. 2021;99:107914.
[30] SBORGI L, RÜHL S, MULVIHILL E, et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35(16):1766-1778.
[31] LIU X, ZHANG Z, RUAN J, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016; 535(7610):153-158.
[32] DUTRA SILVA J, SU Y, CALFEE CS, et al. Mesenchymal stromal cell extracellular vesicles rescue mitochondrial dysfunction and improve barrier integrity in clinically relevant models of ARDS. Eur Respir J. 2021;58(1):2002978.
[33] DOS SANTOS CC, AMATULLAH H, VASWANI CM, et al. Mesenchymal stromal (stem) cell therapy modulates miR-193b-5p expression to attenuate sepsis-induced acute lung injury. Eur Respir J. 2022;59(1): 2004216. |