Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (24): 5254-5262.doi: 10.12307/2025.726
Previous Articles Next Articles
Cathepsins and osteonecrosis: analysis based on European samples from the FinnGen Database and IEU OpenGWAS Database
Chai Jinlian1, Sun Tiefeng2, Li Wei3, Zhang Bochun3, Li Guangzheng4, Shao Xuekun2, Wang Ping2, Liang Xuezhen3, 5
- 1College of Pharmacy, Shandong University of Traditional Chinese Medicine, Jinan 250355, Shandong Province, China; 2Shandong Provincial Research Institute of Traditional Chinese Medicine, Jinan 250014, Shandong Province, China; 3First Clinical Medical College, Shandong University of Traditional Chinese Medicine, Jinan 250014, Shandong Province, China; 4College of Traditional Chinese Medicine, Shandong University of Traditional Chinese Medicine, Jinan 250355, Shandong Province, China; 5Department of Orthopedic Microsurgery, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan 250014, Shandong Province, China
-
Received:2024-08-28Accepted:2024-10-26Online:2025-08-28Published:2025-02-06 -
Contact:Liang Xuezhen, MD, Associate professor, Master’s supervisor, First Clinical Medical College, Shandong University of Traditional Chinese Medicine, Jinan 250014, Shandong Province, China; Department of Orthopedic Microsurgery, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan 250014, Shandong Province, China Co-corresponding author: Wang Ping, MD, Master’s supervisor, Shandong Provincial Research Institute of Traditional Chinese Medicine, Jinan 250014, Shandong Province, China -
About author:Chai Jinlian, MS, College of Pharmacy, Shandong University of Traditional Chinese Medicine, Jinan 250355, Shandong Province, China -
Supported by:National Natural Science Foundation of China, No. 82205154 (to LXZ); Shandong Provincial Natural Science Foundation Youth Project, Nos. ZR2021QH004 and ZR2024MH156 (to LXZ)
CLC Number:
Cite this article
Chai Jinlian, Sun Tiefeng, Li Wei, Zhang Bochun, Li Guangzheng, Shao Xuekun, Wang Ping, Liang Xuezhen. Cathepsins and osteonecrosis: analysis based on European samples from the FinnGen Database and IEU OpenGWAS Database[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(24): 5254-5262.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
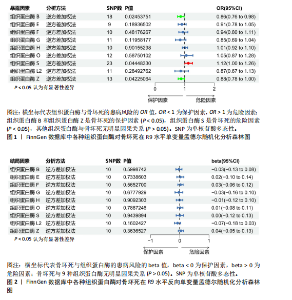
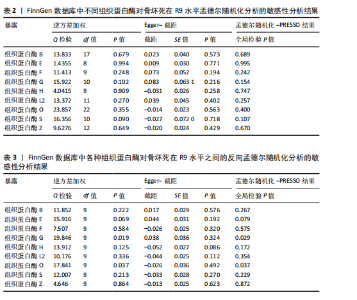
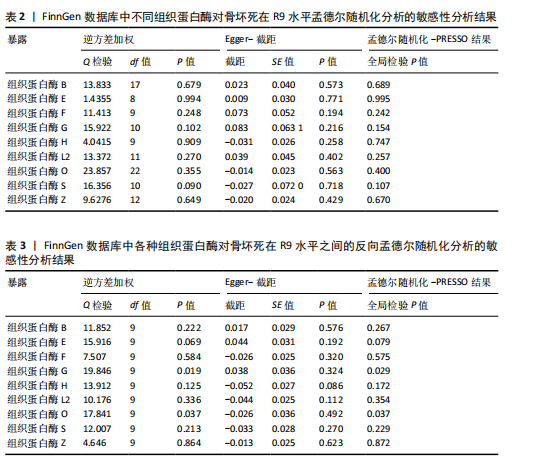
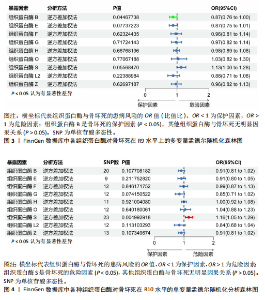
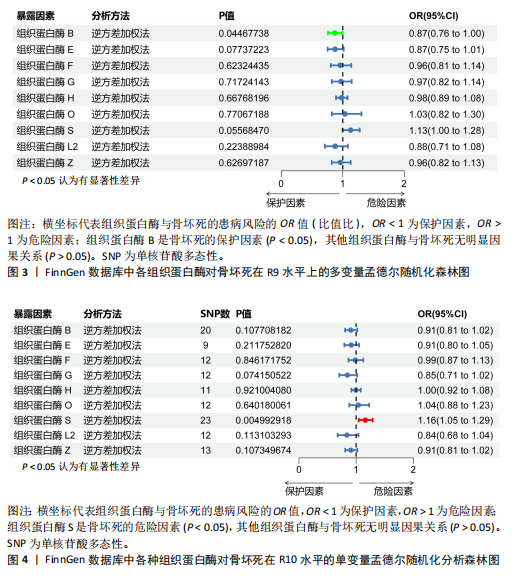
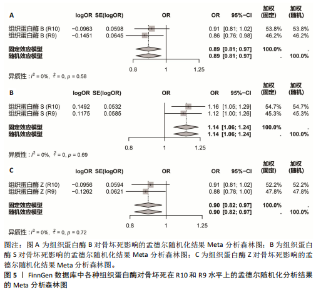
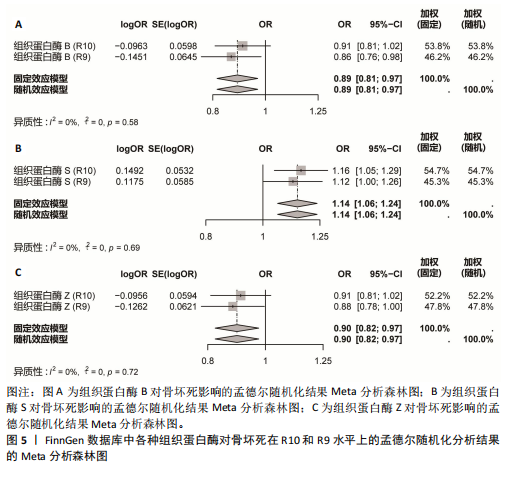
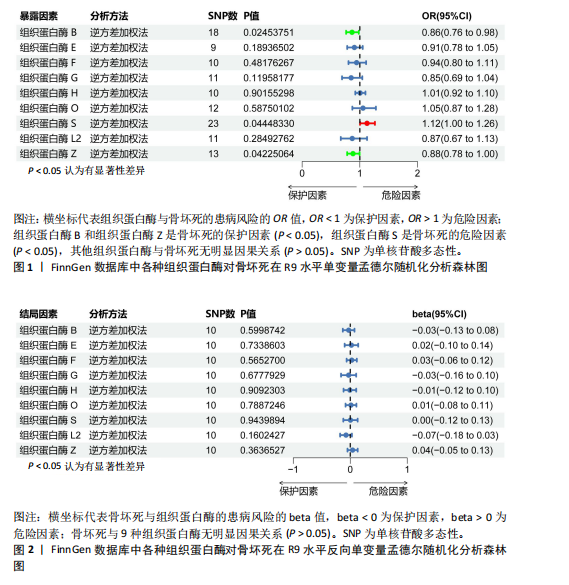
2.1 正向单变量孟德尔随机化分析结果 根据选择标准,纳入了9种组织蛋白酶(B,E,F,G,H,L2,O,S,Z)的工具变量,选取P < 5×10-6的SNP,排除LD中的SNP后,从每种组织蛋白酶的GWAS数据中提取出9-23个SNP作为工具变量,共计117个SNP。计算每个SNP的F统计量,所有SNP的最小F值为20.798。每个工具变量的F统计量大于10,没有SNP被排除为弱工具变量,表明弱工具变量偏倚的证据较低。此外,通过Phenoscanner V2数据库未发现可能影响暴露与结局之间关系的SNP。 单变量孟德尔随机化分析9种组织蛋白酶(组织蛋白酶B,E,F,G,H,O,S,L2,Z)对骨坏死的影响。高水平的组织蛋白酶 S与骨坏死的发病风险增加存在显著的因果关系(逆方差加权:P=0.045,OR=1.125,95%CI:1.003-1.261)。而高水平的组织蛋白酶B (逆方差加权:P=0.025,OR=0.865,95%CI:0.762-0.982)和高水平的组织蛋白酶Z(逆方差加权:P=0.042,OR=0.882,95%CI:0.780-0.996)与骨坏死的发生风险降低相关。对于组织蛋白酶E,F,G,H,O,L2,逆方差加权法与骨坏死发病风险无明显相关性(P > 0.05),比值比接近于0,提示其他类型组织蛋白酶与骨坏死转归无明显因果关系。Cochran’s Q、MR-Egger截距和MR-PRESSO全局检验未发现任何异质性或水平多效性的证据(P > 0.05)。在留一法分析中,没有SNP显著破坏组织蛋白酶S对骨坏死的总体影响;漏斗图的对称性也表明无多效性。上述分析的结果证明了研究结果的稳定性。单变量孟德尔随机化的显著性估计值以森林图的形式在图1中进行了描述,异质性和多效性检验结果见表2。 2.2 反向单变量孟德尔随机化分析结果 为了验证骨坏死对9种组织蛋白酶(B,E,F,G,H,O,S,L2和Z)是否具有反向调控作用,文章进行了反向单变量孟德尔随机化分析。以R9水平的骨坏死作为暴露量,以9种组织蛋白酶作为结局。选择P值小于5×10-6的SNPs并去除LD中的SNPs后,每个骨坏死使用10个SNPs作为工具变量。计算每个SNP的F统计量,所有SNP的最小F值为20.854。每个工具变量的F统计量大于10,表明弱工具变量偏倚的低证据,没有SNP被排除为弱工具变量。此外,通过Phennscanner V2数据库未发现可能影响暴露和结局之间关系的 SNP。 反向单变量孟德尔随机化分析结果未发现骨坏死和9种组织蛋白酶之间存在任何反向因果关系(P > 0.05),除组织蛋白酶G和组织蛋白酶O的Cochran’s Q_P值小于0.05,提示可能存在一定程度的异质性;留一法分析中排除每个SNP后对估计值未见明显影响;使用MR-Egger截距分析均未发现水平多效性的证据(P > 0.05),表明上述分析结果的稳健性。反向单变量孟德尔随机化的显著性估计值以森林图的形式呈现在图2中,异质性和多效性检验结果见表3。 2.3 多变量孟德尔随机化分析结果 在校正其他类型的组织蛋白酶后,进行了多变量孟德尔随机化分析,以验证单变量分析中获得的无偏估计。在多变量孟德尔随机化分析中,评估了不同的组织蛋白酶暴露对骨坏死的影响。经过严格的选择阈值(P=5×10-6, R2=0.001,kb=10 000)后,随后的重复数据删除、聚类和协调程序最终确定了94个SNP为用于多变量孟德尔随机化分析的工具变量。结果显示,在校正其他类型组织蛋白酶后,组织蛋白酶B与骨坏死发生风险呈显著负相关(逆方差加权:P=0.045,OR=0.871,95%CI:0.761 2-0.997),提示其可能对骨坏死具有保护作用;而组织蛋白酶S (逆方差加权:P=0.056,OR=1.128,95%CI:0.997-1.275)和组织蛋白酶Z (逆方差加权:P=0.627,OR=0.961,95%CI:0.819-1.128)水平与骨坏死风险的因果关系在多变量孟德尔随机化分析中不存在。其他组织蛋白酶(F、G、H、O和L2)也与骨坏死无显著相关性,P值超过显著性阈值。Cochran’s Q检验结果显示无异质性证据(P=0.223),MR-Egger截距检验未提示存在水平多效性(P=0.218)。多变量孟德尔随机化的估计值以图3中森林图的形式表示。 2.4 验证分析与Meta分析结果 为了进一步验证文章结论的稳定性和可靠性,在R10水平上重新分析了FinnGen数据库的骨坏死 GWAS数据。结果显示,组织蛋白酶S水平升高与骨坏死发病风险增加存在显著的因果关系(逆方差加权:P=0.005,OR=1.161,95%CI:1.046-1.288)。单变量孟德尔随机化的显著估计值以图4中森林图的形式呈现。 随后进行孟德尔随机化分析,将两种分析的结果合并为Meta分析。Meta分析一致表明,组织蛋白酶B和组织蛋白酶Z对骨坏死的发生具有保护作用,而组织蛋白酶 S与骨坏死发生风险增加相关,这与文章之前的孟德尔随机化分析结果一致,如图5所示。 "
| [1] MOTTA F, TIMILSINA S, GERSHWIN ME, et al. Steroid-induced osteonecrosis. J Transl Autoimmun. 2022;5:100168. [2] BERGMAN J, NORDSTRÖM A, NORDSTRÖM P. Epidemiology of osteonecrosis among older adults in Sweden. Osteoporos Int. 2019;30(5):965-973. [3] CHANG C, GREENSPAN A, GERSHWIN ME. The pathogenesis, diagnosis and clinical manifestations of steroid-induced osteonecrosis. J Autoimmun. 2020;110: 102460. [4] REISER J, ADAIR B, REINHECKEL T. Specialized roles for cysteine cathepsins in health and disease. J Clin Invest. 2010;120(10): 3421-3431. [5] PATEL S, HOMAEI A, EL-SEEDI HR, et al. Cathepsins: proteases that are vital for survival but can also be fatal. Biomed Pharmacother. 2018;105:526-532. [6] RAUNER M, FÖGER-SAMWALD U, KURZ MF, et al. Cathepsin S controls adipocytic and osteoblastic differentiation, bone turnover, and bone microarchitecture. Bone. 2014; 64:281-287. [7] ZHU G, CHEN W, TANG CY, et al. Knockout and Double Knockout of Cathepsin K and Mmp9 reveals a novel function of Cathepsin K as a regulator of osteoclast gene expression and bone homeostasis. Int J Biol Sci. 2022;18(14):5522-5538. [8] CHOI JW, LIM S, JUNG SE, et al. Enhanced osteocyte differentiation: cathepsin d and l secretion by human adipose-derived mesenchymal stem cells. Cells. 2023; 12(24):2852. [9] DUARTE C, YAMADA C, GARCIA C, et al. Crosstalk between dihydroceramides produced by Porphyromonas gingivalis and host lysosomal cathepsin B in the promotion of osteoclastogenesis. J Cell Mol Med. 2022;26(10):2841-2851. [10] Birney E. Mendelian Randomization. Cold Spring Harb Perspect Med. 2022;12(4): a041302. [11] SEKULA P, DEL GRECO MF, PATTARO C, et al. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol. 2016;27(11):3253-3265. [12] LI W, CHAI JL, LI Z, et al. No evidence of genetic causality between diabetes and osteonecrosis: a bidirectional two-sample Mendelian randomization analysis. J Orthop Surg Res. 2023;18(1):970. [13] HUANG X, DENG H, ZHANG B, et al. The causal relationship between cathepsins and digestive system tumors: a Mendelian randomization study. Front Oncol. 2024;14: 1365138. [14] LIN L, WU Z, LUO H, et al. Cathepsin-mediated regulation of alpha-synuclein in Parkinson’s disease: a Mendelian randomization study. Front Aging Neurosci. 2024;16:1394807. [15] YUSUFUJIANG A, ZENG S, LI H. Cathepsins and Parkinson’s disease: insights from Mendelian randomization analyses. Front Aging Neurosci. 2024;16:1380483. [16] ZENG R, ZHOU Z, LIAO W, et al. Genetic insights into the role of cathepsins in cardiovascular diseases: a Mendelian randomization study. ESC Heart Fail. 2024; 11(5):2707-2718. [17] CAO H, LIU B, GONG K, et al. Association between cathepsins and benign prostate diseases: a bidirectional two-sample Mendelian randomization study. Front Endocrinol (Lausanne). 2024;15:1348310. [18] BOEF AG, DEKKERS OM, LE CESSIE S. Mendelian randomization studies: a review of the approaches used and the quality of reporting. Int J Epidemiol. 2015;44(2): 496-511. [19] BURGESS S, LABRECQUE JA. Mendelian randomization with a binary exposure variable: interpretation and presentation of causal estimates. Eur J Epidemiol. 2018; 33(10):947-952. [20] SKRIVANKOVA VW, RICHMOND RC, WOOLF BAR, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR Statement. JAMA. 2021;326(16):1614-1621. [21] SUN BB, MARANVILLE JC, PETERS JE, et al. Genomic atlas of the human plasma proteome. Nature. 2018;558(7708):73-79. [22] KURKI MI, KARJALAINEN J, PALTA P, et al. A. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613(7944):508-518. [23] LI J, TANG M, GAO X, et al. Mendelian randomization analyses explore the relationship between cathepsins and lung cancer. Commun Biol. 2023;6(1):1019. [24] GALA H, TOMLINSON I. The use of Mendelian randomisation to identify causal cancer risk factors: promise and limitations. J Pathol. 2020;250(5):541-554. [25] KAMAT MA, BLACKSHAW JA, YOUNG R, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. 2019;35(22):4851-4853. [26] PIERCE BL, AHSAN H, VANDERWEELE TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol. 2011;40(3):740-752. [27] VERBANCK M, CHEN CY, NEALE B, et al. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693-698. [28] BURGESS S, BUTTERWORTH A, THOMPSON SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658-665. [29] MOUNIER N, KUTALIK Z. Bias correction for inverse variance weighting Mendelian randomization. Genet Epidemiol. 2023; 47(4):314-331. [30] BURGESS S, THOMPSON SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377-389. [31] BOWDEN J, DAVEY SMITH G, HAYCOCK PC, et al. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016; 40(4):304-314. [32] YAVORSKA OO, BURGESS S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46(6):1734-1739. [33] BOWDEN J, DEL GRECO MF, MINELLI C, et al. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med. 2017;36(11):1783-1802. [34] HEMANI G, BOWDEN J, DAVEY SMITH G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet. 2018;27(R2):R195-R208. [35] DELAISSÉ JM, EECKHOUT Y, VAES G. Inhibition of bone resorption in culture by inhibitors of thiol proteinases. Biochem J. 1980;192(1):365-368. [36] UNEMORI EN, WERB Z. Collagenase expression and endogenous activation in rabbit synovial fibroblasts stimulated by the calcium ionophore A23187. J Biol Chem. 1988;263(31):16252-16259. [37] AISA MC, RAHMAN S, SENIN U, et al. Cathepsin B activity in normal human osteoblast-like cells and human osteoblastic osteosarcoma cells (MG-63): regulation by interleukin-1 beta and parathyroid hormone. Biochim Biophys Acta. 1996;1290(1):29-36. [38] CHAE HJ, HA KC, LEE GY, et al. Interleukin-6 and cyclic AMP stimulate release of cathepsin B in human osteoblasts. Immunopharmacol Immunotoxicol. 2007; 29(2):155-172. [39] AISA MC, BECCARI T, COSTANZI E, et al. Biochim Biophys Acta. 2003;1621(2):149-159. [40] PAGE AE, WARBURTON MJ, CHAMBERS TJ, et al. Human osteoclastomas contain multiple forms of cathepsin B. Biochim Biophys Acta. 1992;1116(1):57-66. [41] RIFKIN BR, VERNILLO AT, KLECKNER AP, et al. Cathepsin B and L activities in isolated osteoclasts. Biochem Biophys Res Commun. 1991;179(1):63-69. [42] RIFKIN BR, VERNILLO AT, KLECKNER AP, et al. Cathepsin B and L activities in isolated osteoclasts. Biochem Biophys Res Commun. 1991;179(1):63-69. [43] BAUMGRASS R, WILLIAMSON MK, PRICE PA. Identification of peptide fragments generated by digestion of bovine and human osteocalcin with the lysosomal proteinases cathepsin B, D, L, H, and S. J Bone Miner Res. 1997;12(3):447-455. [44] LAI WF, CHANG CH, TANG Y, et al. Early diagnosis of osteoarthritis using cathepsin B sensitive near-infrared fluorescent probes. Osteoarthritis Cartilage. 2004;12(3):239-244. [45] BAICI A, HÖRLER D, LANG A, et al. Cathepsin B in osteoarthritis: zonal variation of enzyme activity in human femoral head cartilage. Ann Rheum Dis. 1995;54(4):281-288. [46] BAICI A, LANG A, HÖRLER D, et al. Cathepsin B in osteoarthritis: cytochemical and histochemical analysis of human femoral head cartilage. Ann Rheum Dis. 1995;54(4):289-297. [47] MEHRABAN F, TINDAL MH, PROFFITT MM, et al. Temporal pattern of cysteine endopeptidase (cathepsin B) expression in cartilage and synovium from rabbit knees with experimental osteoarthritis: gene expression in chondrocytes in response to interleukin-1 and matrix depletion. Ann Rheum Dis. 1997;56(2):108-115. [48] ZHANG L, QIU J, SHI J, et al. MicroRNA-140-5p represses chondrocyte pyroptosis and relieves cartilage injury in osteoarthritis by inhibiting cathepsin B/Nod-like receptor protein 3. Bioengineered. 2021;12(2):9949-9964. [49] MARTEL-PELLETIER J, CLOUTIER JM, PELLETIER JP. Cathepsin B and cysteine protease inhibitors in human osteoarthritis. J Orthop Res. 1990;8(3):336-344. [50] BERARDI S, LANG A, KOSTOULAS G, et al. Alternative messenger RNA splicing and enzyme forms of cathepsin B in human osteoarthritic cartilage and cultured chondrocytes. Arthritis Rheum. 2001;44(8):1819-1831. [51] HAMER I, DELAIVE E, DIEU M, et al. Up-regulation of cathepsin B expression and enhanced secretion in mitochondrial DNA-depleted osteosarcoma cells. Biol Cell. 2009;101(1):31-41. [52] KRUEGER S, HAECKEL C, BUEHLING F, et al. Inhibitory effects of antisense cathepsin B cDNA transfection on invasion and motility in a human osteosarcoma cell line. Cancer Res. 1999;59(23):6010-6014. [53] HAN SH, CHAE SW, CHOI JY, et al. Acidic pH environments increase the expression of cathepsin B in osteoblasts: the significance of ER stress in bone physiology. Immunopharmacol Immunotoxicol. 2009; 31(3):428-431. |
| [1] | Jiang Yang, Peng Hao, Song Yanping, Yao Na, Song Yueyu, Yin Xingxiao, Li Yanqi, Chen Qigang. Isometric exercise reduces resting blood pressure: a meta-analysis of moderating factors and dose effects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 975-986. |
| [2] | Sun Jiahe, Shi Jipeng, Zhu Tianrui, Quan Helong, Xu Hongqi. Effect of exercise intervention in elderly individuals with sarcopenia and its comorbidities: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 997-1007. |
| [3] | Zeng Hao, Sun Pengcheng, Chai Yuan, Huang Yourong, Zhang Chi, Zhang Xiaoyun. Association between thyroid function and osteoporosis: genome-wide data analysis of European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1019-1027. |
| [4] | Rong Xiangbin, , Zheng Haibo, Mo Xueshen, Hou Kun, Zeng Ping, . Plasma metabolites, immune cells, and hip osteoarthritis: causal inference based on GWAS data from European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1028-1035. |
| [5] | He Qiwang, , , Chen Bo, Liang Fuchao, Kang Zewei, Zhou Yuan, Ji Anxu, Tang Xialin, . Relationship between Alzheimer’s disease and sarcopenia and body mass index: analysis of GWAS datasets for European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1036-1046. |
| [6] | Ding Yu, Chen Jingwen, Chen Xiuyan, Shi Huimin, Yang Yudie, Zhou Meiqi, Cui Shuai, . Circulating inflammatory proteins and myocardial hypertrophy: large sample analysis of European populations from GWAS Catalog and FinnGen databases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1047-1057. |
| [7] | Chen Jiayong, Tang Meiling, Lu Jianqi, Pang Yan, Yang Shangbing, Mao Meiling, Luo Wenkuan, Lu Wei, Zhou Jiatan. Based on Mendelian randomization, the causal relationship between 1400 metabolites and sarcopenia and the correlation analysis of cardiovascular disease were investigated [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-11. |
| [8] | Zhang Yibo, Lu Jianqi, Mao Meiling, Pang Yan, Dong Li, Yang Shangbing, Xiao Xiang. Exploring the causal relationship between rheumatoid arthritis and coronary atherosclerosis: a Mendel randomized study involving serum metabolites and inflammatory factors [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [9] | Dong Tingting, Chen Tianxin, Li Yan, Zhang Sheng, Zhang Lei. Causal relationship between modifiable factors and joint sports injuries [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1953-1962. |
| [10] | Chen Shuai, Jin Jie, Han Huawei, Tian Ningsheng, Li Zhiwei . Causal relationship between circulating inflammatory cytokines and bone mineral density based on two-sample Mendelian randomization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1556-1564. |
| [11] | Wang Yida, Liu Jun, Wang Xiaoling, Wang Liyan, Yang Chengru, Zhang Xuexiao. Effects of wearable electronic device-based interventions on physical activity and sedentary behavior in healthy adolescents: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1693-1704. |
| [12] | Zhang Zixian, Xu Youliang, Wu Shaokui, Wang Xiangying. Effects of blood flow restriction training combined with resistance training on muscle indicators in college athletes: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1705-1713. |
| [13] | Wang Juan, Wang Guanglan, Zuo Huiwu. Efficacy of exercise therapy in the treatment of anterior cruciate ligament reconstruction patients: #br# a network meta-analysis #br# [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1714-1726. |
| [14] |
Zhao Wensheng, Li Xiaolin, Peng Changhua, Deng Jia, Sheng Hao, Chen Hongwei, Zhang Chaoju, He Chuan.
Gut microbiota and osteoporotic fractures #br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1296-1304.
|
| [15] | Ma Haoyu, Qiao Hongchao, Hao Qianqian, Shi Dongbo. Causal effects of different exercise intensities on the risk of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1305-1311. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||