Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (25): 5335-5344.doi: 10.12307/2025.525
Previous Articles Next Articles
Aloin mitigates hypoxic injury in rat cardiomyocytes: inhibiting oxidative stress and ferroptosis
Tan Mingyue1, Jin Yifeng2, Zhang Jun1, Li Hongxia1
- 1Department of Cardiology, 2Department of General Medicine, First Affiliated Hospital of Soochow University, Suzhou 215006, Jiangsu Province, China
-
Received:2024-03-26Accepted:2024-06-11Online:2025-09-08Published:2024-12-21 -
Contact:Li Hongxia, Chief technician, Department of Cardiology, First Affiliated Hospital of Soochow University, Suzhou 215006, Jiangsu Province, China -
About author:Tan Mingyue, Master, Physician, Department of Cardiology, First Affiliated Hospital of Soochow University, Suzhou 215006, Jiangsu Province, China -
Supported by:National Natural Science Foundation of China, No. 81100173 (to LHX)
CLC Number:
Cite this article
Tan Mingyue, Jin Yifeng, Zhang Jun, Li Hongxia. Aloin mitigates hypoxic injury in rat cardiomyocytes: inhibiting oxidative stress and ferroptosis[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(25): 5335-5344.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
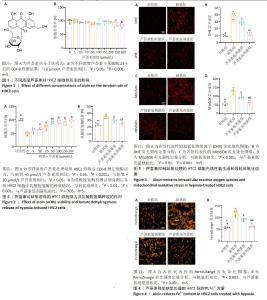
2.1 芦荟素对H9C2细胞活力的影响 芦荟素是从芦荟植物中分离出的蒽醌化合物(分子结构式见图1A),它是食品中常见的“天然香料”[24]。 为明确芦荟素的安全性,使用不同浓度(0,5,10,20,50,100,150,200,250,300 μmol/L)芦荟素对H9C2细胞进行24 h干预。然后,使用CCK-8法检测细胞活力。结果显示,与未接受处理的对照组(0 μmol/L)相比,当芦荟素浓度在0-200 μmol/L 范围内时,细胞活力并未出现显著变化(P > 0.05),见图1B。然而,芦荟素浓度达到250,300 μmol/L时,细胞活力显著下降(P < 0.05,P < 0.001),见图1B。由此可见,在0-200 μmol/L的浓度范围内,芦荟素对H9C2细胞的毒性作用极小。基于这一结果,后续的实验将集中在这一浓度范围内,以探寻芦荟素对H9C2细胞的最佳治疗浓度。 2.2 不同浓度芦荟素对缺氧条件下H9C2细胞活力及乳酸脱氢酶释放的影响 2.2.1 确定药物干预浓度 将缺氧诱导的H9C2细胞与不同浓度的芦荟素(0,5,10,20,50,100,150,200 μmol/L) 共培养24 h,使用CCK-8法检测细胞活力。缺氧导致H9C2细胞活力显著下降(P < 0.001)。与未经芦荟素处理的H9C2细胞相比,当芦荟素浓度处于5 μmol/L时,细胞活力并未出现明显变化(P > 0.05);当芦荟素浓度升至10,20,50,100,150,200 μmol/L时,H9C2细胞活力逐渐恢复(P < 0.05,P < 0.001),见图2A。值得注意的是,在50,100,150,200 μmol/L芦荟素处理组之间,细胞活力未观察到明显差异(P > 0.05)。50 μmol/L处理组的细胞活力优于20 μmol/L处理组(P < 0.05)。因此,选择20,50 μmol/L作为后续实验的芦荟素低、高浓度。 2.2.2 乳酸脱氢酶释放情况 与对照组相比,缺氧组H9C2细胞乳酸脱氢酶释放显著升高(P < 0.001);与缺氧组相比,使用20,50 μmol/L芦荟素处理后乳酸脱氢酶释放明显减少(P < 0.01,P < 0.001),并且芦荟素高剂量组对乳酸脱氢酶释放的抑制作用强于芦荟素低剂量组(P < 0.05),见图2B。 2.3 芦荟素对缺氧条件下H9C2细胞内活性氧生成及线粒体氧化应激的影响 2.3.1 活性氧生成 超氧化物阴离子(DHE)荧光探针检测H9C2心肌细胞活性氧水平,与对照组相比,缺氧组H9C2细胞内荧光强度显著增加(P < 0.001),这说明细胞中活性氧生成显著增加;与缺氧组相比,芦荟素组细胞内荧光强度均明显降低(P < 0.001),且芦荟素高剂量组的荧光强度低于芦荟素低剂量组(P < 0.001),见图3A,B。这一结果说明,20,50 μmol/L芦荟素均能抑制细胞内活性氧生成,且这种抑制作用呈剂量依赖性。 2.3.2 线粒体氧化应激 采用MitoSOX Red荧光探针检测H9C2细胞线粒体中氧化应激情况。MitoSOX染色结果显示,缺氧可诱导H9C2细胞内线粒体氧化应激程度明显升高(P < 0.001),使用芦荟素干预后细胞内线粒体氧化应激相较于缺氧组均降低(P < 0.001);芦荟素高剂量组对线粒体氧化应激的抑制作用强于芦荟素低剂量组(P < 0.01),见图3C,D。 2.4 芦荟素对缺氧条件下H9C2细胞内亚铁含量的影响 细胞内铁超载是铁死亡的驱动因子,也是铁死亡的主要特征之一。利用对Fe2+敏感的荧光染料FerroOrange来检测H9C2细胞内Fe2+的水平。FerroOrange染色结果表明,与对照组相比,缺氧组的H9C2细胞内荧光强度显著增强(P < 0.001),这说明缺氧损伤会导致H9C2细胞内Fe2+含量增加。然而,经20,50 μmol/L芦荟素处理后,H9C2细胞内的荧光强度均明显降低(P < 0.001);芦荟素高剂量组的荧光强度低于芦荟素低剂量组(P < 0.01),见图4。这一结果说明,芦荟素可以有效降低细胞内亚铁累积,而且50 μmol/L芦荟素降低效果更好。"
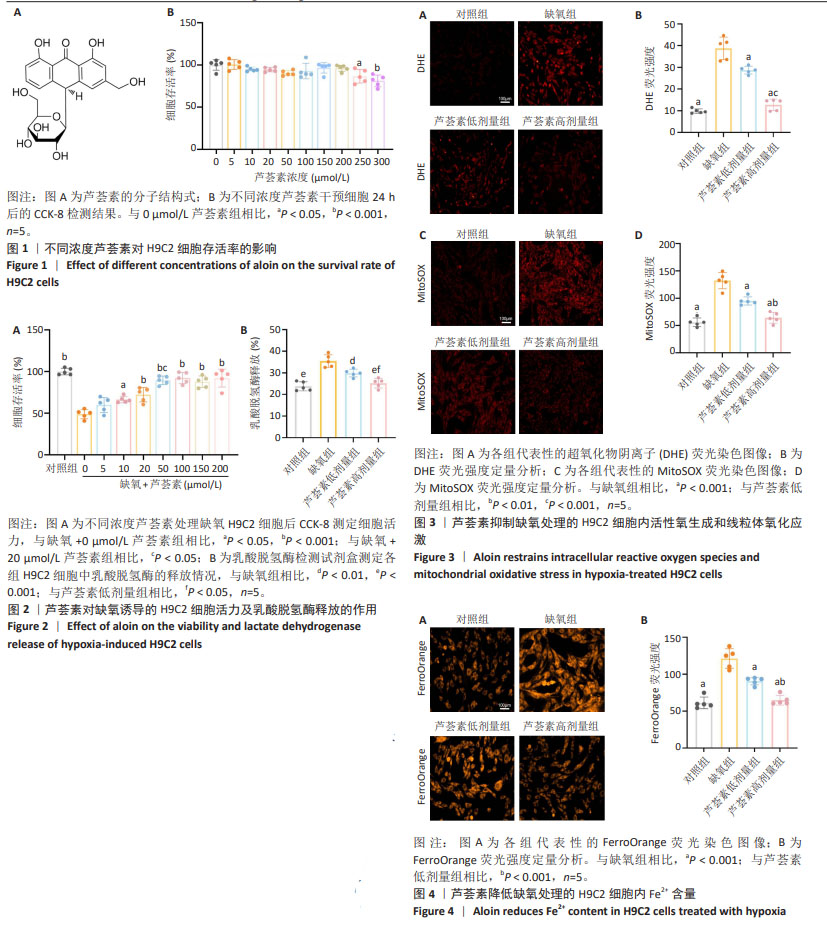
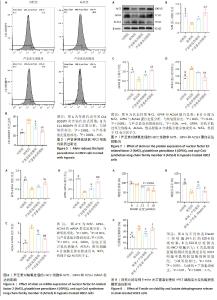
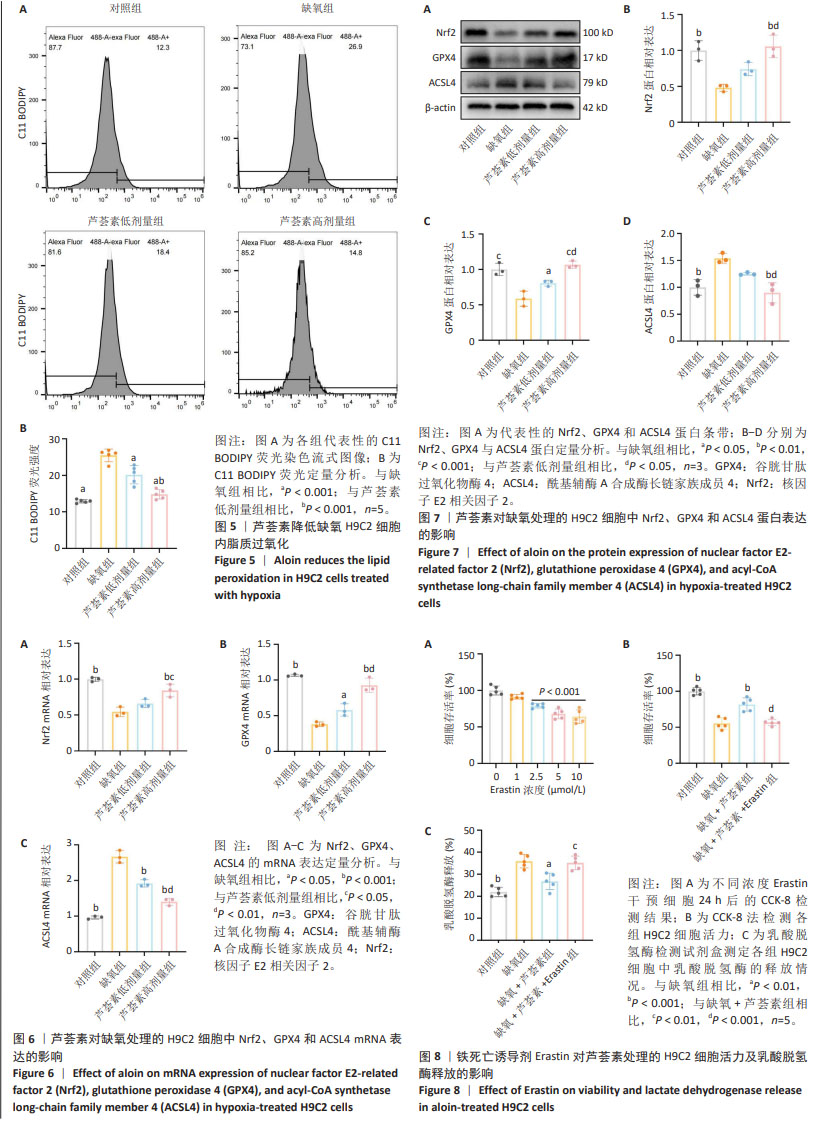
2.5 芦荟素对缺氧处理的H9C2细胞脂质过氧化程度的影响 脂质过氧化累积是铁死亡的核心特征之一。通过脂质过氧化荧光探针C11 BODIPY染色并使用流式细胞术检测荧光强度。结果显示,与对照组相比,缺氧组H9C2细胞内荧光强度显著增强(P < 0.001),这表明缺氧损伤会明显加剧H9C2细胞内的脂质过氧化程度。与缺氧组相比,芦荟素干预明显逆转了脂质过氧化的上升(P < 0.001),其中芦荟素高剂量组的效果优于芦荟素低剂量组(P < 0.001),见图5。 2.6 芦荟素对缺氧条件下H9C2细胞中铁死亡相关mRNA表达的影响 实时荧光定量PCR(RT-qPCR)检测结果显示,与对照组相比,缺氧组细胞中Nrf2、GPX4 mRNA表达水平均显著下调(P < 0.001),而ACSL4 mRNA表达水平明显上调(P < 0.001);芦荟素低剂量组的Nrf2 mRNA表达与缺氧组相比无明显变化(P > 0.05),但芦荟素高剂量组Nrf2 mRNA表达水平显著上升(P < 0.001),并且与低剂量组相比也显示出统计学差异(P < 0.05);与缺氧组相比,芦荟素干预组的GPX4 mRNA表达水平均升高(P < 0.05,P < 0.001),ACSL4 mRNA表达水平均降低(P < 0.001),且芦荟素高剂量组对GPX4和ACSL4的调控效果强于芦荟素低剂量组(P < 0.01),见图6。 2.7 芦荟素对缺氧H9C2细胞中铁死亡相关蛋白表达的影响 Western blot检测结果显示,与对照组相比,缺氧组Nrf2与GPX4的蛋白表达明显降低(P < 0.01,P < 0.001),ACSL4的蛋白表达显著升高(P < 0.01);与缺氧组相比,经芦荟素处理后,GPX4的蛋白表达均升高(P < 0.05,P < 0.001),且这种促进作用呈剂量依赖性(P < 0.05);芦荟素低剂量组的Nrf2与ACSL4蛋白表达相较于缺氧组差异无显著性意义(P > 0.05),芦荟素高剂量组的Nrf2蛋白表达显著升高(P < 0.01),ACSL4蛋白表达明显下降(P < 0.01),见图7。 2.8 芦荟素通过抑制铁死亡改善H9C2细胞缺氧损伤 2.8.1 确定Erastin干预浓度 上述实验明确了芦荟素能够有效抑制由缺氧引发的H9C2细胞铁死亡。为了深入探究芦荟素是否通过抑制铁死亡来保护H9C2细胞,使用铁死亡诱导剂Erastin进行了研究。在实验中,选用了不同浓度的Erastin(0,1,2.5,5,10 μmol/L)对H9C2细胞进行24 h的干预处理。通过CCK-8法检测细胞活力。实验结果显示,与未接受Erastin处理的对照组(0 μmol/L)相比,当Erastin浓度为1 μmol/L时,对细胞活力并无显著影响(P > 0.05);然而,当浓度提高到2.5,5,10 μmol/L时,细胞活力出现明显下降(P < 0.001),见图8A。基于上述结果,选择2.5 μmol/L Erastin进行后续实验。 2.8.2 Erastin对芦荟素干预的H9C2细胞活力及乳酸脱氢酶释放的影响 为了验证铁死亡诱导剂Erastin是否确实能够逆转芦荟素对缺氧细胞的保护作用,在芦荟素(50 μmol/L)治疗的基础上,加入了铁死亡诱导剂Erastin(2.5 μmol/L) 进行干预。与缺氧+芦荟素组对比,缺氧+芦荟素+Erastin组的细胞活力显著下降(P < 0.001),乳酸脱氢酶释放明显升高(P < 0.01),见图8B,C。这一结果表明芦荟素是通过抑制铁死亡改善H9C2细胞的缺氧损伤。"
| [1] LI Z, DAI R, CHEN M, et al. p55γ degrades RIP3 via MG53 to suppress ischaemia-induced myocardial necroptosis and mediates cardioprotection of preconditioning. Cardiovasc Res. 2023;119(14): 2421-2440. [2] BENJAMIN EJ, MUNTNER P, ALONSO A, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139(10):e56-e528. [3] PARK TJ, PARK JH, LEE GS, et al. Quantitative proteomic analyses reveal that GPX4 downregulation during myocardial infarction contributes to ferroptosis in cardiomyocytes. Cell Death Dis. 2019;10(11):835. [4] JIANG Y, QIAO Y, HE D, et al. Adaptor protein HIP-55-mediated signalosome protects against ferroptosis in myocardial infarction. Cell Death Differ. 2023;30(3):825-838. [5] ZHANG B, YANG J, LI X, et al. Tetrahydrocurcumin ameliorates postinfarction cardiac dysfunction and remodeling by inhibiting oxidative stress and preserving mitochondrial function via SIRT3 signaling pathway. Phytomedicine. 2023;121:155127. [6] FANG X, WANG H, HAN D, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A. 2019;116(7): 2672-2680. [7] YU Q, ZHANG N, GAN X, et al. EGCG attenuated acute myocardial infarction by inhibiting ferroptosis via miR-450b-5p/ACSL4 axis. Phytomedicine. 2023;119:154999. [8] FANG X, ARDEHALI H, MIN J, et al. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat Rev Cardiol. 2023;20(1):7-23. [9] BULLUCK H, ROSMINI S, ABDEL-GADIR A, et al. Residual Myocardial Iron Following Intramyocardial Hemorrhage During the Convalescent Phase of Reperfused ST-Segment-Elevation Myocardial Infarction and Adverse Left Ventricular Remodeling. Circ Cardiovasc Imaging. 2016;9(10):e004940. [10] ICHIHARA G, KATSUMATA Y, SUGIURA Y, et al. MRP1-Dependent Extracellular Release of Glutathione Induces Cardiomyocyte Ferroptosis After Ischemia-Reperfusion. Circ Res. 2023;133(10): 861-876. [11] FENG H, STOCKWELL BR. Unsolved mysteries: How does lipid peroxidation cause ferroptosis? PLoS Biol. 2018;16(5):e2006203. [12] HONG M, RONG J, TAO X, et al. The Emerging Role of Ferroptosis in Cardiovascular Diseases. Front Pharmacol. 2022;13:822083. [13] TANG D, KROEMER G. Ferroptosis. Curr Biol. 2020;30(21):R1292-R1297. [14] KOLEINI N, SHAPIRO JS, GEIER J, et al. Ironing out mechanisms of iron homeostasis and disorders of iron deficiency. J Clin Invest. 2021; 131(11):e148671. [15] LI J, CAO F, YIN HL, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11(2):88. [16] JIANG X, STOCKWELL BR, CONRAD M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22(4):266-282. [17] XIE Y, HOU W, SONG X, et al. Ferroptosis: process and function. Cell Death Differ. 2016;23(3):369-379. [18] WANG Y, ZHANG M, BI R, et al. ACSL4 deficiency confers protection against ferroptosis-mediated acute kidney injury. Redox Biol. 2022;51: 102262. [19] ZHANG Y, SWANDA RV, NIE L, et al. mTORC1 couples cyst(e)ine availability with GPX4 protein synthesis and ferroptosis regulation. Nat Commun. 2021;12(1):1589. [20] TAN M, YIN Y, MA X, et al. Glutathione system enhancement for cardiac protection: pharmacological options against oxidative stress and ferroptosis. Cell Death Dis. 2023;14(2):131. [21] DU Y, QIAN B, GAO L, et al. Aloin Preconditioning Attenuates Hepatic Ischemia/Reperfusion Injury via Inhibiting TLR4/MyD88/NF-κB Signal Pathway In Vivo and In Vitro. Oxid Med Cell Longev. 2019;2019:3765898. [22] LEI J, SHEN Y, XV G, et al. Aloin suppresses lipopolysaccharide-induced acute lung injury by inhibiting NLRP3/NF-κB via activation of SIRT1 in mice. Immunopharmacol Immunotoxicol. 2020;42(4): 306-313. [23] SUN W, WANG Z, SUN M, et al. Aloin antagonizes stimulated ischemia/reperfusion-induced damage and inflammatory response in cardiomyocytes by activating the Nrf2/HO-1 defense pathway. Cell Tissue Res. 2021;384(3):735-744. [24] BAI J, QIAN B, CAI T, et al. Aloin Attenuates Oxidative Stress, Inflammation, and CCl4-Induced Liver Fibrosis in Mice: Possible Role of TGF-β/Smad Signaling. J Agric Food Chem. 2023;71(49):19475-19487. [25] SYED AM, KUNDU S, RAM C, et al. Aloin alleviates pathological cardiac hypertrophy via modulation of the oxidative and fibrotic response. Life Sci. 2022;288:120159. [26] DODSON M, CASTRO-PORTUGUEZ R, ZHANG DD. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019; 23:101107. [27] CHANG R, ZHOU R, QI X, et al. Protective effects of aloin on oxygen and glucose deprivation-induced injury in PC12 cells. Brain Res Bull. 2016;121:75-83. [28] ROTH GA, MENSAH GA, JOHNSON CO, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J Am Coll Cardiol. 2020;76(25):2982-3021. [29] KIBEL A, LUKINAC AM, DAMBIC V, et al. Oxidative Stress in Ischemic Heart Disease. Oxid Med Cell Longev. 2020;2020:6627144. [30] ZHANG Q, AWOLAYO AN, NIGHTINGALE M, et al. Kinetics of glauconite dissolution in anoxic conditions as a function of pH and temperature. Geochim Cosmochim Acta. 2022;336:78-91. [31] SAWYER DB, COLUCCI WS. Mitochondrial oxidative stress in heart failure: “oxygen wastage” revisited. Circ Res. 2000;86(2):119-120. [32] PERRELLI MG, PAGLIARO P, PENNA C. Ischemia/reperfusion injury and cardioprotective mechanisms: Role of mitochondria and reactive oxygen species. World J Cardiol. 2011;3(6):186-200. [33] VAN DER POL A, VAN GILST WH, VOORS AA, et al. Treating oxidative stress in heart failure: past, present and future. Eur J Heart Fail. 2019; 21(4):425-435. [34] ZHONG Y, ZHONG P, HE S, et al. Trimetazidine Protects Cardiomyocytes Against Hypoxia/Reoxygenation Injury by Promoting AMP-activated Protein Kinase-dependent Autophagic Flux. J Cardiovasc Pharmacol. 2017;69(6):389-397. [35] PARK MW, CHA HW, KIM J, et al. NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer’s diseases. Redox Biol. 2021;41:101947. [36] HE Y, XI J, FANG J, et al. Aloe-emodin alleviates doxorubicin-induced cardiotoxicity via inhibition of ferroptosis. Free Radic Biol Med. 2023; 206:13-21. [37] LI X, LI Z, DONG X, et al. Astragaloside IV attenuates myocardial dysfunction in diabetic cardiomyopathy rats through downregulation of CD36-mediated ferroptosis. Phytother Res. 2023;37(7):3042-3056. [38] LI H, DING J, LIU W, et al. Plasma exosomes from patients with acute myocardial infarction alleviate myocardial injury by inhibiting ferroptosis through miR-26b-5p/SLC7A11 axis. Life Sci. 2023;322:121649. [39] GARCIA-BERMUDEZ J, BIRSOY K. A mitochondrial gatekeeper that helps cells escape death by ferroptosis. Nature. 2021;593(7860):514-515. [40] TONG X, ZHAO X, DANG X, et al. Predicting Diagnostic Gene Biomarkers Associated With Immune Checkpoints, N6-Methyladenosine, and Ferroptosis in Patients With Acute Myocardial Infarction. Front Cardiovasc Med. 2022;9:836067. [41] LI Y, ZHANG J, ZHANG K, et al. Scutellaria barbata Inhibits Hepatocellular Carcinoma Tumorigenicity by Inducing Ferroptosis of Hepatocellular Carcinoma Cells. Front Oncol. 2022;12:693395. [42] FAN Z, CAI L, WANG S, et al. Baicalin Prevents Myocardial Ischemia/Reperfusion Injury Through Inhibiting ACSL4 Mediated Ferroptosis. Front Pharmacol. 2021;12:628988. [43] ZHANG F, LI Z, GAO P, et al. HJ11 decoction restrains development of myocardial ischemia-reperfusion injury in rats by suppressing ACSL4-mediated ferroptosis. Front Pharmacol. 2022;13:1024292. [44] WANG B, JIN Y, LIU J, et al. EP1 activation inhibits doxorubicin-cardiomyocyte ferroptosis via Nrf2. Redox Biol. 2023;65:102825. [45] GUAN P, LIANG Y, WANG N. Fasudil alleviates pressure overload-induced heart failure by activating Nrf2-mediated antioxidant responses. J Cell Biochem. 2018;119(8):6452-6460. [46] DONG H, QIANG Z, CHAI D, et al. Nrf2 inhibits ferroptosis and protects against acute lung injury due to intestinal ischemia reperfusion via regulating SLC7A11 and HO-1. Aging (Albany NY). 2020;12(13): 12943-12959. [47] MATA A, CADENAS S. The Antioxidant Transcription Factor Nrf2 in Cardiac Ischemia-Reperfusion Injury. Int J Mol Sci. 2021;22(21):11939. [48] YUAN Y, ZHAI Y, CHEN J, et al. Kaempferol Ameliorates Oxygen-Glucose Deprivation/Reoxygenation-Induced Neuronal Ferroptosis by Activating Nrf2/SLC7A11/GPX4 Axis. Biomolecules. 2021;11(7):923. [49] CHEN C, CHEN W, ZHOU X, et al. Hyperbaric oxygen protects HT22 cells and PC12 cells from damage caused by oxygen-glucose deprivation/reperfusion via the inhibition of Nrf2/System Xc-/GPX4 axis-mediated ferroptosis. PLoS One. 2022;17(11):e0276083. [50] CHENG P, WANG X, LIU Q, et al. LuQi formula attenuates Cardiomyocyte ferroptosis via activating Nrf2/GPX4 signaling axis in heart failure. Phytomedicine. 2024;125:155357. |
| [1] | Zhao Jiyu, Wang Shaowei. Forkhead box transcription factor O1 signaling pathway in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1923-1930. |
| [2] | Li Huayuan, Li Chun, Liu Junwei, Wang Ting, Li Long, Wu Yongli. Effect of warm acupuncture on PINK1/Parkin pathway in the skeletal muscle of rats with chronic fatigue syndrome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1618-1625. |
| [3] | Zhu Hanmin, Wang Song, Xiao Wenlin, Zhang Wenjing, Zhou Xi, He Ye, Li Wei, . Mitophagy regulates bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1676-1683. |
| [4] | Zhao Nannan, Li Yanjie, Qin Hewei, Zhu Bochao, Ding Huimin, Xu Zhenhua. Changes in ferroptosis in hippocampal neurons of vascular dementia model rats treated with Tongmai Kaiqiao Pill [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1401-1407. |
| [5] | Zhang Mingyang, Yang Xinling. Verbascoside inhibits Erastin-induced ferroptosis of dopaminergic nerve cell line MN9D cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1408-1413. |
| [6] | Wang Mi, Ma Shujie, Liu Yang, Qi Rui. Identification and validation of characterized gene NFE2L2 for ferroptosis in ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1466-1474. |
| [7] | He Guanghui, Yuan Jie, Ke Yanqin, Qiu Xiaoting, Zhang Xiaoling. Hemin regulates mitochondrial pathway of oxidative stress in mouse chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1183-1191. |
| [8] | He Bo, Chen Wen, Ma Suilu, He Zhijun, Song Yuan, Li Jinpeng, Liu Tao, Wei Xiaotao, Wang Weiwei, Xie Jing . Pathogenesis and treatment progress of flap ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1230-1238. |
| [9] | Gao Yang, Qin Hewei, Liu Dandan. ACSL4 mediates ferroptosis and its potential role in atherosclerotic cardiovascular disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1239-1247. |
| [10] | Liu Lingyun, He Guixin, Qin Weibin, Song Hui, Zhang Liwen, Tang Weizhi, Yang Feifei, Zhu Ziyi, Ou Yangbin . Improvement of myocardial injury by traditional Chinese medicine: mitochondrial calcium homeostasis mediates macrophage autophagy and pyroptosis pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1276-1284. |
| [11] | Lu Ranran, Zhou Xu, Zhang Lijie, Yang Xinling. Dimethyl fumarate alleviates nerve damage in a mouse model of Parkinson’s disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 989-994. |
| [12] | Sima Xinli, Liu Danping, Qi Hui. Effect and mechanism of metformin-modified bone marrow mesenchymal stem cell exosomes on regulating chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7728-7734. |
| [13] | Wang Chen, Zhang Weinan, Shen Jining, Liu Fan, Yuan Jishan, Liu Yake. Inhibitory effect of ferroptosis inhibitor toxicity induced by cobalt nanoparticles through reactive oxygen species [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7310-7317. |
| [14] | Zhang Xiaoyu, Wei Shanwen, Fang Jiawei, Ni Li. Prussian blue nanoparticles restore mitochondrial function in nucleus pulposus cells through antioxidation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7318-7325. |
| [15] | Su Yongkun, Sun Hong, Liu Miao, Yang Hua, Li Qingsong. Development of novel antioxidants and antioxidant combination carried by nano-hydrogel systems in treatment of intervertebral disc degeneration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7376-7384. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||