Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (28): 6127-6137.doi: 10.12307/2025.470
Previous Articles Next Articles
New ideas and opportunities for polyurethane materials in peripheral nerve repair
Lan Xiaoqian1, Feng Guangli1, Qin Shiyi1, Zhong Lianmei2, Li Qing3
- 1First Affiliated Hospital of Kunming Medical University, Kunming 650032, Yunnan Province, China; 2Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing 100053, China; 3Science and Technology Achievement Incubation Center, Kunming Medical University, Kunming 650500, Yunnan Province, China
-
Received:2024-06-04Accepted:2024-07-26Online:2025-10-08Published:2024-12-09 -
Contact:Zhong Lianmei, Chief physician, Professor, Doctoral supervisor, Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing 100053, China Li Qing, Experimenter, Science and Technology Achievement Incubation Center, Kunming Medical University, Kunming 650500, Yunnan Province, China -
About author:Lan Xiaoqian, Doctoral candidate, First Affiliated Hospital of Kunming Medical University, Kunming 650032, Yunnan Province, China Feng Guangli, Master candidate, First Affiliated Hospital of Kunming Medical University, Kunming 650032, Yunnan Province, China
CLC Number:
Cite this article
Lan Xiaoqian, Feng Guangli, Qin Shiyi, Zhong Lianmei, Li Qing. New ideas and opportunities for polyurethane materials in peripheral nerve repair[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(28): 6127-6137.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
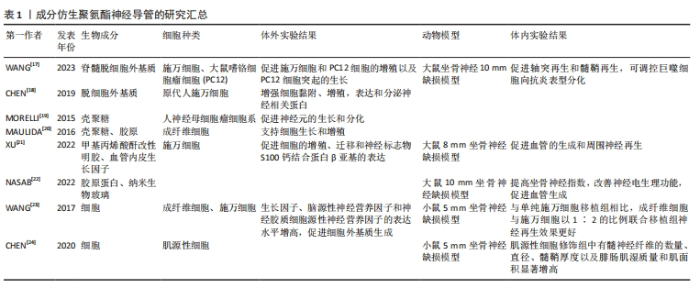
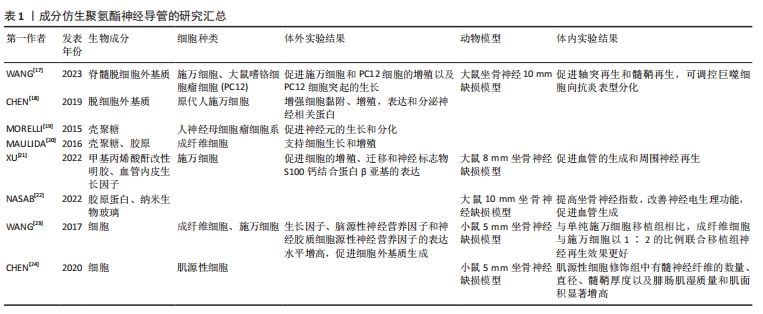
2.1 成分仿生聚氨酯神经导管 通过成分仿生优化聚氨酯神经导管的生物活性,旨在使用合适的细胞外基质成分、神经营养因子和细胞来修饰聚氨酯神经导管,从而改善细胞与神经导管之间的相互作用微环境,促进周围神经损伤修复。细胞外基质是由多种生物大分子组成的复杂网状结构,包括胶原蛋白、糖蛋白(非胶原)、糖胺聚糖和弹性蛋白等,不仅为细胞增殖和活动提供了合适的位点,还通过信号转导系统影响细胞的形状、代谢、功能、迁移、增殖和分化等多种生物学过程[15]。脱细胞细胞外基质可以保留这些关键的生物活性分子以及功能,去除可能引起免疫反应的细胞成分,为再生医学和组织工程提供新的解决方案[16]。WANG等[17]采用脊髓脱细胞外基质修饰可降解水性聚氨酯神经导管,导管在体外促进了施万细胞和大鼠嗜铬细胞瘤细胞(PC12)增殖以及突起的生长;在大鼠坐骨神经切断损伤模型中,神经导管在移植后6周显示出与自体神经移植相似的修复效果,可诱导施万细胞重编程,促进轴突再生和髓鞘再生并调控微环境中的巨噬细胞向抗炎型分化,发挥抗炎功效。另外研究发现,脱细胞外基质增加聚氨酯神经导管的生物活性,促进神经相关蛋白表达,可能在细胞间的化学信号传导中发挥作用[18]。壳聚糖与蛋白多糖的结构相似,可以与细胞外基质中的分子相互作用。研究发现,壳聚糖修饰的聚氨酯神经薄膜可以促进神经元细胞黏附和分化[19]。 MAULIDA等[20]合成壳聚糖包被的聚氨酯-胶原中空神经导管,同时发挥壳聚糖和胶原蛋白的优势,不仅支持细胞的增殖,还有生物相容性好、可再生、易降解等特点。血管内皮生长因子是促进神经损伤修复的一种重要营养因子,XU等[21]制备聚氨酯/甲基丙烯酸酐改性明胶/血管内皮生长因子神经导管,甲基丙烯酸酐改性明胶有利于传递生物活性物质血管内皮生长因子,解决了聚氨酯神经导管植入体内后血管形成缓慢和不足的问题,诱导内皮细胞的生长,影响施万细胞的增殖和迁移,可修复8 mm坐骨神经缺损。细胞外基质中离子成分发挥的作用也不容忽视。研究表明,在聚氨酯/胶原蛋白/纳米生物玻璃新型导管中,胶原蛋白改善了聚氨酯的生物相容性和细胞黏附性,纳米生物玻璃通过释放离子K+、Ca2+、Na+等,一方面促进血管内皮生长因子的分泌进而促进血管生成,另一方面通过钙离子调节轴突的生长;动物实验结果显示,该导管植入后12周,大鼠坐骨神经组织再生率几乎与自体神经移植组相当[22]。上述研究结果表明,细胞外基质衍生物、蛋白和神经营养因子等生物成分能够优化聚氨酯神经导管的生物活性,但需要进一步明确神经再生修复过程中起主要作用的生物活性因子是什么,以及交联哪些生物活性物质治疗的效果最优;另外,细胞外基质中不同离子成分促进神经修复诱发的分子机制值得进一步探索。 神经再生是一个复杂的生物学过程,由施万细胞、巨噬细胞、内皮细胞和成纤维细胞等多种细胞共同调控并影响神经修复微环境。仿生神经再生过程中的细胞微环境非常重要。研究表明,聚氨酯导管作为细胞载体可用于神经组织工程修复[23]。当成纤维细胞与施万细胞以1∶2的比例联合移植时,能产生更多的神经营养因子和细胞外基质,对神经再生和电生理功能恢复有明显的促进作用。CHEN等[24]利用肌源性细胞可分化为施万细胞样细胞的特点,将肌源性细胞注入聚氨酯导管内桥接神经断端后,发现肌源性细胞组术后8,12周再生神经的髓鞘和轴突分布均匀,坐骨神经功能指数较聚氨酯组明显升高,表明肌源性细胞有效地加速了周围神经的再生。上述研究表明,不同细胞在周围神经修复中发挥的作用不同,神经再生微环境中不同类型细胞共同修复神经的效果如何,以及细胞间最适合的比例是多少,也需要更全面的研究。成分仿生聚氨酯神经导管的研究汇总,见表1。"

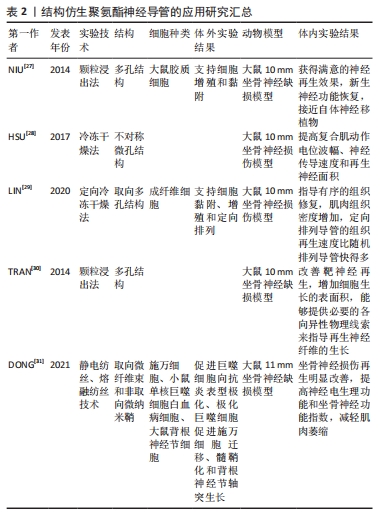
2.2 结构仿生聚氨酯神经导管 理想神经导管的结构设计旨在仿生周围神经组织的拓扑结构,包括必要的多孔结构、管腔内的微观结构和适合的机械性能。生物材料的物理性质和微观结构(如刚度、形貌、孔隙率和尺寸尺度)是控制细胞行为的关键[25]。传统的中空神经导管由于细胞迁移和营养物质运输的限制,在修复周围神经方面效果不理想[26]。非取向多孔设计的神经导管具有更高的空间利用率,并能够增加导管内壁面积,增加细胞的黏附面积和吸附能力,促进物质交换,帮助周围神经再生。颗粒浸出法和冷冻干燥技术可在神经导管内产生高度互联的多孔结构仿生细胞外基质样结构,帮助物质交换。NIU等[27]采用颗粒浸出法制备具有高表面积的聚氨酯多孔导管,便于细胞黏附,并为促进组织再生提供生物化学和形貌信息。研究发现,通过冷冻干燥技术形成具有不对称微孔结构的聚氨酯神经导管,微孔结构可以促进神经纤维增粗[28],提高复合肌动作电位波幅和神经传导速度,增加神经再生面积。 神经导管的非取向多孔结构设计虽然增加导管内壁的表面积和细胞的吸附力,但是在引导细胞定向迁移、增殖、分化等方面的能力还有待提高。研究表明,取向多孔神经导管能更好地为细胞提供定向引导,影响细胞的行为、形态学、迁移、增殖和分化[29]。除了取向多孔结构,神经导管表面凹槽、管道、纳米纤维等结构在引导细胞和轴突定向生长中也发挥了重要作用。TRAN等[30]制备了内部多个定向通道和一个外部无孔的双层聚氨酯神经导管,分别模拟天然的神经内膜微管和神经外膜结构,在体内坐骨神经移植手术中,多纵向通道神经导管显示出与自体神经移植相当的再生效果,在纤维数量、密度以及机械性能方面优于空心管,能够提供必要的定向物理线索来指导再生神经纤维的生长。还有研究利用融纺丝(微米纤维)和静电纺丝(纳米纤维)2种方法设计了由内层定向的微米纤维束芯(即填充剂)和外层随机排列的纳米纤维鞘组成的复合神经导管,发现仿生神经取向微米纤维可影响巨噬细胞的极化,引导巨噬细胞由促炎表型向抗炎表型分化,随后通过细胞-细胞相互作用和分泌旁分泌因子实现了施万细胞的迁移、增殖、成熟和轴突延伸,从而促进神经组织的抗炎和再生[31]。上述研究结果表明,优化聚氨酯神经导管的结构设计能够提供生物引导线索,帮助恢复受损神经。但是值得注意的是,神经导管表面不同类型微/纳米结构,包括柱、管、孔、纤维和沟槽等,最适宜的柱体大小、孔径大小、管道的数量、凹槽的宽度和深浅设计、纤维粗细等的最佳设计参数不同,值得进一步探索。结构仿生聚氨酯神经导管的应用研究汇总,见表2。"
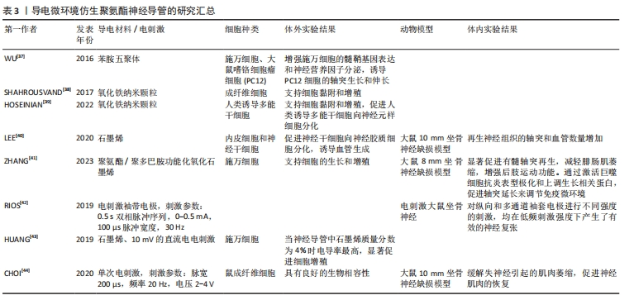
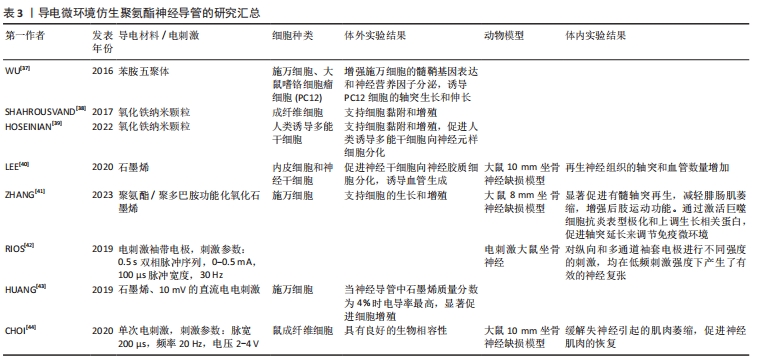
2.3 生物力学仿生聚氨酯神经导管 生物力学仿生侧重于模拟和研究生物系统在机械应力下的力学行为和反应,以改进生物材料在生物医学应用中的性能。细胞外基质的力学特性在调节发育、环境稳态、再生过程和疾病进展中发挥着关键作用。神经导管的力学特性是设计神经导管时应考虑的一个重要参数。为了了解细胞受细胞外基质的刚度和孔隙度、躯体运动以及与周围环境的相互作用引起的机械力的影响,SALA等[32]将神经导管受到的机械应力(不同径向压缩状态)量化为导管孔径大小和表面形貌的函数,以进一步了解导管的形貌结构在引导轴突延伸中的作用,结果发现,增加聚氨酯气凝胶径向压缩程度后,神经突长度增加,细胞体面积增加,神经突数量减少;而表面粗糙度越大,神经突长度越短,神经突密度越大。研究发现,通过调整聚氨酯共聚物材料组分聚己内酯和聚乙二醇的比例,可以使多孔导管的力学性能与周围神经相近,解决神经导管体内降解速率与神经再生过程相匹配的问题,促进血管生成,诱导巨噬细胞由促炎表型转变为抗炎表型并抑制炎症反应,引导神经延伸,增强神经传导功能[33]。WANG等[34]通过调整聚氨酯组分聚乙二醇的含量进而调节多孔聚氨酯神经导管的模量,发现不同模量神经导管都可以诱导巨噬细胞由促炎表型向抗炎表型分化,但对坐骨神经的修复效果不同,并且导管的力学特性通过调节轴突生长的生长相关蛋白43表达影响轴突的形成和延伸,促进周围神经再生。神经导管的力学性能可能在调节免疫反应和促进轴突生长、帮助周围神经损伤修复中起关键作用,在未来的生物材料神经导管设计中应予以考虑,不同结构设计的神经导管是如何通过力学影响细胞行为,其存在的机制是什么,也需要深入研究。 2.4 导电微环境仿生聚氨酯神经导管 神经系统通过电信号传导调节机体的运动和感觉[35]。周围神经损伤后电信号传递受阻,如何重建神经电信号传导通路尤为重要。鉴于神经的电生理特性,物理化学修饰是提高聚氨酯材料生物电活性和调节神经细胞信号传导的重要因素[36]。导电微环境仿生聚氨酯导管旨在通过电活性材料和电刺激帮助神经修复生物电活性。电活性生物材料,如苯胺五聚体、磁铁矿纳米颗粒、石墨烯、聚吡咯等,常被用于修饰神经导管帮助神经电活动恢复。WU等[37]将苯胺五聚体与聚氨酯制备可降解神经导管,研究阐明施万细胞分泌神经营养因子的机制是:由于神经导管具有抑制钙离子敏感受体和磷脂酶β途径的能力,降低细胞内Ca2+水平,从而诱导施万细胞的髓鞘基因表达和神经营养因子分泌。有研究用磁铁矿纳米颗粒Fe2O3修饰聚氨酯,发现随着磁铁矿纳米颗粒数量的增加导致聚合物导电性增强、电阻下降,进而增强了神经导管电信号传导能力,诱导细胞的分化、黏附和增殖[38-39]。研究发现,水性聚氨酯/石墨烯神经导管上调特定的血管和神经相关基因标记物,石墨烯质量分数为5%的聚氨酯神经导管植入动物体内后表现出与正常坐骨神经相似的复合动作电位波 形[40]。为了提高石墨烯的生物相容性,ZHANG等[41]用聚多巴胺修饰氧化石墨烯与聚氨酯反应制备导神经导管,该导管表面施万细胞增殖的标志物S100钙结合蛋白表达增加,导管在体内通过激活巨噬细胞向抗炎表型极化和上调生长相关蛋白43促进轴突延长和连接,进而调节免疫微环境。 电刺激联合神经导管治疗周围神经损伤是一种有效的策略。RIOS等[42]使用铂铱电极和聚氨酯导管制备了袖套电极,发现在低频率电刺激下可产生有效的坐骨神经复张。HUANG等[43]利用石墨烯/热塑性聚氨酯复合材料联合电刺激促进周围神经损伤修复,发现与非导电导管相比,石墨烯质量分数为5%聚氨酯导管联合10 mV的直流电电刺激更适合施万细胞的增殖。与直接电刺激治疗不同,CHOI等[44]以聚己内酯和二异氰酸酯为原料,利用钼金属线圈作为射频信号收集天线,将无线射频信号捕获并转化为交流电能,在单次电刺激、脉宽200 μs、频率 20 Hz、电压2-4 V时可维持神经细胞的电兴奋性,修复坐骨神经10 mm缺损并增强神经肌肉功能恢复的潜力。上述研究表明,应用导电材料和适当的电刺激修饰聚氨酯材料可以帮助恢复神经的电生理功能。但是之前的研究也发现一些问题:部分导电材料的细胞毒性以及不易降解的性质限制了导电神经导管的临床应用,如何对导电材料进行功能修饰,降低生物毒性,优化降解性能则需要进一步研究;具有导电性能的材料非常多,但是哪种材料的性能最优毒性最小、最适合神经损伤修复目前尚不清楚;虽然大量研究发现低频电刺激可以帮助神经损伤修复,但是不同导电材料对应的最佳电刺激参数仍需要大量的实验进一步探索;除了电刺激,磁刺激、光刺激、自发电和复合压电设计在聚氨酯材料中也有很大的应用前景。导电微环境仿生聚氨酯神经导管的研究汇总,见表3。"
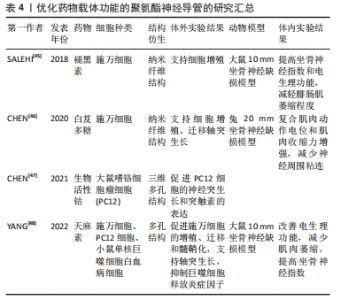
2.5 优化药物载体功能的聚氨酯神经导管 聚氨酯神经导管还被作为药物的载体以开发局部给药系统,改善神经生长、抑制炎症,协助神经断端的修复。化学药物修饰聚氨酯材料往往联合应用结构仿生策略,协同调控神经再生免疫微环境的同时调控药物的释放速率。褪黑素具有神经保护作用,有研究将聚氨酯/明胶/褪黑素/富血小板血浆静电纺丝神经导管植入到动物体内后发现,与对照组相比明显提高了坐骨神经指数和电生理功能、减轻腓肠肌萎缩程度,表现出良好的治疗效果[45];其中,纳米结构增加了导管表面积,明胶显著增加聚氨酯的亲水性,富血小板血浆帮助维持药物的稳定性,在加速聚氨酯降解的同时有助于调控褪黑素释放,促进神经再生。除了神经保护作用,如何减少神经修复中的瘢痕形成也尤为重要。有研究通过添加药物白芨多糖,以异佛尔酮二异氰酸酯和聚乙二醇为原料制备静电纺丝神经导管,移植到兔坐骨损伤模型中后发现,该导管可以减少成纤维细胞的附着和神经瘢痕的形成,同时显著改善了轴突直径和髓鞘厚度,减轻了动物模型的肌肉萎缩[46];同时研究发现,白芨多糖作为一种亲水性聚合物在接触水后大量释放,纳米纤维形成的高表面积和孔隙率进一步促进药物释放,如何调控白芨多糖在聚氨酯神经导管中的缓释也是未来需要攻克的问题。钴是维生素B12的组成部分,对维持神经系统的正常功能具有重要作用。CHEN等[47]采用3D打印技术,利用生物活性钴和海藻酸盐对聚氨酯材料进行修饰,制备了一种具有“珊瑚礁样”表面粗糙纳米拓扑结构的三维多孔材料,发现“珊瑚礁样”凹凸不平的表面拓扑结构和生物活性钴协同促进了导管上PC12细胞的神经突生长和突触素的表达,可用于神经损伤修复。另外,天麻素具有抗炎功效,对修复神经损伤具有重要作用。YANG等[48]选择天麻素对聚氨酯材料进行功能修饰,采用盐浸渍法制备聚氨酯多孔神经导管,用于修复SD大鼠10 mm坐骨神经缺损,高孔隙率和天麻素的亲水性能帮助聚氨酯导管实现天麻素的缓释,促进髓鞘和轴突的生长,减少动物体内炎症反应。基于上述研究,有理由认为合适的化学药物能够通过神经保护作用、抗炎、抗神经瘢痕等方式帮助周围神经重建功能,并且药物的亲疏水性、溶解性、聚氨酯材料表面改性、聚氨酯神经导管的结构等因素都会影响药物的释放,但是如何优化药物释放动力学、实现药物释放与组织生长的时空平衡、解决药物局部释放的同时不影响血药浓度、如何进行药物筛选等问题,都需要进一步研究。优化药物载体功能的聚氨酯神经导管的研究汇总,见表4。"

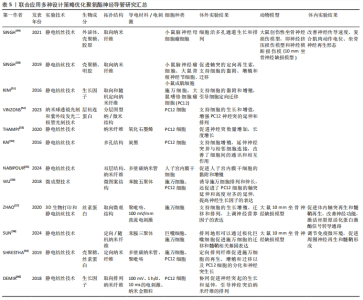
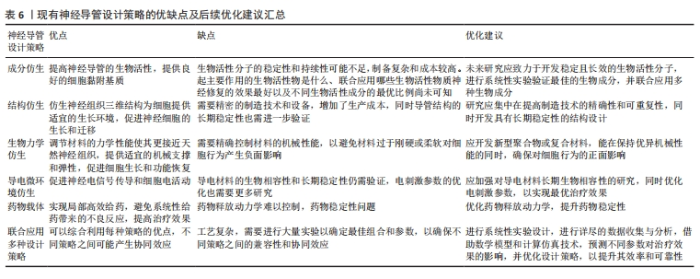
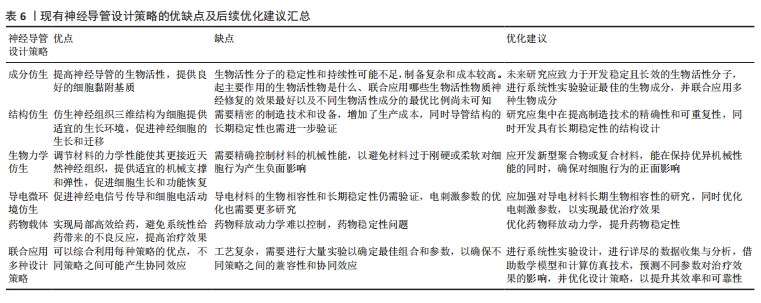
2.6 联合应用多种仿生设计策略优化聚氨酯神经导管 联合应用多种仿生设计策略开发新型神经导管是目前神经组织工程的主流趋势。成分仿生和结构仿生神经策略的联合应用,在仿生细胞外基质的结构基础上赋予聚氨酯材料生物活性。静电纺丝技术可以仿生细胞外基质多孔结构,促进物质交换和组织再生。SINGH等[49-51]发现静电纺丝技术制备的取向纳米纤维,在仿生细胞外基质结构的同时为轴突的定向再生提供地形线索,引导细胞的黏附和迁移,并可通过诱导细胞定向来调控细胞形态;另外,生物活性物质增加了材料的亲水性和生物相容性,协同促进神经再生。不同于孔隙结构,纳米柱和纳米孔结构修饰的聚氨酯/层粘连蛋白聚氨酯材料生物活性提高,并且纳米柱和纳米孔为生长锥丝状伪足提供附着和引导线索,促进其向前移动和轴突的延伸,通过作用于生长锥进而影响PC12细胞的经突长度[52]。 联合应用结构仿生和导电微环境仿生的神经导管设计策略,有望恢复神经电信号传导,引导神经定向生长。研究发现,利用静电纺丝技术制备聚碳酸酯聚氨酯/氧化石墨烯神经薄膜,其导电性能和表面纳米形貌通过提供细胞外基质样微环境来加速神经元再生[53]。KAI等[54]合成了一种由炭黑修饰的取向型新型导电聚氨酯神经导管,支持细胞增殖,促进PC12细胞沿纤维方向伸长,改善了细胞间的通讯和相互作用。NABIPOUR等[55]制备一种双层聚氨酯/聚左旋乳酸/多壁碳纳米管神经导管,研究发现多壁碳纳米管修饰导管后改变了纳米纤维的形貌和力学特性,赋予导管良好的机械性能和导电性能,支持子宫内膜干细胞的黏附、增殖和定向生长,为坐骨神经的再生创造一个理想的环境。WU等[56]通过微成型技术制备具有微图案的导电生物可降解聚氨酯神经导管,不仅可以通过微图案表面诱导施万细胞排列和伸长,还促进了PC12细胞的轴突定向延伸,显著提高了神经生长因子基因的表达。ZHAO 等[57]发现,氧化石墨烯修饰的取向纳米纤维和 10 mV电刺激的协同作用有效增强了细胞的迁移、增殖和神经生长因子表达,并且细胞均匀铺展高度有序。除此之外,导电材料苯胺三聚体联合拓扑结构修饰的聚氨酯神经导管,可以调节免疫微环境、促进巨噬细胞募集、诱导巨噬细胞极化,在体外和体内均促进了施万细胞的迁移和髓鞘形成,促进周围神经再生[58]。 聚氨酯材料联合应用成分仿生、结构仿生和导电微环境仿生的神经导管设计策略,能更全面地仿生细胞外基质环境。SHRESTHA等[59]设计了取向聚氨酯/丝素蛋白/多壁碳纳米神经导管,模拟天然神经导电和细胞外基质样的微环境,显著刺激了施万细胞的生长和增殖,促进PC12细胞的分化和引导神经突沿着纤维排列的方向生长。DEMIR等[60]设计取向规则的金纳米颗粒/聚氨酯神经导管,进一步研究纳米形貌、神经生长因子和电刺激对PC12细胞轴突生长和伸长的协同作用,发现在50 ng/mL神经生长因子和电刺激(100 mV、1 h/d、10 ms)下培养细胞,能更好地促进神经突的生长和伸长。与DEMIR等[60]电刺激参数不同,有研究显示,聚氨酯/聚吡啶/丝素蛋白神经导管体外实验在100 mV/mm参数下电刺激2 h,施万细胞黏附、增殖和分化的情况最好;神经导管还能够成功修复SD大鼠10 mm坐骨神经缺损,促进体内轴突再生和髓鞘再生,激活丝裂原活化蛋白激酶信号转导通路促进神经生长[57]。上述研究结果表明,联合应用多种仿生设计策略的神经导管在周围神经损伤修复中发挥协同作用。但是值得注意的是:不同仿生设计策略联合应用时的治疗效果不尽相同,并且不同组合之间最佳的设计参数也不相同,需要更加全面的实验进一步验证;如何联合应用多种仿生设计策略使之与周围神经修复的时空变化相匹配也是未来研究不能忽视的问题,包括联合应用药物、导管结构、生物成分等在急性期、亚急性期和慢性期阶段分别应对炎症和免疫调控、促进血管生成以及支持髓鞘再生等生物学过程[61]。联合应用多种设计策略优化聚氨酯神经导管研究汇总,见表5。现有神经导管设计策略的优缺点及后续的优化建议汇总,见表6。"
| [1] YANG Y, RAO C, YIN T, et al. Application and underlying mechanism of acupuncture for the nerve repair after peripheral nerve injury: Remodeling of nerve system. Front Cell Neurosci. 2023;17:1253438. [2] WAN T, ZHANG FS, QIN MY, et al. Growth factors: Bioactive macromolecular drugs for peripheral nerve injury treatment - molecular mechanisms and delivery platforms. Biomed Pharmacother. 2024;170: 116024. [3] ZHOU W, RAHMAN MSU, SUN C, et al. Perspectives on the novel multifunctional nerve guidance conduits: From specific regenerative procedures to motor function rebuilding. Adv Mater. 2024;36(14): e2307805. [4] REN J, TANG X, WANG T, et al. A dual-modal magnetic resonance/photoacoustic imaging tracer for long-term high-precision tracking and facilitating repair of peripheral nerve injuries. Adv Healthc Mater. 2022;11(13):e2200183. [5] BEHTAJ S, EKBERG JAK, ST JOHN JA. Advances in electrospun nerve guidance conduits for engineering neural regeneration. Pharmaceutics. 2022;14(2):219. [6] BITTNER GD, BUSHMAN JS, GHERGHEREHCHI CL, et al. Typical and atypical properties of peripheral nerve allografts enable novel strategies to repair segmental-loss injuries. J Neuroinflammation. 2022;19(1):60. [7] MANKAVI F, IBRAHIM R, WANG H. Advances in biomimetic nerve guidance conduits for peripheral nerve regeneration. Nanomaterials (Basel). 2023;13(18):2528. [8] SUN J, CAO W, PAN S, et al. Porous organic materials in tissue engineering: Recent advances and applications for severed facial nerve injury repair. Molecules. 2024;29(3):566. [9] 曹银利,傅锴锴,陈文,等.可生物降解的聚氨酯体内应用进展[J].华中科技大学学报(医学版),2023,52(1):111-116. [10] JIA B, HUANG H, DONG Z, et al. Degradable biomedical elastomers: Paving the future of tissue repair and regenerative medicine. Chem Soc Rev. 2024;53(8):4086-4153. [11] PEDERSEN DD, KIM S, WAGNER WR. Biodegradable polyurethane scaffolds in regenerative medicine: Clinical translation review. J Biomed Mater Res A. 2022;110(8):1460-1487. [12] GU D, XIA Y, DING Z, et al. Inflammation in the peripheral nervous system after injury. Biomedicines. 2024;12(6):1256. [13] MIN Q, PARKINSON DB, DUN XP. Migrating schwann cells direct axon regeneration within the peripheral nerve bridge. Glia. 2021;69(2): 235-254. [14] QIAN Y, LIN H, YAN Z, et al. Functional nanomaterials in peripheral nerve regeneration: Scaffold design, chemical principles and microenvironmental remodeling. Mater Today. 2021;51:165-187. [15] ZHANG CY, FU CP, LI XY, et al. Three-dimensional bioprinting of decellularized extracellular matrix-based bioinks for tissue engineering. Molecules. 2022;27(11):3442. [16] BROWN M, LI J, MORAES C, et al. Decellularized extracellular matrix: New promising and challenging biomaterials for regenerative medicine. Biomaterials. 2022;289:121786. [17] WANG Y, LIN J, CHEN J, et al. Biodegradable polyurethane-incorporating decellularized spinal cord matrix scaffolds enhance schwann cell reprogramming to promote peripheral nerve repair. J Mater Chem B. 2023;11(10):2115-2128. [18] CHEN YW, CHEN CC, NG HY, et al. Additive manufacturing of nerve decellularized extracellular matrix-contained polyurethane conduits for peripheral nerve regeneration. Polymers. 2019;11(10):1612. [19] MORELLI S, PISCIONERI A, MESSINA A, et al. Neuronal growth and differentiation on biodegradable membranes. J Tissue Eng Regen Med. 2015;9(2):106-117. [20] MAULIDA HN, QULUB F, ROSDIANI AF, et al. Hollowfiber polyurethane-collagen coating chitosan as nerve graft for therapy of peripheral nerve injury in extreme paralysis. J Biomim Biomater Biomed Eng. 2016;28:78-84. [21] XU W, ZHANG Z, LU H, et al. Biocompatible polyurethane conduit grafted with vascular endothelial growth factor-loaded hydrogel repairs the peripheral nerve defect in rats. Macromol Biosci. 2022; 22(3):e2100397. [22] NASAB ST, ROODBARI NH, GOODARZI V, et al. Nanobioglass enhanced polyurethane/collagen conduit in sciatic nerve regeneration. J Biomed Mater Res B Appl Biomater. 2022;110(5):1093-1102. [23] WANG Y, LI D, WANG G, et al. The effect of co-transplantation of nerve fibroblasts and schwann cells on peripheral nerve repair. Int J Biol Sci. 2017;13(12):1507-1519. [24] CHEN ZX, LU HB, JIN XL, et al. Skeletal muscle-derived cells repair peripheral nerve defects in mice. Neural Regen Res. 2020;15(1): 152-161. [25] FILIPPI M, GARELLO F, YASA O, et al. Engineered magnetic nanocomposites to modulate cellular function. Small. 2022;18(9): e2104079. [26] DENG P, CHEN F, ZHANG H, et al. Multifunctional double-layer composite hydrogel conduit based on chitosan for peripheral nerve repairing. Adv Healthc Mater. 2022;11(13):e2200115. [27] NIU Y, CHEN KC, HE T, et al. Scaffolds from block polyurethanes based on poly(ɛ-caprolactone) (pcl) and poly(ethylene glycol) (peg) for peripheral nerve regeneration. Biomaterials. 2014;35(14):4266-4277. [28] HSU SH, CHANG WC, YEN CT. Novel flexible nerve conduits made of water-based biodegradable polyurethane for peripheral nerve regeneration. J Biomed Mater Res A. 2017;105(5):1383-1392. [29] LIN W, LAN W, WU Y, et al. Aligned 3d porous polyurethane scaffolds for biological anisotropic tissue regeneration. Regen Biomater. 2020; 7(1):19-27. [30] TRAN RT, CHOY WM, CAO H, et al. Fabrication and characterization of biomimetic multichanneled crosslinked-urethane-doped polyester tissue engineered nerve guides. J Biomed Mater Res A. 2014;102(8): 2793-2804. [31] DONG X, LIU S, YANG Y, et al. Aligned microfiber-induced macrophage polarization to guide schwann-cell-enabled peripheral nerve regeneration. Biomaterials. 2021;272:120767. [32] SALA MR, SKALLI O, LEVENTIS N, et al. Nerve response to superelastic shape memory polyurethane aerogels. Polymers (Basel). 2020; 12(12):2995. [33] NIU Y, GALLUZZI M. A biodegradable block polyurethane nerve-guidance scaffold enhancing rapid vascularization and promoting reconstruction of transected sciatic nerve in sprague-dawley rats. J Mater Chem B. 2020;8(48):11063-11073. [34] WANG Y, LIANG R, LIN J, et al. Biodegradable polyurethane nerve guide conduits with different moduli influence axon regeneration in transected peripheral nerve injury. J Mater Chem B. 2021;9(38): 7979-7990. [35] ZHOU K, WEI W, YANG D, et al. Dual electrical stimulation at spinal-muscular interface reconstructs spinal sensorimotor circuits after spinal cord injury.Nat Commun. 2024;15(1):619. [36] LI L, LI D, WANG Y, et al. Implantable zinc-oxygen battery for in situ electrical stimulation-promoted neural regeneration. Adv Mater. 2023;35(32):e2302997. [37] WU Y, WANG L, GUO B, et al. Electroactive biodegradable polyurethane significantly enhanced schwann cells myelin gene expression and neurotrophin secretion for peripheral nerve tissue engineering. Biomaterials. 2016;87:18-31. [38] SHAHROUSVAND M, HOSEINIAN MS, GHOLLASI M, et al. Flexible magnetic polyurethane/Fe2O3 nanoparticles as organic-inorganic nanocomposites for biomedical applications: Properties and cell behavior. Mater Sci Eng C Mater Biol Appl. 2017;74:556-567. [39] HOSEINIAN MS, POORMOGHADAM D, KHEIROLLAHZADEH F, et al. Improved neural differentiation of human-induced pluripotent stem cell(hipscs) on a novel polyurethane-based scaffold containing iron oxide nanoparticles (Fe2O3 nps). Curr Stem Cell Res Ther. 2022;18(7): 993-1000. [40] LEE TH, YEN CT, HSU SH. Preparation of polyurethane-graphene nanocomposite and evaluation of neurovascular regeneration. ACS Biomater Sci Eng. 2020;6(1):597-609. [41] ZHANG Q, CHEN J, FENG Y, et al. Electroactive scaffolds of biodegradable polyurethane/polydopamine-functionalized graphene oxide regulating the inflammatory response and revitalizing the axonal growth cone for peripheral nerve regeneration. J Mater Chem B. 2023;11(27):6308-6318. [42] RIOS MU, BUCKSOT JE, RAHEBI KC, et al. Protocol for construction of rat nerve stimulation cuff electrodes. Methods Protoc. 2019;2(1):19. [43] HUANG Z, GUO Z, SUN M, et al. A study on graphene composites for peripheral nerve injury repair under electrical stimulation. RSC Adv. 2019;9(49):28627-28635. [44] CHOI YS, HSUEH YY, KOO J, et al. Stretchable, dynamic covalent polymers for soft, long-lived bioresorbable electronic stimulators designed to facilitate neuromuscular regeneration. Nat Commun. 2020;11(1):5990. [45] SALEHI M, NASERI-NOSAR M, EBRAHIMI-BAROUGH S, et al. Polyurethane/gelatin nanofibrils neural guidance conduit containing platelet-rich plasma and melatonin for transplantation of schwann cells. Cell Mol Neurobiol. 2018;38(3):703-713. [46] CHEN SH, CHOU PY, CHEN ZY, et al. An electrospun nerve wrap comprising bletilla striata polysaccharide with dual function for nerve regeneration and scar prevention. Carbohydr Polym. 2020;250:116981. [47] CHEN Y, LONG X, LIN W, et al. Bioactive 3d porous cobalt-doped alginate/waterborne polyurethane scaffolds with a coral reef-like rough surface for nerve tissue engineering application. J Mater Chem B. 2021;9(2):322-335. [48] YANG H, LI Q, LI L, et al. Gastrodin modified polyurethane conduit promotes nerve repair via optimizing schwann cells function. Bioact Mater. 2022;8:355-367. [49] SINGH A, SHIEKH PA, QAYOOM I, et al. Evaluation of polymeric aligned ngcs and exosomes in nerve injury models in diabetic peripheral neuropathy condition. Eur polym J. 2021;146:110256. [50] SINGH A, SHIEKH PA, DAS M, et al. Aligned chitosan-gelatin cryogel-filled polyurethane nerve guidance channel for neural tissue engineering: Fabrication, characterization, and in vitro evaluation. Biomacromolecules. 2019;20(2):662-673. [51] KIM JI, HWANG TI, AGUILAR LE, et al. A controlled design of aligned and random nanofibers for 3d bi-functionalized nerve conduits fabricated via a novel electrospinning set-up. Sci Rep. 2016;6:23761. [52] VINZONS LU, DONG GC, LIN SP. Hierarchically patterned polyurethane microgrooves featuring nanopillars or nanoholes for neurite elongation and alignment. Beilstein J Nanotechnol. 2023;14:1157-1168. [53] THAMPI S, THEKKUVEETTIL A, MUTHUVIJAYAN V, et al. Accelerated outgrowth of neurites on graphene oxide-based hybrid electrospun fibro-porous polymeric substrates. ACS Appl Bio Mater. 2020;3(4): 2160-2169. [54] KAI D, TAN MJ, PRABHAKARAN MP, et al. Biocompatible electrically conductive nanofibers from inorganic-organic shape memory polymers. Colloids Surf B Biointerfaces. 2016;148:557-565. [55] NABIPOUR M, MELLATI A, ABASI M, et al. Preparation of bilayer tissue-engineered polyurethane/poly-l-lactic acid nerve conduits and their in vitro characterization for use in peripheral nerve regeneration. J Biol Eng. 2024;18(1):16. [56] WU Y, WANG L, HU T, et al. Conductive micropatterned polyurethane films as tissue engineering scaffolds for schwann cells and pc12 cells. J Colloid Interface Sci. 2018;518:252-262. [57] ZHAO Y, LIANG Y, DING S, et al. Application of conductive ppy/sf composite scaffold and electrical stimulation for neural tissue engineering . Biomaterials. 2020;255:120164. [58] SUN Y, ZHANG Y, GUO Y, et al. Electrical aligned polyurethane nerve guidance conduit modulates macrophage polarization and facilitates immunoregulatory peripheral nerve regeneration. J Nanobiotechnology. 2024;22(1):244. [59] SHRESTHA S, SHRESTHA BK, LEE J, et al. A conducting neural interface of polyurethane/silk-functionalized multiwall carbon nanotubes with enhanced mechanical strength for neuroregeneration. Mater Sci Eng C Mater Biol Appl. 2019;102:511-523. [60] DEMIR US, SHAHBAZI R, CALAMAK S, et al. Gold nano-decorated aligned polyurethane nanofibers for enhancement of neurite outgrowth and elongation. J Biomed Mater Res A. 2018;106(6):1604-1613. [61] SHAN Y, XU L, CUI X, et al. A responsive cascade drug delivery scaffold adapted to the therapeutic time window for peripheral nerve injury repair. Mater Horiz. 2024;11(4):1032-1045. |
| [1] | Lai Pengyu, Liang Ran, Shen Shan. Tissue engineering technology for repairing temporomandibular joint: problems and challenges [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [2] | Cao Yue, Ye Xinjian, Li Biyao, Zhang Yining, Feng Jianying. Effect of extracellular vesicles for diagnosis and therapy of oral squamous cell carcinoma [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1523-1530. |
| [3] | Sun Yuting, Wu Jiayuan, Zhang Jian. Physical factors and action mechanisms affecting osteogenic/odontogenic differentiation of dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1531-1540. |
| [4] | Ding Zhili, Huang Jie, Jiang Qiang, Li Tusheng, Liu Jiang, Ding Yu. Constructing rabbit intervertebral disc degeneration models by different methods under X-ray guidance: a comparative study [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 995-1002. |
| [5] | Li Shuai, Liu Hua, Shang Yonghui, Liu Yicong, Zhao Qihang, Liu Wen. Stress distribution on the maxilla when wearing the Twin-block appliance for Class II malocclusion [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 881-887. |
| [6] | Xiao Fang, Huang Lei, Wang Lin. Magnetic nanomaterials and magnetic field effects accelerate bone injury repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 827-838. |
| [7] | Wang Sifan, He Huiyu, Yang Quan, Han Xiangzhen. miRNA-378a overexpression of macrophage cell line composite collagen sponge: anti-inflammation and tissue repair promotion [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 789-799. |
| [8] | Li Mingzhe, Ye Xiangling, Wang Bing, Yu Xiang. Preparation and osteogenic properties of liquid crystal display light-cured polylactic acid scaffold loaded with nano-tantalum [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 670-677. |
| [9] | Yu Shuangqi, Ding Fan, Wan Song, Chen Wei, Zhang Xuejun, Chen Dong, Li Qiang, Lin Zuoli. Effects of polylactic acid-glycolic acid copolymer/lysine-grafted graphene oxide nanoparticle composite scaffolds on osteogenic differentiation of MC3T3 cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 707-712. |
| [10] | Dang Xiaowen, Huang Hailiang, Huang Lei, Wang Yajie . Research frontiers and hotspots of carbon nanomaterials in biomedical field over the past 10 years [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 752-760. |
| [11] | Yi Xiaoding, Zhang Di, Guo Hong, Qing Liang, Zhao Tianyu. Decellularized tendon scaffold: a biomedical material for tendon injury repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7385-7392. |
| [12] | Wu Ziwei, Luo Yicai, Wei Yinge, Liao Hongbing. Application of poly(lactic-co-glycolic acid) copolymer in stomatology [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7393-7404. |
| [13] | Li Zhongzheng, Chen Zhenghao, Tang Ziyou, Lou Kaiyang, Zhang Rui, Liu Qi, Zhao Na, Yang Kun. Effects of scaffold materials combined with biological factors on biological characteristics of dental follicle cell proliferation and osteogenic differentiation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7405-7414. |
| [14] | Liu Xun, Ouyang Hougan, Pan Rongbin, Wang Zi, Yang Fen, Tian Jiaxuan . Optimal parameters for physical interventions in bone marrow mesenchymal stem cell differentiation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6727-6732. |
| [15] | Wang Zhao, Gong Lin, Piao Yongjun. Research on hair follicle organoids: current status, challenges and prospects [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6733-6742. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||