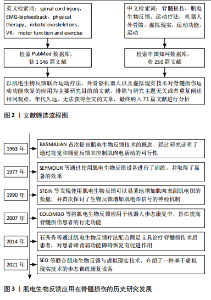
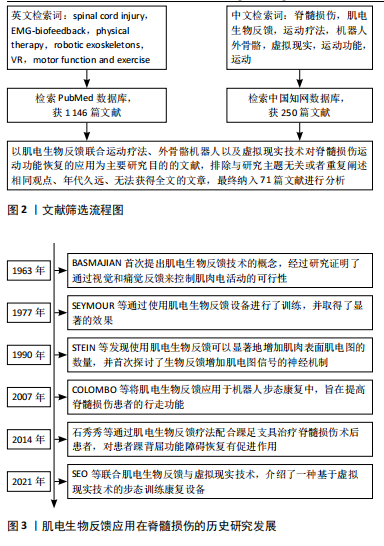
Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (14): 3002-3010.doi: 10.12307/2025.624
Previous Articles Next Articles
Electromyographic biofeedback therapy and motor function recovery after spinal cord injury
Liang Jiajia1, Sun Jiaojiao1, Liu Wenjie1, Xing Zheng2, Li Qi2, Li Qingwen1, Chu Xiaolei2
- 1School of Sports and Health, Tianjin University of Sport, Tianjin 301617, China; 2Tianjin Hospital, Tianjin 300211, China
-
Received:2024-06-07Accepted:2024-07-22Online:2025-05-18Published:2024-09-29 -
Contact:Li Qingwen, PhD, Professor, School of Sports and Health, Tianjin University of Sport, Tianjin 301617, China -
About author:Liang Jiajia, Master candidate, School of Sports and Health, Tianjin University of Sport, Tianjin 301617, China -
Supported by:the National Key Research and Development Program of China under the Key Special Project of “Biology and Information Convergence (BT and IT Convergence),” No. 2023YFF1205200 (to XZ [project participant]); Tianjin Municipal Natural Science Foundation (General Program), Nos. 22JCYBJC00210 (to LQ) and 22JCYBJC00220 (to CXL)
CLC Number:
Cite this article
Liang Jiajia, Sun Jiaojiao, Liu Wenjie, Xing Zheng, Li Qi, Li Qingwen, Chu Xiaolei . Electromyographic biofeedback therapy and motor function recovery after spinal cord injury[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(14): 3002-3010.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
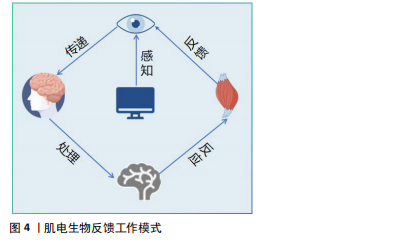
2.2 肌电生物反馈概述 2.2.1 定义和原理 肌电生物反馈是将生物反馈疗法与传统康复的电刺激相结合,本质上属于生物反馈疗法中的一种,通过表面电极测量肌肉活动产生的电信号,然后对其进行放大数据处理,以视觉或听觉模式实时反馈给大脑中枢,使患者了解肌肉的收缩状态,逐步训练和调节患者的肌肉活动情况,使大脑中枢逐渐恢复对瘫痪肌肉的控制,提高患者的主动控制能力,达到恢复运动功能的目的[9]。肌电生物反馈治疗安全、无创,可以用于治疗各种心理和身体健康问题,迫使患者积极参与到实现健康的行动中来[10]。不同于单纯的肌肉电刺激和功能性电刺激治疗手段,肌电生物反馈根据患者残存的肌肉主动收缩参与成分进行反馈刺激,进行主动收缩同时给予相应强度的肌肉电刺激以增加中枢输入、重塑肢体功能[11]。 生物反馈的工作模式涉及多种生理学和神经学过程,主要涉及感知、传递、处理、反应、反馈5个环节(图4)。当肌肉受到刺激时神经肌肉系统会产生电信号,这些信号通过神经纤维传递到脊髓和大脑进行加工处理;肌电生物反馈技术通过捕捉这些电信号,将其转化为可感知的反馈信号,使患者能够感知到自身肌肉活动的状态,并据此调整肌肉活动的策略。一方面,这种方法可以将患者自身的身体信息以图形界面或声音的形式直观地显示出来,使患者能够自我感知自己的运动情况;另一方面,对没有活动或肌肉力量明显减少的肌肉进行电刺激可以"
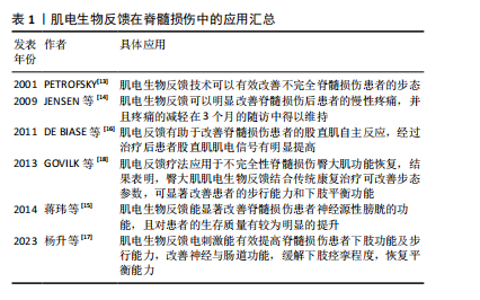
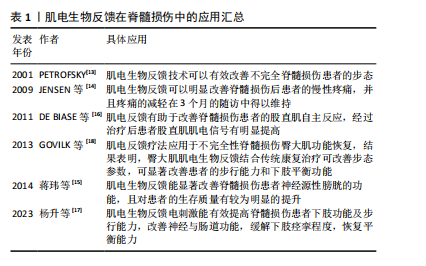
触发肌电信号,或者对运动意识较弱的肌电信号进行放大以增加运动范围[12]。这种反馈机制有助于患者建立正确的肌肉活动模式,促进肌肉功能的恢复。 当前,肌电生物反馈技术在脊髓损伤运动功能恢复领域已经取得了显著进展,其在临床中的应用越来越广泛(表1)[13-18]。PETROFSKY[13]早在2001年便指出对于脊髓损伤等神经系统疾病患者,肌电生物反馈可以作为一种重要的康复手段。近年来,肌电生物反馈逐渐应用于脊髓损伤患者的功能康复中,例如:JENSEN等[14]的一项研究表明,肌电生物反馈可以明显改善脊髓损伤后患者的慢性疼痛,并且该作用可持续3个月;蒋玮等[15]提出,肌电生物反馈能显著改善脊髓损伤患者神经源性膀胱的功能,并且可明显提升患者的生活质量;DE BIASE 等[16]的研究发现,肌电反馈有助于改善脊髓损伤患者的股直肌自主反应,经过治疗后患者股直肌肌电信号有明显提高;杨升等[17]指出,肌电生物反馈电刺激能有效提高脊髓损伤患者下肢功能及步行能力,改善神经与肠道功能,缓解下肢痉挛程度,恢复平衡能力;GOVIL等[18]将肌电反馈疗法应用于不完全性脊髓损伤患者臀大肌的功能恢复,结果表明臀大肌肌电生物反馈结合传统康复治疗可改善患者步态参数(步长、步行速度等),显著改善患者的步行能力和下肢平衡功能。"
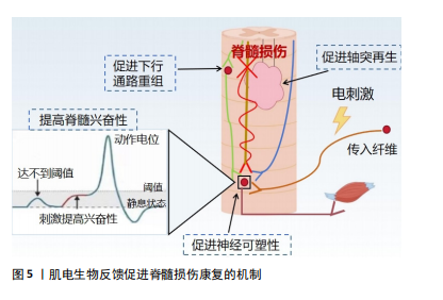
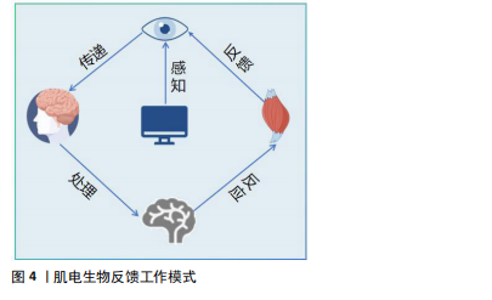
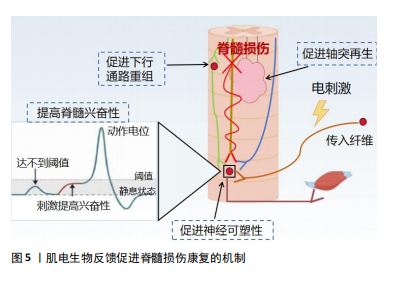
近年来,肌电生物反馈技术迅猛发展,其在临床中的应用越来越广泛,在各种疾病的治疗中也取得了良好的效果[19],如肌肉骨骼系统疾病[20]、神经系统疾病导致的吞咽功能障碍[21] 、呼吸系统疾病等。现有的国内外研究结果存在争议:一些研究表明,肌电生物反馈在肌肉激活、减轻疼痛和步态改善方面有好处;也有研究发现肌电生物反馈与传统锻炼计划相比没有显著优势,因此仍需要进一步的研究来更好地了解肌电图生物反馈在脊髓损伤康复和其他疾病中的机制和疗效。 2.2.2 肌电生物反馈在脊髓损伤中的历史研究发展 20世纪60年代,肌电生物反馈技术的概念首次被提出,BASMAJIAN[22]被认为是肌电生物反馈领域的先驱之一,其研究证明了通过视觉和听觉反馈来控制肌肉电活动的可行性,自此开始探索通过监测和反馈肌肉电活动来帮助患者恢复运动控制。1977年,SEYMOUR等[23]通过肌电生物反馈提高了患者的肌肉力量和行走功能,这一时期的研究主要集中在单独利用生物反馈改善肌肉控制和功能恢复。1990年,STEIN等[24]发现生物反馈可以显著增加不完全性脊髓损伤患者的肌肉表面肌电图数量,并首次探讨了生物反馈增加肌电图信号的神经机制,通过肌电生物反馈详细地检查单个运动神经元的电学和收缩性质。21世纪,随着科技的发展,肌电生物反馈逐渐与人工智能和机器学习相结合,提供更加个性化和精确的康复方案。2007年,LüNENBURGER等[25]将肌电生物反馈应用于机器人步态康复中,旨在提高脊髓损伤患者的步行功能、增加患者的训练时间和强度。2014年,石秀秀等[26]研究表明,通过肌电生物反馈疗法配合踝足支具能明显提高患者的胫前肌自主肌电信号及肌力。肌电生物反馈技术逐渐成为综合康复的一部分,与物理治疗、工学设备等结合使用,提高了脊髓损伤患者的康复效果。2021年,SEO等[27]联合肌电生物反馈与虚拟现实技术,开发了一种基于虚拟现实技术的步态训练康复设备,通过同步行走速度和虚拟现实视觉效果为患者提供身临其境的感觉,从而提高患者的参与度和积极性。 2.3 肌电生物反馈促进脊髓损伤康复的机制 肌电生物反馈通过放大患者在正常情况下没有意识到的肌肉组织的生物电活动,将信号作为直观的视觉和听觉信号反馈给人体,并反馈到中枢,引导患者主动运动。肌电生物反馈疗法可以在被动训练(电刺激)的基础上施加主动训练(反馈),可以很好地调动患者的内在潜能,因此可以通过特定任务的完成促进脊髓中的轴突再生和神经回路重组[28]。中枢神经系统根据反馈信号调节肌肉收缩和舒张强度,并接受主动康复训练,以达到治疗的目标。现国内外关于肌电生物反馈促进脊髓损伤运动功能恢复机制的报道较少,该文将其分为促进神经可塑性变化、增强神经肌肉连接和改善运动模式2部分,分析探讨肌电生物反馈的可能神经生理机制。 促进神经可塑性变化:脊髓损伤后神经修复依赖于神经可塑性,包括损伤直接诱导的自发可塑性和依赖于训练任务的可塑性。肌电生物反馈技术通过促进这一过程帮助恢复受损的运动功能,它通过激励残存的神经通路促使未受损的神经元发挥作用,从而在一定程度上替代受损神经元的功能,增强神经元之间的联系,提高肌肉的控制力和运动能力。肌电生物反馈疗法结合了电刺激与生物反馈两者的优势。电刺激对神经元具有广泛影响,包括在损伤部位引入电场以促进轴突生长和可塑性,增强脊髓回路功能、保留运动通路的强度和功效[29-30],特别是在联合治疗中,电刺激可能更能促进轴突再生。现有研究表明,经皮电刺激通过脊髓背根的感觉通路激活脊髓,为损伤远端脊髓内的中间神经元和运动神经元提供阈下兴奋。接近阈值的运动神经元更容易被来自大脑的完整但休眠的残余下行通路激活,恢复对运动的意识控制。在刺激过程中重新获得的活动能力使患者能够积极参与康复训练,从而诱导脊髓网络的重组,加强突触连接,并通过神经可塑性导致功能的长期恢复[31-32]。因此,肌电生物反馈的可能作用机制是:促进局部神经可塑性,加强运动神经元从传入或下行输入激活或促进下行通路的重组,电刺激可以提高脊髓的兴奋性,使亚损伤电路对微弱的残留脊髓上输入作出反应,进而恢复功能[33-34](图5)。因此,长期使用肌电生物反馈训练可以促进大脑和脊髓中的可塑性变化,增强损伤后残存神经网络的功能[34];此外,肌电生物反馈疗法还需指导患者主动运动,在训练过程中患者的运动能力得到提升,使肌电生物反馈得以逐渐被身体自然反应所代替。一方面,中枢性突触由于电刺激的作用被激活,建立了新的感觉兴奋;另一方面,主动运动通过患者的积极配合减少反馈使用,逐渐实现运动、步行功能的恢复[12]。"
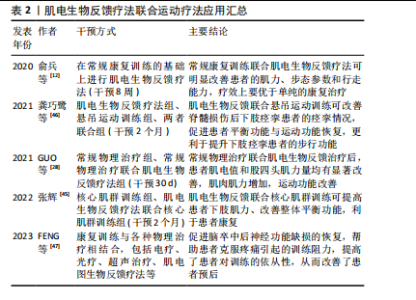
增强神经肌肉连接和改善运动模式:通过提供实时的肌电活动反馈,患者可以学习如何更有效地激活和控制残存的肌肉群,这种练习增强神经肌肉之间的连接,改善运动控制。电刺激直接刺激患者肌肉,能够激活肌肉活性、提高肌肉张力;同时电刺激引起的肌肉运动会向中枢系统提供传入冲动[35],使大脑高级中枢渐渐恢复对瘫痪肌肉的控制,缓解肌肉痉挛、肌肉瘫痪等[36]。电刺激还可提高神经肌肉兴奋度,改善血液循环[37]。因此,肌电生物反馈可以减少肌肉痉挛和不自主运动,通过肌肉活动模式训练来提高运动的平滑度和准确性,放松肌肉,改善患者舒适度和活动范围。此外,肌电生物反馈通过提供肌电活动的视觉或听觉反馈,增强了患者对其肌肉控制的意识,有助于提高参与训练的积极性和康复效果。 2.4 肌电生物反馈疗法在脊髓损伤后运动功能恢复中的应用 2.4.1 肌电生物反馈疗法联合运动疗法治疗不完全性脊髓损伤 运动疗法已成为康复治疗的核心治疗手段,具有效果好、风险小、不易出现并发症的优点,能改善身体功能,增强患者的活动能力和社会参与能力[38]。运动疗法已被证明在脊髓损伤后可改变结构的可塑性和改善微环境[39]。现有证据表明,长期运动可改变循环生长因子、胰岛素样生长因子和血管内皮生长因子的水平,促进神经胶质、突触以及血管生成,这些都是神经可塑性的重要过程[40]。运动通过增加脑血流量促进血管生成[41-42],支持神经元生长和突触形成,改善认知和运动功能。运动疗法已广泛应用于脊髓损伤患者的康复中,例如:NANDAKUMAR 等[43]研究表明,运动疗法指导胸中段脊髓损伤后的皮质重组,以增强对下胸肌的控制,改善平衡相关的肢体协调,增强姿势控制,改善运动功能;WINCEK等[44]提出运动疗法不仅改善运动功能,而且有助于感觉功能改善。 运动疗法可增加肌肉群的收缩效能,促进神经功能的修复,但这种方法存在潜在的受伤风险。肌电生物反馈疗法通过监测患者的生理指标可以帮助识别潜在的运动过程中的风险因素,并及时调整治疗方案,以减少受伤的可能性。GUO等[28]探讨了肌电图生物反馈训练对改善不完全性脊髓损伤患者股四头肌运动功能的影响,对照组接受定期运动疗法,包括股四头肌力量训练、坐姿踩踏、站立训练等,而干预组在此基础上增加肌电生物反馈训练,持续治疗30 d后发现,干预组患者表面肌电图值和股四头肌力量均有显著改善,并且股四头肌肌电图值增高早于股四头肌肌力,说明肌电图肌电生物反馈疗法联合运动能够增加肌肉肌力,改善运动功能。张辉[45]将核心肌群训练与肌电生物反馈疗法联合,探究其在不完全性脊髓损伤患者中的效果,结果显示肌电生物反馈联合核心肌训练使股四头肌和腘绳肌力量评分升高,提高了脊髓损伤患者下肢肌力,改善平衡功能。这种联合治疗脊髓损伤的方式不仅优于单独的运动疗法,并且能够增强患者主动性,更直观地观察康复疗效。龚巧鹭等[46]将悬吊运动训练结合肌电生物反馈技术治疗脊髓损伤后下肢痉挛患者,发现患者功能性步行能力评分、改良Ashworth 痉挛评分、Berg平衡评分及Fugl-Meyer运动功能评分均优于干预前,提示肌电生物反馈及悬吊运动训练对脊髓损伤后下肢痉挛患者痉挛情况、平衡功能、运动功能均具有改善作用。俞兵等[12]指出,与传统的步态训练相比,肌电生物反馈疗法的加入对不完全性脊髓损伤患者下肢运动功能的恢复具有显著的促进作用,可有效改善患者下肢主要肌群自主肌电指标、肌力及步态参数,对患者行走能力的预后有很大帮助。还有研究指出,肌电图生物反馈疗法结合康复训练是改善脑卒中后肩手综合征患者上肢运动功能和疼痛的有效物理治疗选择[47],同时还激发了患者的训练欲望,将被动康复转变为主动康复,提高了患者对训练的依从性,从而改善了患者预后。 肌电生物反馈疗法联合运动疗法应用汇总,见表2。 综上所述,结合使用肌电生物反馈和运动疗法可以更有效地提高患者的肌肉力量、运动协调性和运动功能,"
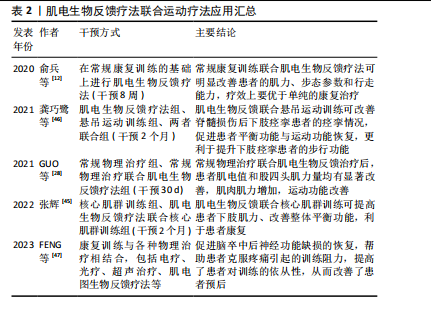
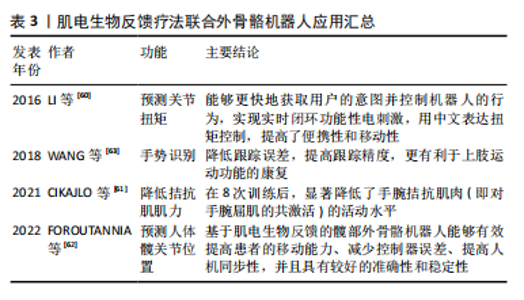
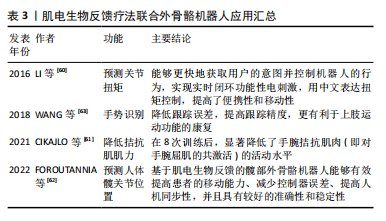
相比单独使用任一方法更为有效。由于运动可以促进脊髓损伤的神经可塑性[39],肌电生物反馈也可以促进这一过程,两者联合双管齐下,帮助恢复受损的运动功能;同时,两者结合能够加强神经与肌肉的联系,由被动变为主动,激发患者康复的信心,最大限度调动积极性,最终使患者恢复运动功能。但是目前国内外两者结合的研究具有局限性:样本量较小,缺乏为其疗效提供有力证据的大规模随机对照试验;许多研究集中于短期效果,对长期康复效果的研究相对较少,不足以全面评估其长期效益;目前尚没有标准化的评估标准评价两者结合后的效果;现有的研究可能还忽略了损伤严重程度和恢复潜力的个体差异,由于患者对肌电生物反馈训练的反应存在个体差异,一些患者可能无法有效地使用肌电信号来改善肌肉控制;不同患者的损伤程度和康复需求各不相同,现有的肌电生物反馈系统可能无法充分适应每个患者的具体需求,虽然一些设备提供了一定程度的个性化设置,但在应对广泛的个体差异方面仍显不足。未来的研究应开展更多大规模、随机对照试验,积累广泛的临床证据,验证肌电生物反馈技术的有效性和安全性,同时提高个性化和智能化,能够根据患者的具体情况进行个性化调整,提供定制化的康复方案。 2.4.2 肌电生物反馈疗法联合外骨骼机器人治疗不完全性脊髓损伤 外骨骼机器人集传感、力学、仿生学、控制理论、通信技术和信息处理技术等于一体,是专为个人佩戴而设计的机器人系统,通过患者控制的动作完成特定任务[48-49],不仅提供了精确控制的物理互动,帮助患者进行重复性的运动训练,并且使患者能够在日常活动中进行运动,减轻因关节僵硬和肌肉挛缩而导致的功能障碍[50]。外骨骼系统有助于改善患者预后、恢复患者的活动能力、改善其生活质量[51-52]。近年来,下肢行走外骨骼被广泛应用于脊髓损伤的研究中[53],现有研究表明,下肢外骨骼机器人辅助步态训练在步行距离、腿部力量、活动能力和独立性方面有显著改善效果,可以增强不完全脊髓损伤患者的心肺功能、提高平衡能力、减轻疼痛、改善肠道和膀胱功能以及提供积极的情绪等[54-55]。此外,Lokomat外骨骼也显示出广泛的应用,包括对不同水平脊髓损伤患者的下肢运动功能产生积极影响,具有降低不完全性脊髓损伤患者的痉挛、提高痉挛足踝部的意志控制以及减少神经肌肉特性异常调节的作用[56-57]。 肌电图信号被广泛应用于估算肌肉力矩[58],使用肌电图信号来控制机器人的协助可以显著减少患者的运动阻力,保持运动的准确性和方向[59]。LI等[60]提出一种基于肌电信号实时处理的无线便携式刺激器,用于预测关节扭矩,该方法利用了生物电信号的快速响应特性。与传统的扭矩估计算法相比,这种方式具有实时性高、灵敏性强、可扩展性高等优点。CIKAJLO等[61]探讨了在机器人手腕运动训练中使用肌电生物反馈对降低拮抗肌肉协同激活的效果,通过可视化反馈系统,参与者可以更直观地了解自己的肌肉活动情况,更容易掌握正确的运动技巧。 FOROUTANNIA等[62]提出了一种使用肌电信号的深度学习策略来预测人体髋关节位置,并指出与机械位置信号相比,肌电信号表现出更短的时间延迟和更高的信噪比,肌肉激活发生在运动前20-100 ms,可以更好地估计关节位置,实现人机交互体验。这种方式能够辅助运动障碍患者进行步行训练,适用于脊髓损伤或脑卒中患者。在一项手部外骨骼机器人结合肌电生物反馈在神经康复的应用研究中,将肌电图生物反馈集成到手势识别中的主动辅助控制算法,通过算法捕获和识别手势收集来自前臂的肌电信号,进行分析后将位置和扭矩融合到控制回路中,实现了降低跟踪误差、提高跟踪精度,更有利于上肢运动功能的康复[63]。这些研究共同表明,外骨骼机器人与肌电图生物反馈的结合为增强脊髓损伤患者的运动功能恢复提供了一条新的途径。虽然证据支持这些干预措施的有效性,但还需要进一步研究以探索这些治疗的最佳组合、参数和时机,以最大限度地提高患者预后。 肌电生物反馈疗法联合外骨骼机器人应用汇总,见表3。"
| [1] ACKERY A, TATOR C, KRASSIOUKOV A. A global perspective on spinal cord injury epidemiology. J Neurotrauma. 2004;21(10):1355-1370. [2] ARORA M, CRAIG AR. Spinal Cord Injuries: Advances in Rehabilitation. J Clin Med. 2024;13(6):1782. [3] THOMAS A, TOWNSEND O, EVANS N. Spinal cord injury–basic principles and management. Surgery (Oxford). 2024;46(6):443-450. [4] DE ALMEIDA FM, MARQUES SA, DOS SANTOS ACR, et al. Molecular approaches for spinal cord injury treatment. Neural Regen Res. 2023; 18(1):23-30. [5] COVELL MM, NAIK A, SHAFFER A, et al. Social determinants of health impact spinal cord injury outcomes in low-and middle-income countries: a meta-epidemiological study. Neurosurgery. 2024;94(5):893-902. [6] KETCHUM JM, CUTHBERT JP, DEUTSCH A, et al. Representativeness of the spinal cord injury model systems national database. Spinal Cord. 2018.56(2):126-132. [7] BAROUDI M, REZK A, DAHER M, et al. Management of traumatic spinal cord injury: A current concepts review of contemporary and future treatment. Injury. 2024;55(6):111472. [8] SIMIĆ M, STOJANOVIĆ GM. Wearable Device for Personalized EMG Feedback-based Treatments. Results Eng. 2024;23:102472. [9] CINNERA AM, MORONE G, BISIRRI A, et al. Headaches treatment with EMG biofeedback: a focused systematic review and meta-analysis. Eur J Phys Rehabil Med. 2023;59(6):697. [10] 杨菊贤,杜勤.生物反馈治疗在心身疾病的应用[J].中国行为医学科学,2004,13(1):122-124. [11] 马利娜,方力争.肌电生物反馈技术在脑卒中领域的应用进展[J].全科医学临床与教育,2018,16(4):417-420. [12] 俞兵,周涛,吴健,等.肌电生物反馈联合康复训练对不完全性脊髓损伤下肢肌力及步态的影响[J].临床骨科杂志,2020,23(5):618-621. [13] PETROFSKY J. The use of electromyogram biofeedback to reduce Trendelenburg gait. Eur J Appl Physiol. 2001;85:491-495. [14] JENSEN MP, BARBER J, ROMANO JM, et al. Effects of self-hypnosis training and EMG biofeedback relaxation training on chronic pain in persons with spinal-cord injury. Int J Clin Exp Hypn. 2009;57(3):239-268. [15] 蒋玮,张茂舒,谭波涛,等.盆底肌生物反馈电刺激对脊髓损伤后神经源性膀胱功能恢复的临床研究[J].第三军医大学学报,2014, 36(16):1725-1728. [16] DE BIASE MEM, POLITTI F, PALOMARI ET, et al. Increased EMG response following electromyographic biofeedback treatment of rectus femoris muscle after spinal cord injury. Physiotherapy. 2011;97(2):175-179. [17] 杨升,吴博中.肌电生物反馈电刺激改善脊髓损伤患者下肢功能及步行能力的研究[J].现代医学与健康研究电子杂志,2023,7(24):48-51. [18] GOVIL K, NOOHU MM. Effect of EMG biofeedback training of gluteus maximus muscle on gait parameters in incomplete spinal cord injury. NeuroRehabilitation. 2013;33(1):147-152. [19] 吴豫昌,李天禹,张明秀.生物反馈治疗技术在临床应用中的研究进展[J].齐齐哈尔医学院学报,2022,43(17):1668-1673. [20] KARABORKLU ARGUT S, CELIK D, YASACı Z. Effectiveness of therapeutic electromyographic biofeedback after orthopedic knee surgeries: a systematic review. Disabil Rehabil. 2022;44(14):3364-3372. [21] ALBUQUERQUE LCA, PERNAMBUCO L, DA SILVA CM, et al. Effects of electromyographic biofeedback as an adjunctive therapy in the treatment of swallowing disorders: a systematic review of the literature. Eur Arch Otorhinolaryngol. 2019;276:927-938. [22] BASMAJIAN JV. Control and training of individual motor units. Science. 1963;141(3579):440-441. [23] SEYMOUR RJ, BASSLER CR. Electromyographic biofeedback in the treatment of incomplete paraplegia. Phys Ther. 1977;57(10):1148-1150. [24] STEIN RB, BRUCKER BS, AYYAR DR. Motor units in incomplete spinal cord injury: electrical activity, contractile properties and the effects of biofeedback. J Neurol Neurosurg Psychiatry. 1990;53(10):880-885. [25] LÜNENBURGER L, COLOMBO G, RIENER R. Biofeedback for robotic gait rehabilitation. J Neuroeng Rehabil. 2007;4:1. [26] 石秀秀,唐金树,秦江,等.肌电生物反馈疗法配合踝足支具治疗脊髓损伤患者术后踝背屈功能障碍的疗效[J].中国骨与关节杂志, 2014,3(9):661-664. [27] SEO JW, KIM DH, JUNG J, et al. Development of Immersive VR Device for Gait Training Rehabilitation with Biofeedback System-Preliminary Study. Appl Sci. 2021;11(21):10394.
[28] GUO Y, GAO F, LI J, et al. Effect of electromyographic biofeedback training on motor function of quadriceps femoris in patients with incomplete spinal cord injury: A randomized controlled trial. NeuroRehabilitation. 2021;48(3):345-351. [29] JARA JS, AGGER S, HOLLIS ER. Functional electrical stimulation and the modulation of the axon regeneration program. Front Cell Dev Biol. 2020;8:736. [30] ZHANG H, LIU Y, ZHOU K, et al. Restoring sensorimotor function through neuromodulation after spinal cord injury: progress and remaining challenges. Front Neurosci. 2021;15:749465. [31] INANICI F, BRIGHTON LN, SAMEJIMA S, et al. Transcutaneous spinal cord stimulation restores hand and arm function after spinal cord injury. IEEE Trans Neural Syst Rehabil Eng. 2021;29:310-319. [32] MORITZ C, FIELD-FOTE EC, TEFERTILLER C, et al. Non-invasive spinal cord electrical stimulation for arm and hand function in chronic tetraplegia: a safety and efficacy trial. Nat Med. 2024;30(5):1276-1283. [33] JACK AS, HURD C, MARTIN J, et al. Electrical stimulation as a tool to promote plasticity of the injured spinal cord. J Neurotrauma. 2020; 37(18):1933-1953. [34] DORRIAN RM, BERRYMAN CF, LAUTO A, et al. Electrical stimulation for the treatment of spinal cord injuries: A review of the cellular and molecular mechanisms that drive functional improvements. Front Cell Neurosci. 2023;17:1095259. [35] ARMAGAN O, TASCIOGLU F. Electromyographic biofeedback in the treatment of the hemiplegic hand: a placebo-controlled study. Am J Phys Med Rehabil. 2003;82(11):856-861. [36] 吴豫昌,李天禹,张明秀.生物反馈治疗技术在临床应用中的研究进展[J].齐齐哈尔医学院学报,2022,43(17):1668-1673. [37] 张梦月,冯加彬,张蕊,等.肌电生物反馈结合肌内效贴治疗脑卒中偏瘫患者肩关节半脱位的研究进展[J].按摩与康复医学,2019, 10(2):48-49. [38] PHUPHANICH ME, DROESSLER J, ALTMAN L, et al. Movement-based therapies in rehabilitation. Phys Med Rehabil Clin N Am. 2020;31(4): 577-591. [39] BILCHAK JN, CARON G, COTE MP. Exercise-Induced Plasticity in Signaling Pathways Involved in Motor Recovery after Spinal Cord Injury. Int J Mol Sci. 2021;22(9):48-58. [40] LIU K, TRONSTAD O, FLAWS D, et al. From bedside to recovery: exercise therapy for prevention of post-intensive care syndrome. J Intensive Care. 2024;12(1):11. [41] KISSANE RWP, WRIGHT O, AL’JOBOORI YD, et al. Effects of treadmill training on microvascular remodeling in the rat after spinal cord injury. Muscle Nerve. 2019;59(3):370-379. [42] VIVODTZEV I, TAYLOR JA. Cardiac, autonomic, and cardiometabolic impact of exercise training in spinal cord injury: a qualitative review. J Cardiopulm Rehabil Prev. 2021;41:6-12. [43] NANDAKUMAR B, BLUMENTHAL GH, DISSE GD, et al. Exercise therapy guides cortical reorganization after midthoracic spinal contusion to enhance control of lower thoracic muscles, supporting functional recovery. Exp Neurol. 2023;364:114394. [44] WINCEK A, HUBER J, LESZCZYNSKA K, et al. Results of a long-term uniform system of neurorehabilitation in patients with incomplete thoracic spinal cord injury. Ann Agric Environ Med. 2022;29(1):94-102. [45] 张辉.肌电生物反馈疗法联合核心肌群训练在不完全性脊髓损伤患者康复中的应用[J].黑龙江医学,2022,46(4):471-473. [46] 龚巧鹭,张建梅.肌电生物反馈及悬吊运动训练对脊髓损伤后下肢痉挛患者步行功能的影响[J].中国医药导报,2021,18(2):110-113. [47] FENG S, TANG M, HUANG G, et al. EMG biofeedback combined with rehabilitation training may be the best physical therapy for improving upper limb motor function and relieving pain in patients with the post-stroke shoulder-hand syndrome: a Bayesian network meta-analysis. Front Neurol. 2023;13:1056156. [48] CHU X, LIU S, ZHAO X, et al. Case report: Virtual reality-based arm and leg cycling combined with transcutaneous electrical spinal cord stimulation for early treatment of a cervical spinal cord injured patient. Front Neurosci. 2024;18:1380467. [49] HE Y, XU Y, HAI M, et al. Exoskeleton-assisted rehabilitation and neuroplasticity in spinal cord injury. World Neurosurg. 2024;185:45-54. [50] RODRIGUEZ TAPIA G, DOUMAS I, LEJEUNE T, et al. Wearable powered exoskeletons for gait training in tetraplegia: a systematic review on feasibility, safety and potential health benefits. Acta Neurol Belg. 2022; 122(5):1149-1162 [51] LOUIE DR, ENG JJ. Powered robotic exoskeletons in post-stroke rehabilitation of gait: a scoping review. J Neuroeng Rehabil. 2016;13:1-10. [52] CONTRERAS-VIDAL JL, BHAGAT NA, BRANTLEY J, et al. Powered exoskeletons for bipedal locomotion after spinal cord injury. J Neural Eng. 2016;13(3):031001. [53] HU X, LU J, WANG Y, et al. Effects of a lower limb walking exoskeleton on quality of life and activities of daily living in patients with complete spinal cord injury: A randomized controlled trial. Technol Health Care. 2023;32(1):243-253. [54] 张立新,白定群,白玉龙,等.下肢康复机器人临床应用专家共识[J].康复学报,2023,33(5):383-396. [55] ALMUTAIRI S, SWANK C, WANG-PRICE S, et al. Walking with and without a robotic exoskeleton in people with incomplete spinal cord injury compared to a typical gait pattern. NeuroRehabilitation. 2021;49(4):585-596. [56] ALCOBENDAS-MAESTRO M, ESCLARÍN-RUZ A, CASADO-LÓPEZ RM, et al. Lokomat robotic-assisted versus overground training within 3 to 6 months of incomplete spinal cord lesion: randomized controlled trial. Neurorehabil Neural Repair. 2012;26(9):1058-1063. [57] ALASHRAM AR, ANNINO G, PADUA E. Robot-assisted gait training in individuals with spinal cord injury: A systematic review for the clinical effectiveness of Lokomat. J Clin Neurosci. 2021;91:260-269. [58] KAO PC, LEWIS CL, FERRIS DP. Invariant ankle moment patterns when walking with and without a robotic ankle exoskeleton. J Biomech. 2010;43(2):203-209. [59] LENZI T, DE ROSSI SMM, VITIELLO N, et al. Intention-based EMG control for powered exoskeletons. IEEE Trans Biomed Eng. 2012;59(8):2180-2190. [60] LI Z, GUIRAUD D, ANDREU D, et al. Real-time estimation of FES-induced joint torque with evoked EMG: Application to spinal cord injured patients. J Neuroeng Rehabil. 2016;13(1):1-11. [61] CIKAJLO I, ZADRAVEC M, MATJACIC Z, et al. High-density electromyography biofeedback during robotic wrist exercises for reducing co-activation of antagonist muscles: a case report. Int J Rehabil Res. 2021;44(1):92-97. [62] FOROUTANNIA A, AKBARZADEH-T MR, AKBARZADEH A. A deep learning strategy for EMG-based joint position prediction in hip exoskeleton assistive robots. Biomed Signal Proces. 2022;75:103557. [63] WANG P, WANG Y, HUANG H, et al. The use of a cascaded Kinect and electromyography gesture decoding algorithm in an initial robot-aided hand neurorehabilitation. Adv Mech Eng. 2018;10(1): 1687814017751967. [64] LEEMHUIS E, ESPOSITO RM, DE GENNARO L, et al. Go Virtual to Get Real: Virtual Reality as a Resource for Spinal Cord Treatment. Int J Environ Res Public Health. 2021;18(4):1819. [65] DE MIGUEL-RUBIO A, MUÑOZ-PÉREZ L, ALBA-RUEDA A, et al. A Therapeutic Approach Using the Combined Application of Virtual Reality with Robotics for the Treatment of Patients with Spinal Cord Injury: A Systematic Review. Int J Environ Res Public Health. 2022; 19(14):8772. [66] AZURDIA D, ACUÑA SM, NARASAKI-JARA M, et al. Effects of virtual reality-based aerobic exercise on perceptions of pain and fatigue in individuals with spinal cord injury. Games Health J. 2022;11(4):236-241. [67] AN Y, PARK C. The effects of virtual soccer game on balance, gait function, and kick speed in chronic incomplete spinal cord injury: a randomized controlled trial. Spinal Cord. 2022;60(6):504-509. [68] TRAN Y, AUSTIN P, LO C, et al. An exploratory EEG analysis on the effects of virtual reality in people with neuropathic pain following spinal cord injury. Sensors. 2022;22(7):2629. [69] XIAO Y, BAI H, GAO Y, et al. Interactive Virtual Ankle Movement Controlled by Wrist sEMG Improves Motor Imagery: An Exploratory Study. IEEE Trans Vis Comput Graph. 2024;30(8):5507-5524. [70] LIN M, HUANG J, FU J, et al. A VR-based motor imagery training system with EMG-based real-time feedback for post-stroke rehabilitation. IEEE Trans Neural Syst Rehabil Eng. 2022;31:1-10. [71] 胡凤丹,蔡华安,胡德,等.表面肌电在临床康复中的应用进展[J].赣南医学院学报,2021,41(7):740-744. |
| [1] | Wang Juan, Wang Guanglan, Zuo Huiwu. Efficacy of exercise therapy in the treatment of anterior cruciate ligament reconstruction patients: #br# a network meta-analysis #br# [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1714-1726. |
| [2] | Zhao Ruihua, Chen Sixian, Guo Yang, Shi Lei, Wu Chengjie, Wu Mao, Yang Guanglu, Zhang Haoheng, Ma Yong. Wen-Shen-Tong-Du Decoction promoting spinal cord injury repair in mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1118-1126. |
| [3] | Wang Rongrong, Huang Yushan, Li Xiangmiao, Bai Jinzhu. Prostaglandin E1 regulates vascular-related factors and protects microcirculatory function during the acute phase of traumatic spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 958-967. |
| [4] | Guo Jia, Ren Yafeng, Li Bing, Huang Jing, Shang Wenya, Yang Yike, Liu Huiyao. Action mechanism of mesenchymal stem cell-derived exosomes carrying miRNAs in improving spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7827-7838. |
| [5] | Zhu Chuanxi, Qiu Long, Li Lingxu, Ji Guangcheng. Chinese herbal prescription combined with head acupuncture exercise therapy improves limb spasticity in rats with ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7571-7577. |
| [6] | Xu Biao, Dong Yuzhen, Lu Tan. Effect of dihydroquercetin on the expression of inflammatory response markers in rats with spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6843-6850. |
| [7] | Wang Ziheng, Wu Shuang. Oxidative stress-related genes and molecular mechanisms after spinal cord injury: data analysis and verification based on GEO database [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6893-6904. |
| [8] | Wang Xuesong, Wang Yue, Xu Yan, Zeng Wenhui, Lu Wenming, Tang Xingkun, Chen Wenjie, Ye Junsong. Brain-computer interface combined with different therapies for limb dysfunction in stroke patients: effectiveness and mechanism analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6538-6546. |
| [9] | Guan Zhenjie, Li Wenyuan, Geng Rui, Wang Ying. Regulatory mechanisms of the corticospinal tract after spinal cord injury: combined therapeutic strategies targeting transcription factors and signaling pathways [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(24): 5158-5170. |
| [10] | Hu Tong, Li Xuan, Yuan Jing, Wang Wei. Different electromagnetic stimulation programs improve post-stroke dysphagia: a network Meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(24): 5224-5236. |
| [11] | Wu Yue, Ren Shuang, Huang Hongshi, Dai Ruilan, Ao Yingfang, Gou Bo. Gluteal muscle activation exercise therapy improves lower limb muscle strength in young male patients with anterior knee pain [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(18): 3798-3803. |
| [12] | Yang Yike, Ren Yafeng, Li Bing, Shang Wenya, Huang Jing, Guo Jia, Liu Huiyao . Regulatory mechanisms and therapeutic strategies for cellular autophagy after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(18): 3885-3896. |
| [13] | Zhang Xiaowei, Yan Binghan, Qiu Daodi, Xue Haipeng, Tan Guoqing, Xu Zhanwang . Natural products regulate oxidative stress in the treatment of spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(12): 2560-2568. |
| [14] | Song Haiwang, Jiang Guanhua, Mu Yingying, Fu Shanyu, Sun Baofei, Li Yumei, Yu Zijiang, Yang Dan. Repetitive trans-spinal magnetic stimulation promotes motor function recovery in mice after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(11): 2252-2260. |
| [15] | Zhang Jiale, Wang Fusen, Qiu Zhenrui, Fan Xinming, Zou Jilong, Bi Zhenggang, Sun Jiabing. Exercise therapy for the treatment of chronic nonspecific lower back pain through mechanical-chemical coupling [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(11): 2377-2384. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||