Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (23): 4841-4850.doi: 10.12307/2025.091
Fe3O4@ZIF-8 nanoparticles affect osteogenic differentiation of bone marrow mesenchymal stem cells under magnetic stimulation
Chen Pinrui1, 2, Xue Yiyuan1, 3, Pei Xibo1, 2
- 1Stomatology and Frontier Medical Innovation Center, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China; 2Department of Prosthodontics, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China; 3Department of Prosthodontics, Jinjiang Outpatient Department, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China
-
Received:2023-12-02Accepted:2024-05-20Online:2025-08-18Published:2024-09-24 -
Contact:Pei Xibo, Associate professor, Master’s supervisor, Stomatology and Frontier Medical Innovation Center, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China; Department of Prosthodontics, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China -
About author:Chen Pinrui, Master candidate, Stomatology and Frontier Medical Innovation Center, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China; Department of Prosthodontics, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China -
Supported by:Sichuan Natural Science Foundation of China, No. 2022NSFSC1364 (to XYY); National Natural Science Foundation of China-Youth Fund Project, No. 82301150 (to XYY); National Natural Science Foundation of China, No. 82271016 (to PXB); Central Guidance Local - Free Exploration Project, No. 2023ZYD0109 (to PXB)
CLC Number:
Cite this article
Chen Pinrui, Xue Yiyuan, Pei Xibo. Fe3O4@ZIF-8 nanoparticles affect osteogenic differentiation of bone marrow mesenchymal stem cells under magnetic stimulation[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(23): 4841-4850.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
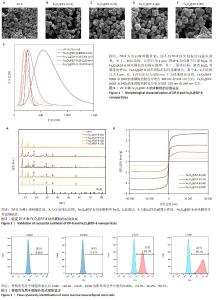
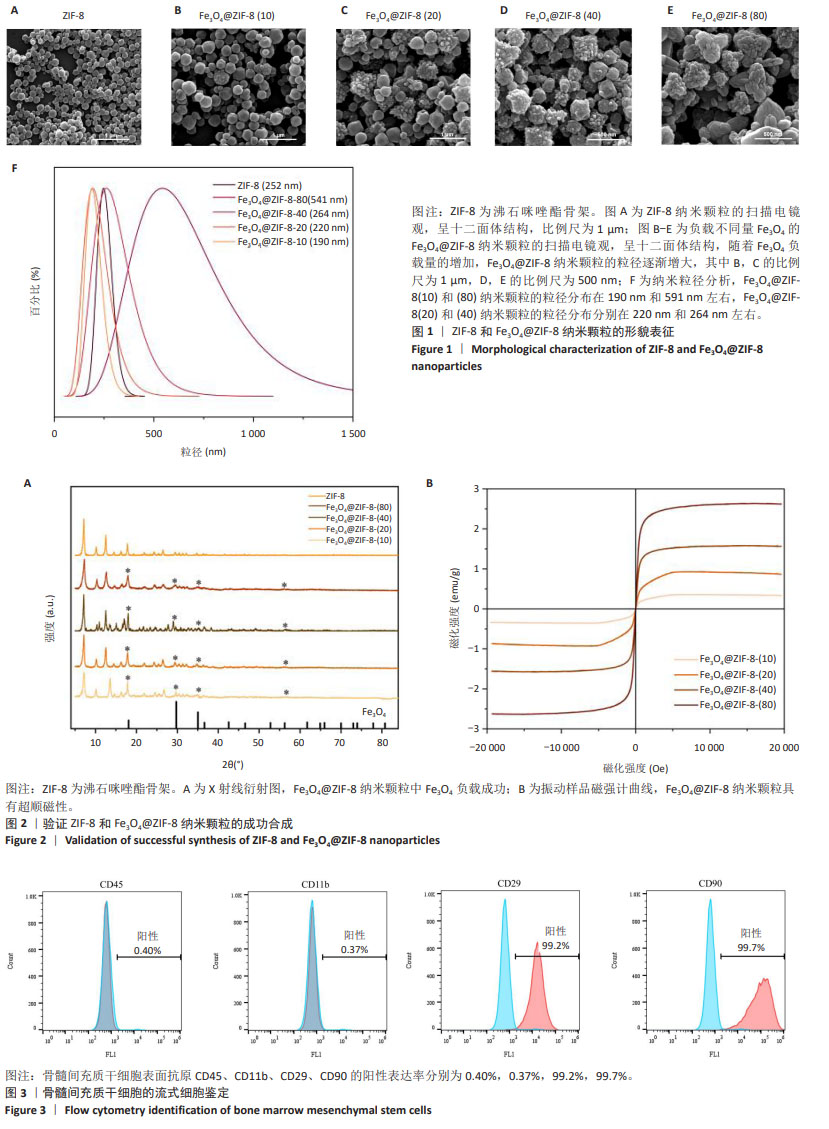
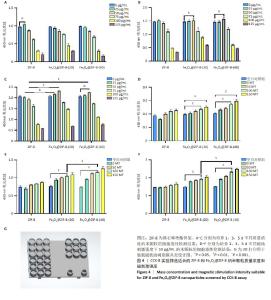
2.1 ZIF-8和Fe3O4@ZIF-8纳米颗粒的形貌及粒径分析结果 实验采用水热法合成ZIF-8纳米颗粒,扫描电镜观察其形态,ZIF-8纳米颗粒呈220 nm的十二面体结构,见图1A所示。采用一锅法合成不同比例的Fe3O4@ ZIF-8纳米颗粒,随着Fe3O4纳米颗粒负载量的增加,Fe3O4@ZIF-8纳米颗粒的粒径不断变大,见图1B-E所示,这可能与Fe3O4纳米颗粒本身容易团聚的特性相关。根据纳米粒径分析测试结果,Fe3O4@ZIF-8(10)和(80)纳米颗粒的粒径分布在190 nm和591 nm左右,而Fe3O4@ZIF-8(20)和(40)纳米颗粒的粒径分布分别在220 nm和264 nm左右,见图1F所示,研究发现纳米颗粒的功能性及生物安全性在250 nm处较为稳定[11],因此初步筛选出Fe3O4@ZIF-8(20)和(40)纳米颗粒作为后续实验的对象。 2.2 Fe3O4@ZIF-8纳米颗粒的理化特性表征结果 X射线衍射分析结果显示,ZIF-8纳米颗粒符合(011)(002)(112)(022)(013)和(222)的ZIF-8晶胞结构,说明样品为纯ZIF-8相,结晶度较高;Fe3O4@ZIF-8与Fe3O4晶体(PDF#19-0629)的晶胞结构匹配,18.1°,29.7°,35.5°,42.5°,52.7°,56.2°和61.7°的衍射峰分别对应于磁铁矿的(111)(220)(311)(400)(422)(511)和(440)晶面,说明Fe3O4纳米颗粒负载成功,然而Fe3O4@ZIF-8纳米颗粒各组间无明显差异,这可能是负载量较少导致,见图2A。 使用振动样品磁强计检测Fe3O4@ZIF-8纳米颗粒的磁性,测量了磁化强度与外加磁场强度的关系曲线,见图2B所示,随着Fe3O4负载量的增加,Fe3O4@ZIF-8纳米颗粒饱和磁化强度更高;曲线穿过零点,说明没有任何的矫顽力或剩余磁化强度,即Fe3O4@ZIF-8纳米颗粒具有超顺磁性。上述检测验证了超顺磁性Fe3O4@ZIF-8纳米颗粒的成功合成。 2.3 骨髓间充质干细胞的流式细胞鉴定结果 流式细胞仪测定骨髓间充质干细胞表面抗原CD45、CD11b、CD29、CD90的阳性表达率分别为0.40%,0.37%,99.2%,99.7%,见图3,证明了骨髓间充质干细胞的纯度可满足实验要求。"
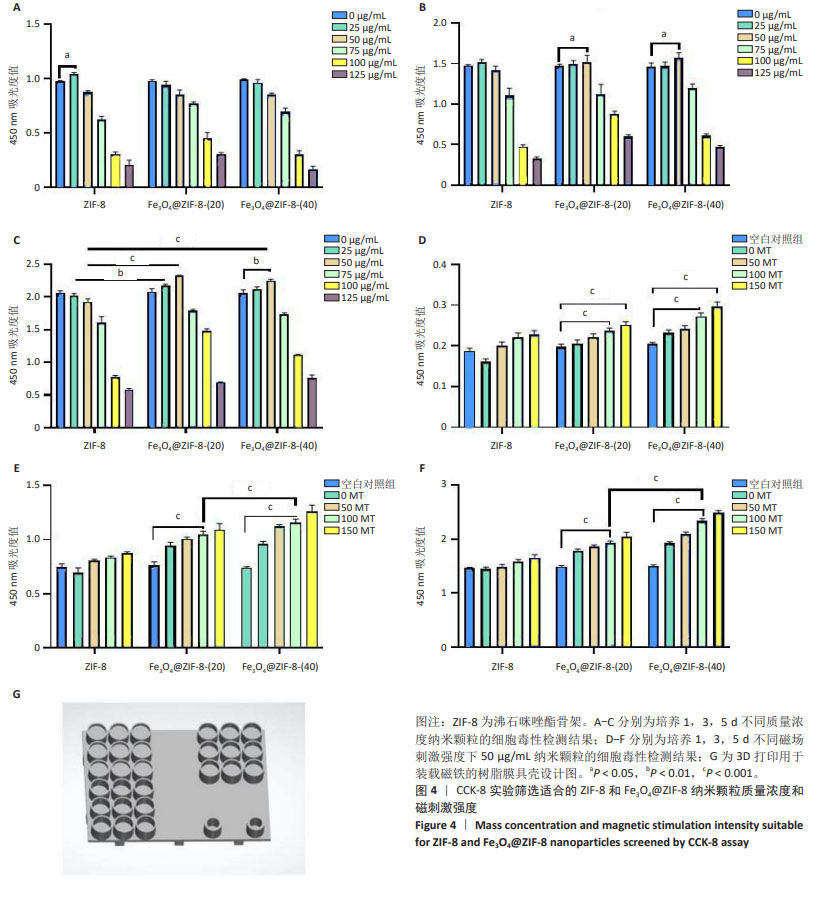
2.4 Fe3O4@ZIF-8纳米颗粒对骨髓间充质干细胞活性的影响 CCK-8检测结果显示,培养1,3,5 d,在25,50 μg/mL 质量浓度下,ZIF-8组、Fe3O4@ZIF-8(20)组、Fe3O4@ZIF-8(40)组骨髓间充质干细胞都有较好的细胞活性,并且培养3,5 d的50 μg/mL组细胞吸光度值高于同组内的其他质量浓度组,因此选择50 μg/mL质量浓度进行后续检测;值得注意的是,在第5天25,50 μg/mL Fe3O4@ZIF-8组的细胞吸光度值高于对应质量浓度的ZIF-8组,见图4A-C,说明Fe3O4@ZIF-8纳米颗粒的细胞相容性优于ZIF-8纳米颗粒,表明Fe3O4纳米颗粒对细胞增殖有促进作用,为后续进一步成骨分化检测提供了支撑。 CCK-8检测结果显示,相较于空白对照组,Fe3O4@ZIF-8(20)组、Fe3O4@ZIF-8(40)组暴露在50,100 MT磁场下的细胞吸光度值升高(P < 0.001),说明低强度静磁场对成骨细胞的增殖具有促进作用;并且暴露在相同磁场强度下,随着纳米颗粒中Fe3O4负载量的增加,细胞吸光度值升高,见图4D-F,说明更高的Fe3O4纳米颗粒负载量对外部磁场的敏感性更强。"
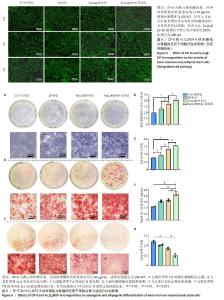
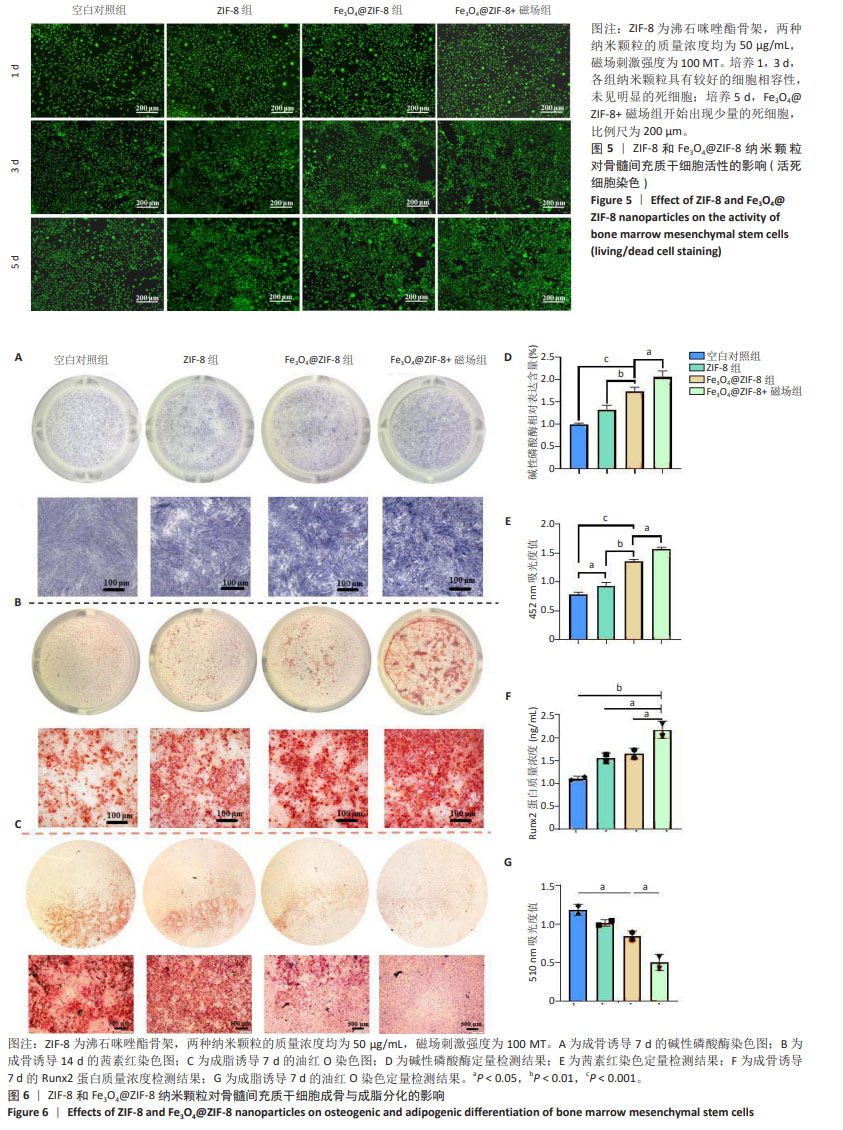
考虑到成骨诱导分化实验中细胞培养周期所需时间较长,而细胞长期暴露在磁场条件下可能存在潜在的负向作用,有研究发现200 MT以上的磁场对成骨有抑制作用[12],尽管150 MT磁场下也能促进骨髓间充质干细胞的增殖活性,但最终筛选出更加安全的100 MT磁刺激作用于50 μg/mL的Fe3O4@ZIF-8(40)纳米颗粒和50 μg/mL的ZIF-8纳米颗粒,作为最适的体外成骨实验条件。 见图5所示,培养1,3 d的活死细胞染色显示,各组纳米颗粒具有较好的细胞相容性,未见明显的死细胞;培养5 d,Fe3O4@ZIF-8+磁场组开始出现少量的死细胞,说明磁力刺激对细胞有潜在的毒性危害。 2.5 Fe3O4@ZIF-8纳米颗粒对骨髓间充质干细胞成脂与成骨分化的影响 见图6。 成骨诱导第7天的碱性磷酸酶染色结果见图6A所示,Fe3O4@ZIF-8+磁场组阳性染色最深,Fe3O4@ZIF-8组阳性染色深度强于ZIF-8组,说明Fe3O4@ZIF-8纳米颗粒对骨髓间充质干细胞的成骨分化具有更好的促进作用,特别是在100 MT磁场作用下进一步加速了骨髓间充质干细胞的成骨分化。成骨诱导第14天,利用茜素红染色评估成骨分化后期的细胞外基质矿化程度,见图6B所示,Fe3O4@ZIF-8+磁场组表现出最大的矿化程度,其次是Fe3O4@ZIF-8组。 骨髓间充质干细胞的碱性磷酸酶活性水平越高,成骨分化越明显,见图6D所示,Fe3O4@ZIF-8+磁场组与其他3组相比表现出最好的成骨细胞分化和功能状态。茜素红定量分析结果也显示Fe3O4@ZIF-8+磁场组表现出最高的细胞外基质矿化程度,其次是Fe3O4@ZIF-8组,见图6E,与碱性磷酸酶活性检测结果一致。 成骨诱导第7天,Fe3O4@ZIF-8+磁场组Runx2蛋白质量浓度高于其他3组,如图6F所示。 成脂诱导第7天的油红O染色结果显示,Fe3O4@ZIF-8+磁场组脂滴形成最少,其次是Fe3O4@ZIF-8组,空白对照组脂滴形成最多,比色定量结果也显示出相同的趋势,见图6C,G,可能提示磁场条件对成脂分化产生了抑制作用。 综合以上结果,Fe3O4@ZIF-8纳米颗粒能够促进骨髓间充质干细胞的成骨分化,并在100 MT磁场作用下成骨效果更加显著。"
| [1] LEW WZ, FENG SW, LEE SY, et al. The Review of Bioeffects of Static Magnetic Fields on the Oral Tissue-Derived Cells and Its Application in Regenerative Medicine. Cells. 2021;10(10):2662. [2] LIU C, LUO JW, LIANG T, et al. Matrix stiffness regulates the differentiation of tendon-derived stem cells through FAK-ERK1/2 activation. Exp Cell Res. 2018;373(1-2):62-70. [3] ZHAO YZ, CHEN R, XUE PP, et al. Magnetic PLGA microspheres loaded with SPIONs promoted the reconstruction of bone defects through regulating the bone mesenchymal stem cells under an external magnetic field. Mater Sci Eng C. 2021;122:111877. [4] SHOKROLLAHI H. Structure, synthetic methods, magnetic properties and biomedical applications of ferrofluids. Mater Sci Eng C. 2013;33(5): 2476-2487. [5] FERNANDES MM, CORREIA DM, RIBEIRO C, et al. Bioinspired Three-Dimensional Magnetoactive Scaffolds for Bone Tissue Engineering. ACS Appl Mater Interface. 2019;11(48):45265-45275. [6] HAO L, LI L, WANG P, et al. Synergistic osteogenesis promoted by magnetically actuated nano-mechanical stimuli. Nanoscale. 2019; 11(48):23423-23437. [7] REN N, LIANG N, DONG M, et al. Stem Cell Membrane-Encapsulated Zeolitic Imidazolate Framework-8: A Targeted Nano-Platform for Osteogenic Differentiation. Small. 2022;18(26):e2202485. [8] XUE Y, ZHU Z, ZHANG X, et al. Accelerated Bone Regeneration by MOF Modified Multifunctional Membranes through Enhancement of Osteogenic and Angiogenic Performance. Adv Healthc Mater. 2021; 10(6):e2001369. [9] LIU Y, ZHU Z, PEI X, et al. ZIF-8-Modified Multifunctional Bone-Adhesive Hydrogels Promoting Angiogenesis and Osteogenesis for Bone Regeneration. ACS Appl Mater Interfaces. 2020;12(33): 36978-36995. [10] OBEID M, SABER SEL D, ISMAEL AEL D, et al. Mesenchymal stem cells promote hard-tissue repair after direct pulp capping. J Endod. 2013; 39(5):626-631. [11] ULLRICH SJ, FREEDMAN-WEISS M, AHLE S, et al. Nanoparticles for delivery of agents to fetal lungs. Acta Biomater. 2021;123:346-353. [12] CHEN G, ZHUO Y, TAO B, et al. Moderate SMFs attenuate bone loss in mice by promoting directional osteogenic differentiation of BMSCs. Stem Cell Res Ther. 2020;11(1):487. [13] MARYCZ K, ALICKA M, KORNICKA-GARBOWSKA K, et al. Promotion through external magnetic field of osteogenic differentiation potential in adipose-derived mesenchymal stem cells: Design of polyurethane/poly(lactic) acid sponges doped with iron oxide nanoparticles. J Biomed Mater Res B Appl Biomater. 2020;108(4):1398-1411. [14] ABDEEN AA, LEE J, BHARADWAJ NA, et al. Temporal Modulation of Stem Cell Activity Using Magnetoactive Hydrogels. Adv Healthc Mater. 2016;5(19):2536-2544. [15] YAN Z, SUN T, TAN W, et al. Magnetic Field Boosts the Transmembrane Transport Efficiency of Magnesium Ions from PLLA Bone Scaffold. Small. 2023;19(40):e2301426. [16] HUANG Z, HE Y, CHANG X, et al. A Magnetic Iron Oxide/Polydopamine Coating Can Improve Osteogenesis of 3D-Printed Porous Titanium Scaffolds with a Static Magnetic Field by Upregulating the TGFbeta-Smads Pathway. Adv Healthc Mater. 2020;9(14):e2000318. [17] XIA Y, SUN J, ZHAO L, et al. Magnetic field and nano-scaffolds with stem cells to enhance bone regeneration. Biomaterials. 2018;183: 151-170. [18] Wu D, Chang X, Tian J, et al. Bone mesenchymal stem cells stimulation by magnetic nanoparticles and a static magnetic field: release of exosomal miR-1260a improves osteogenesis and angiogenesis. J Nanobiotechnology. 2021;19(1):209. [19] FOWLKES JL, BUNN RC, THRAILKILL KM. Contributions of the Insulin/Insulin-Like Growth Factor-1 Axis to Diabetic Osteopathy. J Diabetes Metab. 2011;1(3):S1-003. [20] WU C, CHUNG AE, MCDONALD JA. A novel role for alpha 3 beta 1 integrins in extracellular matrix assembly. J Cell Sci. 1995;108( Pt 6): 2511-2523. [21] WANG H, LI J, ZHANG X, et al. Priming integrin alpha 5 promotes the osteogenic differentiation of human periodontal ligament stem cells due to cytoskeleton and cell cycle changes. J Proteomics. 2018;179:122-130. [22] SHAO PL, WU SC, LIN ZY, et al. Alpha-5 Integrin Mediates Simvastatin-Induced Osteogenesis of Bone Marrow Mesenchymal Stem Cells. Int J Mol Sci. 2019;20(3):506. [23] VENTOLA CL. Progress in Nanomedicine: Approved and Investigational Nanodrugs. P T. 2017;42(12):742-755. [24] CHEN X, ZHUANG Y, RAMPAL N, et al. Formulation of Metal-Organic Framework-Based Drug Carriers by Controlled Coordination of Methoxy PEG Phosphate: Boosting Colloidal Stability and Redispersibility. J Am Chem Soc. 2021;143(34):13557-13572. [25] ABU LA, KIWADA H, ISHIDA T. The accelerated blood clearance (ABC) phenomenon: clinical challenge and approaches to manage. J Control Release. 2013;172(1):38-47. [26] LONGO N, HARDING CO, BURTON BK, et al. Single-dose, subcutaneous recombinant phenylalanine ammonia lyase conjugated with polyethylene glycol in adult patients with phenylketonuria: an open-label, multicentre, phase 1 dose-escalation trial. Lancet. 2014; 384(9937):37-44. [27] LI S, ZHANG L, LIANG X, et al. Tailored synthesis of hollow MOF/polydopamine Janus nanoparticles for synergistic multi-drug chemo-photothermal therapy. Chem Eng J. 2019;378:122175. [28] LI L, DUAN Y, LIAO S, et al. Adsorption and separation of propane/propylene on various ZIF-8 polymorphs: Insights from GCMC simulations and the ideal adsorbed solution theory (IAST). Chem Eng J. 2020;386:123945. [29] NASSER ABDELHAMID H, MATHEW AP. Cellulose-zeolitic imidazolate frameworks (CelloZIFs) for multifunctional environmental remediation: Adsorption and catalytic degradation. Chem Eng J. 2021;426:131733. [30] TAO B, ZHAO W, LIN C, et al. Surface modification of titanium implants by ZIF-8@Levo/LBL coating for inhibition of bacterial-associated infection and enhancement of in vivo osseointegration. Chem Eng J. 2020;390:124621. [31] ZHANG Z, JIA B, YANG H, et al. Zn0.8Li0.1Sr-a biodegradable metal with high mechanical strength comparable to pure Ti for the treatment of osteoporotic bone fractures: In vitro and in vivo studies. Biomaterials. 2021;275:120905. [32] 王姗姗,舒晴,田峻.物理因子促进干细胞的成骨分化[J].中国组织工程研究,2024,28(7):1083-1090. [33] 汪文妮,陈超群,顾新华.磁性纳米粒子复合支架及外加磁场影响成骨作用的研究进展[J]. 浙江大学学报(医学版),2022,51(1): 102-107. [34] 卓祥龙,胡建中.脉冲电磁场成骨生物学效应的研究进展[J].医学综述,2014(23):4239-4241. |
| [1] | Liu Qi, Li Linzhen, Li Yusheng, Jiao Hongzhuo, Yang Cheng, Zhang Juntao. Icariin-containing serum promotes chondrocyte proliferation and chondrogenic differentiation of stem cells in the co-culture system of three kinds of cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1371-1379. |
| [2] | Aikepaer · Aierken, Chen Xiaotao, Wufanbieke · Baheti. Osteogenesis-induced exosomes derived from human periodontal ligament stem cells promote osteogenic differentiation of human periodontal ligament stem cells in an inflammatory microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1388-1394. |
| [3] | Zhang Zhenyu, Liang Qiujian, Yang Jun, Wei Xiangyu, Jiang Jie, Huang Linke, Tan Zhen. Target of neohesperidin in treatment of osteoporosis and its effect on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1437-1447. |
| [4] | Sun Yuting, Wu Jiayuan, Zhang Jian. Physical factors and action mechanisms affecting osteogenic/odontogenic differentiation of dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1531-1540. |
| [5] | Yang Zhihang, Sun Zuyan, Huang Wenliang, Wan Yu, Chen Shida, Deng Jiang. Nerve growth factor promotes chondrogenic differentiation and inhibits hypertrophic differentiation of rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1336-1342. |
| [6] | Sun Xianjuan, Wang Qiuhua, Zhang Jinyi, Yang Yangyang, Wang Wenshuang, Zhang Xiaoqing. Adhesion, proliferation, and vascular smooth muscle differentiation of bone marrow mesenchymal stem cells on different electrospinning membranes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 661-669. |
| [7] | Ge Xiao, Zhao Zhuangzhuang, Guo Shuyu, Xu Rongyao. HOXA10 gene-modified bone marrow mesenchymal stem cells promote bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7701-7708. |
| [8] | Zhang Xiongjinfu, Chen Yida, Cheng Xinyi, Liu Daihui, Shi Qin . Exosomes derived from bone marrow mesenchymal stem cells of young rats to reverse senescence in aged rat bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7709-7718. |
| [9] | Sima Xinli, Liu Danping, Qi Hui. Effect and mechanism of metformin-modified bone marrow mesenchymal stem cell exosomes on regulating chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7728-7734. |
| [10] | Tian Jinxin, Zhao Yuxin, Hu Tong, Cui Tiantian, Ma Lihong. Effects of different transcranial magnetic stimulation modes on refractory depression in adults: a network meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7639-7648. |
| [11] | Liu Chengyuan, Guo Qianping. Differential effects of kartogenin on chondrogenic and osteogenic differentiation of rat and rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7490-7498. |
| [12] | Tang Haoxu, Liang Yingjie, Li Ce, Ding Penglin, Qian Minlong, Yuan Lingli. Deferoxamine alleviates the inhibitory effect of glucocorticoids on osteogenic differentiation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6821-6827. |
| [13] | Shao Xuekun, Shi Dianhua, Ding Zhiping, Qiu Zhuoya, Wang Ping, Wang Yi, Wang Cheng, Ding Xiaoyan, Sun Tiefeng. Calcined deer antler slices promote proliferation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6601-6608. |
| [14] | Lin Shuqian, Zhao Xilong, Gao Jing, Pan Xinghua, Li Zian, Ruan Guangping. Comparison of biological characteristics of mouse bone marrow mesenchymal stem cells after interference and overexpression of telomere Cajal body protein-1 [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6616-6624. |
| [15] | Liu Xun, Ouyang Hougan, Pan Rongbin, Wang Zi, Yang Fen, Tian Jiaxuan . Optimal parameters for physical interventions in bone marrow mesenchymal stem cell differentiation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6727-6732. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||