Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (19): 4102-4110.doi: 10.12307/2025.067
Previous Articles Next Articles
Adult stem cells from different germ layers applied in peripheral nerve injury repair
Zheng Jiachen1, Yang Entong1, Zhu Yizhou1, Liu Fang2
- 1Basic Medical College of Naval Medical University, Shanghai 200433, China; 2Department of Human Anatomy, Naval Medical University, Shanghai 200433, China
-
Received:2024-02-29Accepted:2024-04-26Online:2025-07-08Published:2024-09-13 -
Contact:Liu Fang, Professor, Department of Human Anatomy, Naval Medical University, Shanghai 200433, China -
About author:Zheng Jiachen, Basic Medical College of Naval Medical University, Shanghai 200433, China -
Supported by:National Natural Science Foundation of China, No. 81571211 (to LF)
CLC Number:
Cite this article
Zheng Jiachen, Yang Entong, Zhu Yizhou, Liu Fang. Adult stem cells from different germ layers applied in peripheral nerve injury repair[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(19): 4102-4110.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
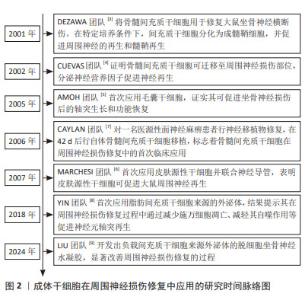
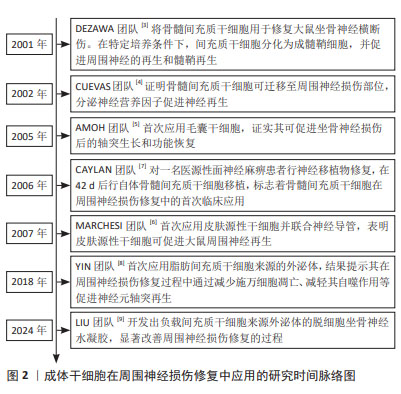
自2001年DEZAWA等[3]将骨髓间充质干细胞应用于坐骨神经损伤模型,证明间充质干细胞可分化为成髓鞘细胞并促进周围神经的再生和髓鞘形成,成体干细胞疗法逐渐成为周围神经损伤治疗研究领域的新方向。相关研究证明了骨髓间充质干细胞在损伤部位的迁移以及对周围神经修复的促进作用[4],随后,各种来源的成体干细胞开始陆续应用于周围神经损伤研究领域[5-6]。与此同时,成体干细胞疗法的临床研究也在逐步开展中。在2006年,CAYLAN等[7]先对1例医源性面神经麻痹患者行神经移植物修复,并在42 d后行自体骨髓间充质干细胞移植,标志着骨髓间充质干细胞在周围神经损伤修复中的首次临床应用。成体干细胞疗法研究逐步深入[8-9],具体时间脉络见图2。"
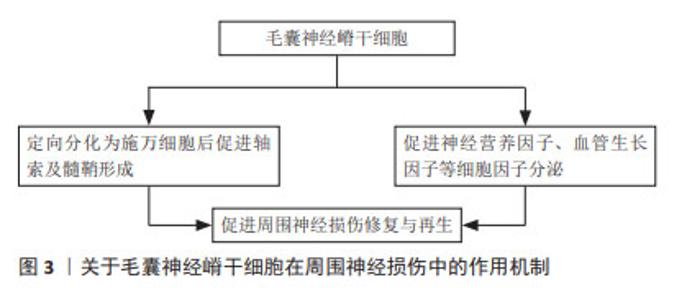
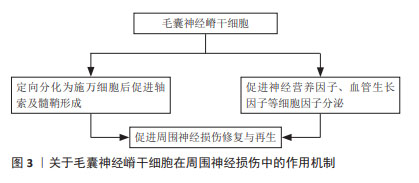
2.1 外胚层来源成体干细胞 胚胎三胚层分化过程中,在脊索的诱导下,相应的外胚层细胞增生,分化形成表皮及附属器和神经系统。当神经沟闭合为神经管时,神经板外侧缘的细胞迁移至神经管背外侧形成2条纵行细胞索,即神经嵴。神经嵴又称“第四胚层”,是周围神经系统的原基,具有很强的迁移能力,可分化为多种特定的细胞和组织。 2.1.1 毛囊神经嵴干细胞 起源于胚胎神经嵴,表达巢蛋白(Nestin)、Sox10和未成熟的神经嵴细胞标志物,具有定向分化为神经元、平滑肌细胞、施万细胞、黑素细胞和软骨细胞的能力[10]。研究表明,毛囊神经嵴干细胞可促进周围神经损伤的修复,并可能通过定向分化、调节相关细胞因子分泌等多种机制发挥作用,见图3。LIN等[11]认为其作用机制是定向分化为施万细胞后促进轴索及髓鞘形成,而HU等[12]发现毛囊神经嵴干细胞能促进神经营养因子、血管生长因子以及金属蛋白酶的分泌。唐笠等[13]以静脉导管桥接SD大鼠面神经干缺损,探究毛囊表皮神经嵴干细胞(epidermal neural crest stem cells,EPI-NCSCs)对面神经损伤的调节作用,发现术后毛囊EPI-NCSCs组的肿瘤坏死因子α表达水平显著降低,而白细胞介素4表达水平和面神经功能评分显著增高,表明毛囊EPI-NCSCs对面神经损伤修复早期局部肿瘤坏死因子α和白细胞介素4表达具有调节作用,能促进面神经功能和形态恢复。可见毛囊EPI-NCSCs能通过调节细胞因子水平促进周围神经损伤后轴突的生长和神经功能恢复。最新研究显示,PAN等[14]提取毛囊EPI-NCSCs来源的外泌体促进面神经功能恢复,结果表明其外泌体有助于脱细胞同种异体神经移植物修复面神经缺损,并有可能在临床上取代自体移植疗法。"
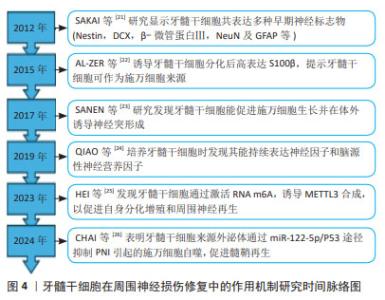
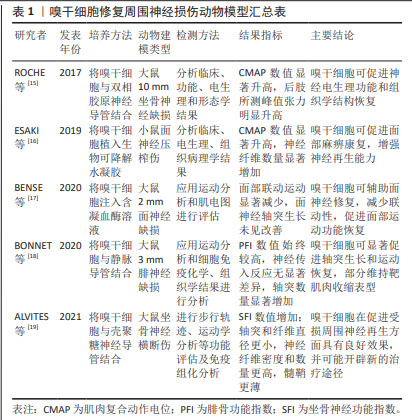
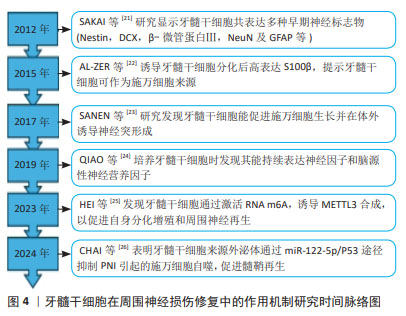
ROCHE等[15]将嗅干细胞与双相胶原神经导管结合,治疗大鼠坐骨神经缺损,发现嗅干细胞可促进神经电生理功能和组织学结构的恢复。ESAKI等[16]应用嗅干细胞治疗小鼠面神经压榨伤,发现其有助于促进面部麻痹康复,并增强神经再生能力。ENTEZARI等[20]研究发现,嗅干细胞相比脂肪间充质干细胞和施万细胞,具有更显著的分裂活性和增殖速度;同时,嗅干细胞可表达施万细胞标志物(SOX10,p75,S100,GFAP和MBP)并分泌神经营养因子(脑源性神经营养因子和神经生长因子),提示嗅干细胞能够自发分化为施万细胞样细胞表型并通过分泌神经营养因子促进神经突生长。嗅干细胞易于从嗅黏膜活检中获得,且风险较低,可以作为组织工程神经的种子细胞,用于基础乃至临床试验的研究。 2.1.3 牙外胚层干细胞 包括牙髓干细胞、脱落乳牙干细胞、根尖乳头干细胞和牙周韧带干细胞等,其与神经嵴干细胞有着相同的起源,具有神经源性。目前对牙髓干细胞的研究较为全面[21-26],见图4。牙髓干细胞可在体外分化为施万细胞,表达多种早期神经细胞标志物并释放生长因子,促进体内血管形成、促进神经生长以及髓鞘形成和轴突再生[27]。体内移植的牙髓干细胞可能同时发挥多种作用,如牙髓干细胞通过向施万细胞前体细胞分化并分泌神经营养因子3和脑源性神经营养因子等促进坐骨神经再生和再髓鞘化[28]。PEREIRA等[29]将脱落乳牙干细胞注入聚乙醇酸导管,治疗大鼠面神经缺损,发现脱落乳牙干细胞同样可以在体内分化为施万细胞,促进神经再生。可见牙外胚层干细胞不仅具有多能分化潜力,还展现出很好的再生能力,为其应用于周围神经损伤修复提供了依据。"
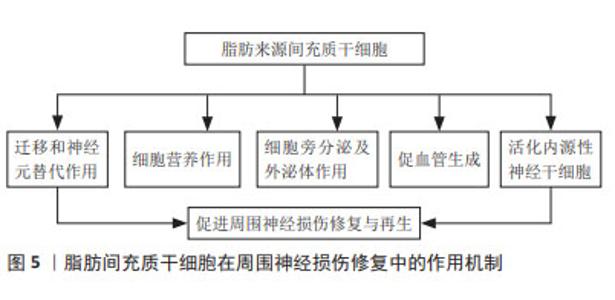
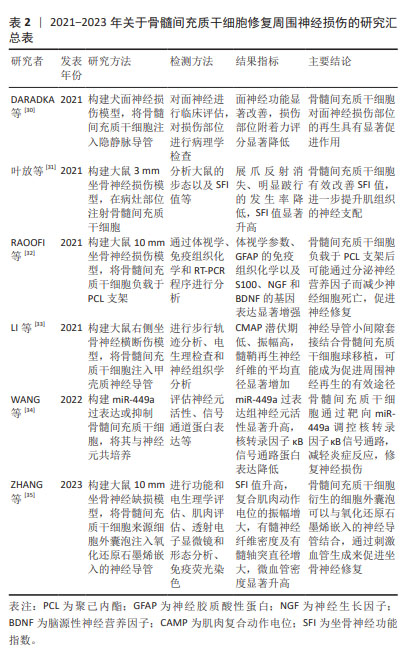
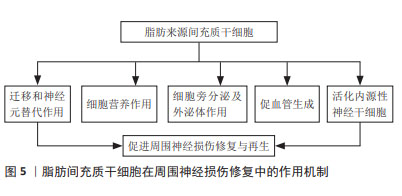
DARADKA等[30]发现在犬面神经损伤、移植自体隐静脉模型中,骨髓间充质干细胞注射组的神经功能恢复、粘连减少程度以及胶原沉积程度均有显著性改善。叶放等[31]将骨髓间充质干细胞注入大鼠坐骨神经3 mm缺损模型,观察各项指标发现,注射骨髓间充质干细胞所产生的细胞外基质,相较于施万细胞、皮肤前体细胞分化的施万细胞和成纤维细胞,能为神经功能恢复提供更为有利的微环境,同时减少炎症反应的发生[36]。LI等[37]研究显示,骨髓间充质干细胞来源外泌体在大鼠坐骨神经损伤模型中可促进M2巨噬细胞极化、轴突再生和重建,加速轴突功能恢复。最新研究发现,骨髓间充质干细胞可通过靶向miR-449a调控核转录因子κB信号通路,从而减轻周围神经损伤的炎症反应,修复神经损伤[34]。相比于神经干细胞以及施万细胞,骨髓间充质干细胞取材更为便捷,体外扩增能力较强,在伦理学中具有较强优势,是目前研究的热点。 2.2.2 脂肪来源间充质干细胞 脂肪在人体内含量丰富,且提取纯化后的脂肪间充质干细胞纯度高、活性好、扩增能力强,具有分化为施万细胞样细胞(SC like cells,SCLCs)和抑制炎症反应的能力,成为临床治疗与再生医学应用的理想种子细胞。近年来,研究者们不断探究脂肪来源间充质干细胞在神经再生领域的作用机制,见图5。研究显示,该干细胞向神经分化后由长梭形转变为类圆形胞体,延伸出突起,并表达神经细胞特异性蛋白NSE和Nestin[38]。移植至损伤部位的脂肪来源间充质干细胞可受施万细胞产生的外泌体诱导而向其分化[39],并通过诱导神经营养因子及生长因子的旁分泌,促进损伤部位施万细胞的生理作用。YAL??N等[40]研究发现脂肪来源间充质干细胞可增加受损神经syndecan-1和热休克蛋白70水平,显著改善功能恢复和促进神经再生,其产生的外泌体可通过内含的miRNA-26b下调Kpna水平,以抑制施万细胞自噬作用,促进坐骨神经损伤修复[41]。工程化细胞技术同样得到应用,HSU等[42]以基因编辑的方式多路径激活脂肪来源间充质干细胞,延长神经因子的作用时间,促进大鼠坐骨神经再生。脂肪来源间充质干细胞获得率高、体外大量扩增所耗费的成本低,在所有非神经源性干细胞中更具吸引力,因此更有可能广泛应用于临床,促进周围神经损伤修复与再生。"

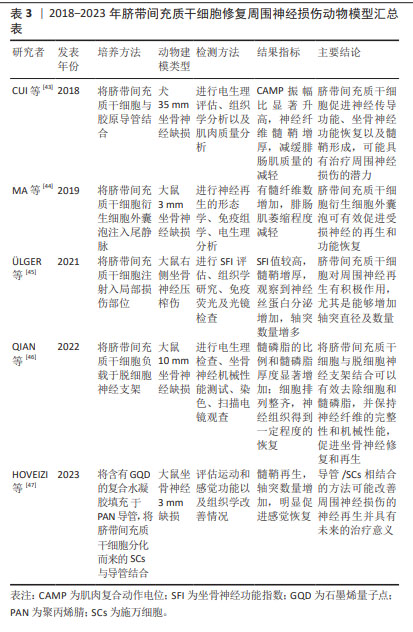
2.2.3 脐带间充质干细胞 是存在于新生儿脐带组织中的一种多能干细胞,可在体内和体外分化为不同胚层的细胞类型。目前的研究多集中于脐带间充质干细胞在周围神经损伤动物模型中的作用[43-47],见表3。研究显示,脐带间充质干细胞不表达或低表达相关主要组织相容性复合体Ⅱ,并且释放各类细胞因子、生长因子和神经营养因子,具有旁分泌、免疫调节、抗氧化以及炎症调节等特性[44]。将脐带间充质干细胞衍生出的SCLCs组装成3D水凝胶球,移植至压榨伤的大鼠坐骨神经中,发现3D-SCLCs水凝胶球有效地促进了大鼠运动功能的恢复,增强神经再生能力。与骨髓间充质干细胞相比,脐带间充质干细胞的自我更新能力和旁分泌作用更强[48],且获取过程简单。然而,人们通常难以收集到足量的脐带间充质干细胞进行移植治疗,移植后的有效浓度可能较低,这也是其主要的不足与劣势。"
| [1] 龚超,张玉强,王伟.细胞治疗周围神经损伤的作用及机制[J].中国组织工程研究,2022,26(13):2114-2119. [2] LI Y, KAMEI Y, KAMBE M, et al. Peripheral nerve regeneration using different germ layer-derived adult stem cells in the past decade. Behav Neurol. 2021;2021:5586523. [3] DEZAWA M, TAKAHASHI I, ESAKI M, et al. Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. Eur J Neurosci. 2001;14(11):1771-1776. [4] CUEVAS P, CARCELLER F, DUJOVNY M, et al. Peripheral nerve regeneration by bone marrow stromal cells. Neurol Res. 2002;24(7): 634-638. [5] AMOH Y, LI L, CAMPILLO R, et al. Implanted hair follicle stem cells form Schwann cells that support repair of severed peripheral nerves. Proc Natl Acad Sci U S A. 2005;102(49):17734-17738. [6] MARCHESI C, PLUDERI M, COLLEONI F, et al. Skin-derived stem cells transplanted into resorbable guides provide functional nerve regeneration after sciatic nerve resection. Glia. 2007;55(4): 425-438. [7] CAYLAN R, BEKTAS D, DIKMEN T, et al. Mesenchymal stem cells in iatrogenic facial nerve paralysis: a possible role in the future. Eur Arch Otorhinolaryngol. 2006;263(10):963-967. [8] YIN G, LIU C, LIN Y, et al. Effect of exosomes from adipose-derived stem cells on peripheral nerve regeneration. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018;32(12):1592-1596. [9] LIU B, ALIMI O A, WANG Y, et al. Differentiated mesenchymal stem cells-derived exosomes immobilized in decellularized sciatic nerve hydrogels for peripheral nerve repair. J Control Release. 2024;368:24-41. [10] SALEHI MS, PANDAMOOZ S, SAFARI A, et al. Epidermal neural crest stem cell transplantation as a promising therapeutic strategy for ischemic stroke. CNS Neurosci Ther. 2020;26(7):670-681. [11] LIN H, LIU F, ZHANG C, et al. Pluripotent hair follicle neural crest stem-cell-derived neurons and schwann cells functionally repair sciatic nerves in rats. Mol Neurobiol. 2009;40(3):216-223. [12] HU Y F, GOURAB K, WELLS C, et al. Epidermal neural crest stem cell (EPI-NCSC)--mediated recovery of sensory function in a mouse model of spinal cord injury. Stem Cell Rev Rep. 2010;6(2):186-198. [13] 唐笠, 潘瑶, 朱国臣.毛囊表皮神经嵴干细胞调节面神经损伤后局部炎症因子的表达水平[J].中国组织工程研究,2023,27(33): 5249-5255. [14] PAN Y, TANG L, DONG S, et al. Exosomes from hair follicle epidermal neural crest stem cells promote acellular nerve allografts to bridge rat facial nerve defects. Stem Cells Dev. 2022;32(1-2):1-11. [15] ROCHE P, ALEKSEEVA T, WIDAA A, et al. Olfactory derived stem cells delivered in a biphasic conduit promote peripheral nerve repair in vivo. Stem Cells Transl Med. 2017;6(10):1894-1904. [16] ESAKI S, KATSUMI S, HAMAJIMA Y, et al. Transplantation of olfactory stem cells with biodegradable hydrogel accelerates facial nerve regeneration after crush injury. Stem Cells Transl Med. 2019;8(2): 169-178. [17] BENSE F, MONTAVA M, DUCLOS C, et al. Syngeneic transplantation of rat olfactory stem cells in a vein conduit improves facial movements and reduces synkinesis after facial nerve injury. Plast Reconstr Surg. 2020;146(6):1295-1305. [18] BONNET M, GUIRAUDIE-CAPRAZ G, MARQUESTE T, et al. Immediate or delayed transplantation of a vein conduit filled with nasal olfactory stem cells improves locomotion and axogenesis in rats after a peroneal nerve loss of substance. Int J Mol Sci. 2020;21(8):2670. [19] ALVITES RD, BRANQUINHO MV, SOUSA AC, et al. Combined use of chitosan and olfactory mucosa mesenchymal stem/stromal cells to promote peripheral nerve regeneration in vivo. Stem Cells Int. 2021;2021:6613029. [20] ENTEZARI M, BAKHtTIARI M, MORADI F, et al. Human olfactory ecto-mesenchymal stem cells displaying Schwann-cell-like phenotypes and promoting neurite outgrowth in vitro. Basic Clin Neurosci. 2023; 14(1):31-42. [21] SAKAI K, YAMAMOTO A, MATSUBARA K, et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest. 2012;122(1):80-90. [22] AL-ZER H, APEL C, HEILAND M, et al. Enrichment and Schwann cell differentiation of neural crest-derived dental pulp stem cells. In Vivo. 2015;29(3):319-326. [23] SANEN K, MARTENS W, GEORGIOU M, et al. Engineered neural tissue with Schwann cell differentiated human dental pulp stem cells: potential for peripheral nerve repair? J Tissue Eng Regen Med. 2017;11(12):3362-3372. [24] QIAO W, LU L, WU G, et al. DPSCs seeded in acellular nerve grafts processed by myroilysin improve nerve regeneration. J Biomater Appl. 2019;33(6):819-833. [25] HEI WH, DU MY, HE H. Effects of RNA m6A writer METTL3 and hDPSCs on the peripheral nerve regeneration: In vitro and in vivo study. Neurosci Lett. 2023;812:137384. [26] CHAI Y, LIU Y, LIU Z, et al. Study on the role and mechanism of exosomes derived from dental pulp stem cells in promoting regeneration of myelin sheath in rats with sciatic nerve injury. Mol Neurobiol. 2024. doi: 10.1007/s12035-024-03960-9. [27] CARNEVALE G, PISCIOTTA A, RICCIO M, et al. Human dental pulp stem cells expressing STRO-1, c-kit and CD34 markers in peripheral nerve regeneration. J Tissue Eng Regen Med. 2018;12(2):e774-e785. [28] MU XD, LIU HH, LI YF, et al. Research progress of dental pulp stem cells for peripheral nerve injury repair. Zhonghua Kou Qiang Yi Xue Za Zhi. 2022;57(2):196-201. [29] PEREIRA LV, BENTO RF, CRUZ DB, et al. Stem cells from human exfoliated deciduous teeth (SHED) differentiate in vivo and promote facial nerve regeneration. Cell Transplant. 2019;28(1):55-64. [30] DARADKA MH, BANI ISMAIL ZA, IRSHEID MA. Peripheral nerve regeneration: a comparative study of the effects of autologous bone marrow-derived mesenchymal stem cells, platelet-rich plasma, and lateral saphenous vein graft as a conduit in a dog model. Open Vet J. 2021;11(4):686-694. [31] 叶放,王彦生,于宁,等.骨髓间充质干细胞在大鼠坐骨神经损伤修复中的作用[J].社区医学杂志,2021,19(6):338-341. [32] RAOOFI A, SADEGHI Y, PIRYAEI A, et al. Bone marrow mesenchymal stem cell condition medium loaded on PCL nanofibrous scaffold promoted nerve regeneration after sciatic nerve transection in male rats. Neurotox Res. 2021;39(5):1470-1486. [33] LI C, ZHANG M, LIU SY, et al. Chitin nerve conduits with three-dimensional spheroids of mesenchymal stem cells from SD rats promote peripheral nerve regeneration. Polymers (Basel). 2021;13(22): 3957. [34] WANG H, WANG F, WANG Y, et al. Study on the mechanism of BMSCs in regulating NF-κB signal pathway by targeting miR-449a to improve the inflammatory response to peripheral nerve injury. J Musculoskelet Neuronal Interact. 2022;22(4):546-561. [35] ZHANG W, FANG XX, LI QC, et al. Reduced graphene oxide-embedded nerve conduits loaded with bone marrow mesenchymal stem cell-derived extracellular vesicles promote peripheral nerve regeneration. Neural Regen Res. 2023;18(1):200-206. [36] WANG S, ZHU C, ZHANG B, et al. BMSC-derived extracellular matrix better optimizes the microenvironment to support nerve regeneration. Biomaterials. 2022;280:121251. [37] LI C, LI X, SHI Z, et al. Exosomes from LPS-preconditioned bone marrow MSCs accelerated peripheral nerve regeneration via M2 macrophage polarization: involvement of TSG-6/NF-κB/NLRP3 signaling pathway. Exp Neurol. 2022;356:114139. [38] 杨贺然,李兴江,胡嘉航,等.Ghrelin对脂肪间充质干细胞神经分化的影响[J].吉林大学学报(医学版),2022,48(6):1490-1497. [39] ZHOU N, XU Z, LI X, et al. Schwann cell-derived exosomes induce the differentiation of human adipose-derived stem cells into schwann cells. Front Mol Biosci. 2021;8:835135. [40] YALÇİN MB, BORA ES, ERDOĞAN MA, et al. The Effect of adipose-derived mesenchymal stem cells on peripheral nerve damage in a rodent model. J Clin Med. 2023;12(19):6411. [41] YIN G, YU B, LIU C, et al. Exosomes produced by adipose-derived stem cells inhibit Schwann cells autophagy and promote the regeneration of the myelin sheath. Int J Biochem Cell Biol. 2021;132:105921. [42] HSU MN, LIAO HT, TRUONG VA, et al. CRISPR-based activation of endogenous neurotrophic genes in adipose stem cell sheets to stimulate peripheral nerve regeneration. Theranostics. 2019;9(21): 6099-6111. [43] CUI Y, YAO Y, ZHAO Y, et al. Functional collagen conduits combined with human mesenchymal stem cells promote regeneration after sciatic nerve transection in dogs. J Tissue Eng Regen Med. 2018;12(5): 1285-1296. [44] MA Y, DONG L, ZHOU D, et al. Extracellular vesicles from human umbilical cord mesenchymal stem cells improve nerve regeneration after sciatic nerve transection in rats. J Cell Mol Med. 2019;23(4):2822-2835. [45] ÜLGER M, SEZER G, ÖZYAZGAN İ, et al. The effect of erythropoietin and umbilical cord-derived mesenchymal stem cells on nerve regeneration in rats with sciatic nerve injury. J Chem Neuroanat. 2021;114:101958. [46] QIAN C, ZHANG Z, ZHAO R, et al. Effect of acellular nerve scaffold containing human umbilical cord-derived mesenchymal stem cells on nerve repair and regeneration in rats with sciatic nerve defect. Ann Transl Med. 2022;10(8):483. [47] HOVEIZI E. Enhancement of nerve regeneration through Schwann cell-mediated healing in a 3D printed polyacrylonitrile conduit incorporating hydrogel and graphene quantum dots: a study on rat sciatic nerve injury model. Biomed Mater. 2023. doi: 10.1088/1748-605X/ad1576. [48] BOJANIC C, TO K, ZHANG B, et al. Human umbilical cord derived mesenchymal stem cells in peripheral nerve regeneration. World J Stem Cells. 2020;12(4):288-302. [49] TORRE P, FLORES AI. Current status and future prospects of perinatal stem cells. Genes (Basel). 2020;12(1):6. [50] PAN HC, CHENG FC, CHEN CJ, et al. Post-injury regeneration in rat sciatic nerve facilitated by neurotrophic factors secreted by amniotic fluid mesenchymal stem cells. J Clin Neurosci. 2007;14(11):1089-1098. [51] PAN HC, YANG DY, HO SP, et al. Escalated regeneration in sciatic nerve crush injury by the combined therapy of human amniotic fluid mesenchymal stem cells and fermented soybean extracts, Natto. J Biomed Sci. 2009;16(1):75. [52] CHIANG CY, LIU SA, SHEU ML, et al. Feasibility of human amniotic fluid derived stem cells in alleviation of neuropathic pain in chronic constrictive injury nerve model. PLoS One. 2016;11(7):e0159482. [53] 陈木彬,李青青,柴辉辉,等.不同TGF-β表达量的hAMSCs尾静脉移植对异种周围神经移植小鼠坐骨神经功能的影响[J].中华神经医学杂志,2020,19(1):9-16. [54] HU T, CHANG S, QI F, et al. Neural grafts containing exosomes derived from Schwann cell-like cells promote peripheral nerve regeneration in rats. Burns Trauma. 2023;11:tkad013. [55] LIU QW, HUANG QM, WU HY, et al. Characteristics and therapeutic potential of human amnion-derived stem cells. Int J Mol Sci. 2021; 22(2):970. [56] ZHOU X, YU M, CHEN D, et al. Chitosan nerve grafts incorporated with SKP-SC-EVs induce peripheral nerve regeneration. Tissue Eng Regen Med. 2023;20(2):309-322. [57] WU X, WANG L, CONG M, et al. Extracellular vesicles from skin precursor-derived Schwann cells promote axonal outgrowth and regeneration of motoneurons via Akt/mTOR/p70S6K pathway. Ann Transl Med. 2020;8(24):1640. [58] ZHANG Q, NGUYEN PD, SHI S, et al. Neural crest stem-like cells non-genetically induced from human gingiva-derived mesenchymal stem cells promote facial nerve regeneration in rats. Mol Neurobiol. 2018;55(8):6965-6983. [59] ZHANG Q, NGUYEN P, BURRELL J C, et al. Harnessing 3D collagen hydrogel-directed conversion of human GMSCs into SCP-like cells to generate functionalized nerve conduits. NPJ Regen Med. 2021;6(1):59. [60] RAO F, ZHANG D, FANG T, et al. Exosomes from human gingiva-derived mesenchymal stem cells combined with biodegradable chitin conduits promote rat sciatic nerve regeneration. Stem Cells Int. 2019; 2019:2546367. [61] 龚超,智晓东,张玉强,等.睫状神经营养因子复合毛喉素及3-异丁基-1-甲基黄嘌呤通过环磷酸腺苷信号通路诱导肌源性干细胞分化为许旺细胞表型[J].中国组织工程研究,2022,26(19): 2970-2977. [62] XUN H, YESANTHARAO P, MUSAVI L, et al. The efficacy of Schwann-like differentiated muscle-derived stem cells in treating rodent upper extremity peripheral nerve injury. Plast Reconstr Surg. 2021;148(4): 787-798. [63] MAKI D, TAMAKI T, FUKUZAWA T, et al. Peripheral nerve regeneration using a cytokine cocktail secreted by skeletal muscle-derived stem cells in a mouse model. J Clin Med. 2021;10(4):824. [64] 陈蒙蒙.肺成体干细胞的研究进展[J].国际呼吸杂志,2013,33(20): 1589-1593. [65] BERTONCELLO I, MCQUALTER JL. Endogenous lung stem cells: what is their potential for use in regenerative medicine? Expert Rev Respir Med. 2010;4(3):349-362. [66] 延祝,夏育民.TWEAK/Fn14信号调控干细胞增殖与分化的作用与机制[J].中华细胞与干细胞杂志(电子版),2020,10(1):57-62. [67] 段德庆,王鹏,张红艳.恒牙牙髓干细胞在周围神经疾病中的应用研究进展[J].南昌大学学报(医学版),2021,61(6):76-79, 100. [68] 胡增祥,赵琪,唐井钢,等.自体骨髓干细胞移植治疗糖尿病周围神经病变临床研究[J].白求恩军医学院学报,2010(2):102-103. [69] 郑培,安沂华,王晓东,等.脐带间充质干细胞移植治疗糖尿病周围神经病变的疗效观察[J].武警医学,2013,24(5):398-401. |
| [1] | Yang Zhihang, Sun Zuyan, Huang Wenliang, Wan Yu, Chen Shida, Deng Jiang. Nerve growth factor promotes chondrogenic differentiation and inhibits hypertrophic differentiation of rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1336-1342. |
| [2] | Liu Qi, Li Linzhen, Li Yusheng, Jiao Hongzhuo, Yang Cheng, Zhang Juntao. Icariin-containing serum promotes chondrocyte proliferation and chondrogenic differentiation of stem cells in the co-culture system of three kinds of cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1371-1379. |
| [3] | Zhang Zhenyu, Liang Qiujian, Yang Jun, Wei Xiangyu, Jiang Jie, Huang Linke, Tan Zhen. Target of neohesperidin in treatment of osteoporosis and its effect on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1437-1447. |
| [4] | Zhao Xiaoxuan, Liu Shuaiyi, Li Qi, Xing Zheng, Li Qingwen, Chu Xiaolei. Different exercise modalities promote functional recovery after peripheral nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1248-1256. |
| [5] | Liu Zan, An Ran, Li Baocheng. Effect of pravastatin on functional recovery from sciatic nerve crush injury in rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 942-950. |
| [6] | Sun Xianjuan, Wang Qiuhua, Zhang Jinyi, Yang Yangyang, Wang Wenshuang, Zhang Xiaoqing. Adhesion, proliferation, and vascular smooth muscle differentiation of bone marrow mesenchymal stem cells on different electrospinning membranes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 661-669. |
| [7] | Ge Xiao, Zhao Zhuangzhuang, Guo Shuyu, Xu Rongyao. HOXA10 gene-modified bone marrow mesenchymal stem cells promote bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7701-7708. |
| [8] | Zhang Xiongjinfu, Chen Yida, Cheng Xinyi, Liu Daihui, Shi Qin . Exosomes derived from bone marrow mesenchymal stem cells of young rats to reverse senescence in aged rat bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7709-7718. |
| [9] | Sima Xinli, Liu Danping, Qi Hui. Effect and mechanism of metformin-modified bone marrow mesenchymal stem cell exosomes on regulating chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7728-7734. |
| [10] | Liu Chengyuan, Guo Qianping. Differential effects of kartogenin on chondrogenic and osteogenic differentiation of rat and rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7490-7498. |
| [11] | He Changliang, Wang Yan, Luo Ling, Liu Jian. Human umbilical cord-derived mesenchymal stem cells thwart pyroptosis of lung tissue cells in septic mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6642-6648. |
| [12] | Liu Xun, Ouyang Hougan, Pan Rongbin, Wang Zi, Yang Fen, Tian Jiaxuan . Optimal parameters for physical interventions in bone marrow mesenchymal stem cell differentiation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6727-6732. |
| [13] | Zhao Yihan, Sun Xuhang, Zhao Lin, Jiang Shiqing. Effects and mechanisms of exosomal miRNA in treatment of multiple myeloma [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6743-6752. |
| [14] | Shao Xuekun, Shi Dianhua, Ding Zhiping, Qiu Zhuoya, Wang Ping, Wang Yi, Wang Cheng, Ding Xiaoyan, Sun Tiefeng. Calcined deer antler slices promote proliferation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6601-6608. |
| [15] | Lin Shuqian, Zhao Xilong, Gao Jing, Pan Xinghua, Li Zian, Ruan Guangping. Comparison of biological characteristics of mouse bone marrow mesenchymal stem cells after interference and overexpression of telomere Cajal body protein-1 [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6616-6624. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||