Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (13): 2669-2674.doi: 10.12307/2025.059
Previous Articles Next Articles
Protective effect of mangiferin on oxidative stress injury in rat bone marrow mesenchymal stem cells
Li Xiaofeng, Zhao Duo, Ouyang Qin, Pang Zixiang, Li Yuquan, Chen Qianfen
- Department of Spine and Osteopathy Surgery, Second Affiliated Hospital of Guangxi Medical University, Nanning 530007, Guangxi Zhuang Autonomous Region, China
-
Received:2024-01-12Accepted:2024-04-13Online:2025-05-08Published:2024-09-11 -
Contact:Chen Qianfen, PhD, Chief physician, Department of Spine and Osteopathy Surgery, Second Affiliated Hospital of Guangxi Medical University, Nanning 530007, Guangxi Zhuang Autonomous Region, China -
About author:Li Xiaofeng, PhD, Associate chief physician, Department of Spine and Osteopathy Surgery, Second Affiliated Hospital of Guangxi Medical University, Nanning 530007, Guangxi Zhuang Autonomous Region, China -
Supported by:Regional High-Incidence Disease Research Joint Special Foundation of Guangxi Natural Science Foundation, No. 2024GXNSFAA010029 (to CQF); Regional High-Incidence Disease Research Joint Special Foundation of Guangxi Natural Science Foundation, No. 2024GXNSFAA010010 (to LXF); Open Project of Guangxi Key Laboratory of Regenerative Medicine, No. 202205 (to LXF); Guangxi Autonomous Region Health Commission Self-Financing Research Project, No. Z-A20230699 (to LXF)
CLC Number:
Cite this article
Li Xiaofeng, Zhao Duo, Ouyang Qin, Pang Zixiang, Li Yuquan, Chen Qianfen. Protective effect of mangiferin on oxidative stress injury in rat bone marrow mesenchymal stem cells[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(13): 2669-2674.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
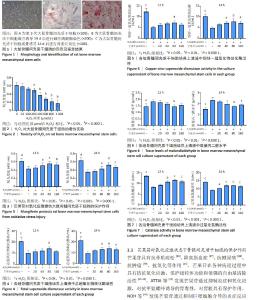
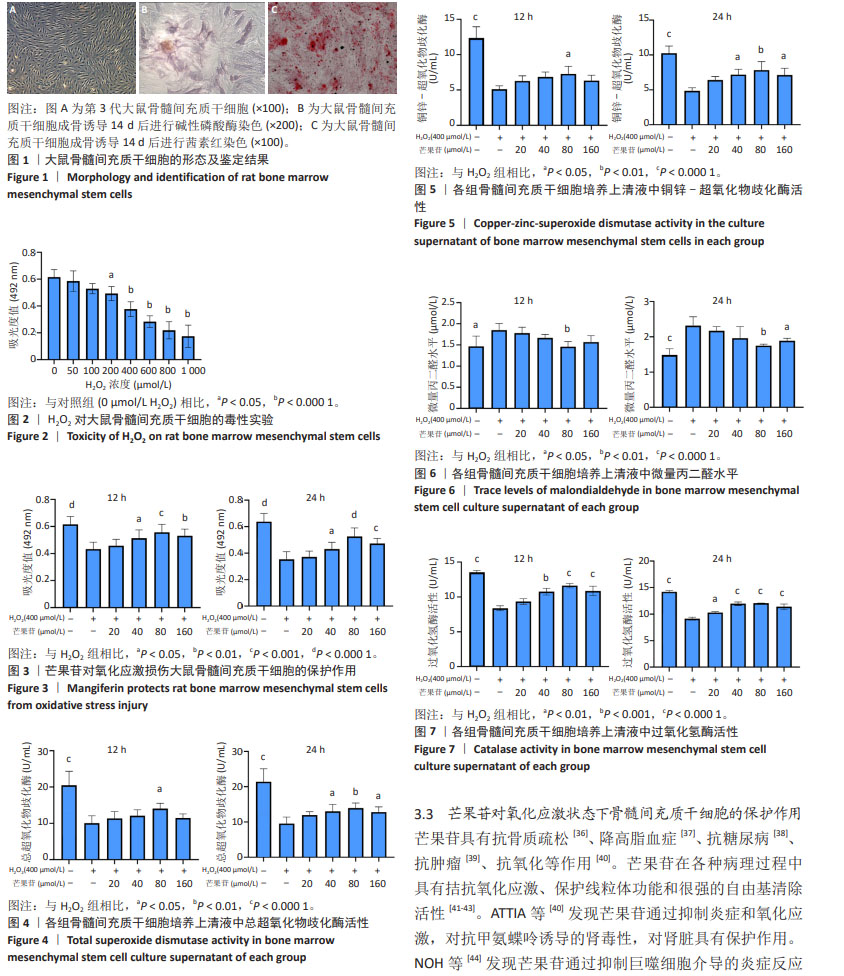
2.1 大鼠骨髓间充质干细胞的形态及鉴定结果 倒置显微镜下观察,第3代大鼠骨髓间充质干细胞贴壁生长,排列紧密,部分呈漩涡状生长,细胞饱满,见图1A。第3代大鼠骨髓间充质干细胞成骨诱导14 d后进行碱性磷酸酶染色,倒置显微镜下观察部分细胞形态呈多角形,胞膜及胞浆蓝染,染色呈阳性反应,见图1B。第3代大鼠骨髓间充质干细胞成骨诱导14 d后进行茜素红染色,倒置显微镜下观察可见大量红染的矿化结节形成,见图1C。 2.2 H2O2对骨髓间充质干细胞的细胞毒性实验 通过MTT实验证实,H2O2能显著抑制骨髓间充质干细胞的生长,并且随着H2O2浓度的增加,抑制作用显著增强,呈明显的剂量相关性,见图2。与对照组相比,H2O2浓度在200 μmol/L时开始出现显著性差异(P < 0.05),400 μmol/L时开始出现非常显著差异(P < 0.000 1)。400 μmol/L H2O2对细胞伤害接近细胞半数中毒浓度(CC50),因此,后续研究应用400 μmol/L H2O2用于氧化应激损伤造模。 2.3 氧化应激模型下芒果苷对大鼠骨髓间充质干细胞的保护作用 MTT实验结果证实,在H2O2构建的氧化应激模型基础上,随着加入芒果苷浓度升高,骨髓间充质干细胞的增殖能力逐渐升高,以80 μmol/L芒果苷组细胞增殖能力最强,见图3。 2.4 各组骨髓间充质干细胞培养上清液中超氧化物歧化酶活性 在氧化应激状态下,细胞上清液中总超氧化物歧化酶活性和铜锌-超氧化物歧化酶活性下降。随着芒果苷浓度的升高,细胞上清液中总超氧化物歧化酶活性和铜锌-超氧化物歧化酶活性逐渐升高,以80 μmol/L芒果苷组超氧化物歧化酶活性最强,见图4,5。 2.5 各组骨髓间充质干细胞培养上清液中微量丙二醛水平 在氧化应激状态下,细胞培养上清液中丙二醛水平上升,随着芒果苷浓度的升高,丙二醛水平逐渐降低,以80 μmol/L芒果苷组丙二醛水平最低,见图6。 2.6 各组骨髓间充质干细胞培养上清液中过氧化氢酶活性 在氧化应激状态下,细胞培养上清液中过氧化氢酶活性下降。随着芒果苷浓度的升高,上清液中过氧化氢酶活性逐渐升高,以80 μmol/L芒果苷组过氧化氢酶活性最强,见图7。"
| [1] ELI I, LERNER DP, GHOGAWALA Z. Acute Traumatic Spinal Cord Injury. Neurol Clin. 2021;39(2):471-488. [2] SZYMONIUK M, LITAK J, SAKWA L, et al. Molecular Mechanisms and Clinical Application of Multipotent Stem Cells for Spinal Cord Injury. Cells. 2022;12(1):120. [3] PAREDES-ESPINOSA MB, PALUH JL. Human stem cell-derived neurons and neural circuitry therapeutics: Next frontier in spinal cord injury repair. Exp Biol Med (Maywood). 2022;247(23):2142-2151. [4] LERMAN LO. Cell-based regenerative medicine for renovascular disease. Trends Mol Med. 2021;27(9):882-894. [5] SHANG Z, WANG M, ZHANG B, et al. Clinical translation of stem cell therapy for spinal cord injury still premature: results from a single-arm meta-analysis based on 62 clinical trials. BMC Med. 2022; 20(1):284. [6] ZHANG Y, HE Y, DENG R, et al. Multifaceted Characterization of Human Embryonic Stem Cell-Derived Mesenchymal Stem/Stromal Cells Revealed Amelioration of Acute Liver Injury in NOD-SCID Mice. Cell Transplant. 2024;33:9636897231218383. [7] ANDRZEJEWSKA A, DABROWSKA S, LUKOMSKA B, et al. Mesenchymal Stem Cells for Neurological Disorders. Adv Sci (Weinh). 2021;8(7): 2002944. [8] MORSY S, MANSOUR MF, ABDO M, et al. Can mobilization of bone marrow stem cells be an alternative regenerative therapy to stem cell injection in a rat model of chronic kidney disease? Physiol Rep. 2022;10(17):e15448. [9] 程建文,赵劲民,李晓峰,等.芒果苷对缺氧损伤骨髓间充质干细胞的保护作用[J].中国组织工程研究,2014,18(32):5091-5096. [10] 李晓峰,罗世兴,赵劲民,等.芒果苷对缺氧损伤骨髓间充质干细胞凋亡的保护[J].中国组织工程研究,2013,17(49): 8481-8487. [11] 李晓峰,赵劲民,苏伟,等.大鼠骨髓间充质干细胞的培养与鉴定[J].中国组织工程研究与临床康复,2011,15(10):1721-1725. [12] LV B, ZHANG X, YUAN J, et al. Biomaterial-supported MSC transplantation enhances cell-cell communication for spinal cord injury. Stem Cell Res Ther. 2021;12(1):36. [13] NAM SH, LEE Y, KIM CH, et al. The complex of miRNA2861 and cell-penetrating, dimeric α-helical peptide accelerates the osteogenesis of mesenchymal stem cells. Biomater Res. 2022;26(1):90. [14] LI B, LIU S, HE Z, et al. The role of zinc finger proteins in the fate determination of mesenchymal stem cells during osteogenic and adipogenic differentiation. Int J Biochem Cell Biol. 2024;167: 106507. [15] ZUNCHEDDU D, DELLA BELLA E, PETTA D, et al. Effect of glucose depletion and fructose administration during chondrogenic commitment in human bone marrow-derived stem cells. Stem Cell Res Ther. 2022;13(1):533. [16] LIU W, LUO F, WU H, et al. Noggin Protein can Induce the Differentiation of Rat Bone Marrow Mesenchymal Stem Cells to Neurons and Repair Spinal Cord Injury. Discov Med. 2023;35(179):956-964. [17] WANG LT, LIU KJ, SYTWU HK, et al. Advances in mesenchymal stem cell therapy for immune and inflammatory diseases: Use of cell-free products and human pluripotent stem cell-derived mesenchymal stem cells. Stem Cells Transl Med. 2021;10(9):1288-1303. [18] RAGHAVAN S, MALAYAPERUMAL S, MOHAN V, et al. A comparative study on the cellular stressors in mesenchymal stem cells (MSCs) and pancreatic β-cells under hyperglycemic milieu. Mol Cell Biochem. 2021;476(1):457-469. [19] ZHAO M, LIU S, WANG C, et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Mitochondrial Damage and Inflammation by Stabilizing Mitochondrial DNA. ACS Nano. 2021;15(1): 1519-1538. [20] TANG LX, WEI B, JIANG LY, et al. Intercellular mitochondrial transfer as a means of revitalizing injured glomerular endothelial cells. World J Stem Cells. 2022;14(9):729-743. [21] TANG F, TANG J, ZHAO Y, et al. Long-term clinical observation of patients with acute and chronic complete spinal cord injury after transplantation of NeuroRegen scaffold. Sci China Life Sci. 2022;65(5):909-926. [22] LI M, CHEN H, ZHU M. Mesenchymal stem cells for regenerative medicine in central nervous system. Front Neurosci. 2022;16:1068114. [23] AFSARTALA Z, HADJIGHASSEM M, SHIRIAN S, et al. Advances in Management of Spinal Cord Injury Using Stem Cell-derived Extracellular Vesicles: A Review Study. Basic Clin Neurosci. 2023;14(4):443-451. [24] QIN H, ZHAO A. Mesenchymal stem cell therapy for acute respiratory distress syndrome: from basic to clinics. Protein Cell. 2020;11(10): 707-722. [25] PRAKASH R, FAUZIA E, SIDDIQUI AJ, et al. Oxidative Stress-induced Autophagy Compromises Stem Cell Viability. Stem Cells. 2022;40(5): 468-478. [26] LI H, XIANG D, GONG C, et al. Naturally derived injectable hydrogels with ROS-scavenging property to protect transplanted stem cell bioactivity for osteoarthritic cartilage repair. Front Bioeng Biotechnol. 2023;10:1109074. [27] DENG H, CHEN Y, LIU H, et al. Study of the effect of keap1 on oxidative stress in human umbilical cord mesenchymal stem cells. Mol Biol Rep. 2024;51(1):67. [28] VATNER SF, ZHANG J, OYDANICH M, et al. Healthful aging mediated by inhibition of oxidative stress. Ageing Res Rev. 2020;64:101194. [29] WENG Z, WANG Y, OUCHI T, et al. Mesenchymal Stem/Stromal Cell Senescence: Hallmarks, Mechanisms, and Combating Strategies. Stem Cells Transl Med. 2022;11(4):356-371. [30] DENU RA, HEMATTI P. Optimization of oxidative stress for mesenchymal stromal/stem cell engraftment, function and longevity. Free Radic Biol Med. 2021;167:193-200. [31] HAJAM YA, RANI R, GANIE SY, et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells. 2022;11(3):552. [32] AKKI R, SIRACUSA R, CORDARO M, et al. Adaptation to oxidative stress at cellular and tissue level. Arch Physiol Biochem. 2022;128(2): 521-531. [33] LI X, SU Z, SHEN K, et al. Eugenol-Preconditioned Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Antioxidant Capacity of Tendon Stem Cells In Vitro and In Vivo. Oxid Med Cell Longev. 2022; 2022:3945195. [34] XIA C, DAI Z, JIN Y, et al. Emerging Antioxidant Paradigm of Mesenchymal Stem Cell-Derived Exosome Therapy. Front Endocrinol (Lausanne). 2021;12:727272. [35] HU C, WU Z, LI L. Pre-treatments enhance the therapeutic effects of mesenchymal stem cells in liver diseases. J Cell Mol Med. 2020; 24(1):40-49. [36] DENG X, LIN B, WANG F, et al. Mangiferin attenuates osteoporosis by inhibiting osteoblastic ferroptosis through Keap1/Nrf2/SLC7A11/GPX4 pathway. Phytomedicine. 2024;124:155282. [37] MINNITI G, LAURINDO LF, MACHADO NM, et al. Mangifera indica L., By-Products, and Mangiferin on Cardio-Metabolic and Other Health Conditions: A Systematic Review. Life (Basel). 2023;13(12):2270. [38] YOOPUM S, WONGMANEE N, ROJANAVERAWONG W, et al. Mango (Mangifera indica L.) seed kernel extract suppresses hyperglycemia by modulating pancreatic β cell apoptosis and dysfunction and hepatic glucose metabolism in diabetic rats. Environ Sci Pollut Res Int. 2023;30(59):123286-123308. [39] RAHMANI AH, ALMATROUDI A, ALLEMAILEM KS, et al. Role of Mangiferin in Management of Cancers through Modulation of Signal Transduction Pathways. Biomedicines. 2023;11(12):3205. [40] ATTIA SH, ELSHAZLY SM, ABDELAAL MM, et al. Reno-protective effect of mangiferin against methotrexate-induced kidney damage in male rats: PPARγ-mediated antioxidant activity. Saudi Pharm J. 2022;30(9): 1252-1261. [41] CHANG CC, TSAI KL, CHENG HC, et al. Mangiferin Protects against Angiotensin-II-Enhanced Hypertrophic Markers and Apoptosis in H9c2 Cardiomyocytes. Am J Chin Med. 2023;51(7):1865-1878. [42] AIN QU, IQBAL MO, KHAN IA, et al. Phytochemical, antioxidant, antipyretic and anti-inflammatory activities of aqueous-methanolic leaf extract of Mangifera indica. Am J Transl Res. 2023;15(7):4533-4543. [43] WU Y, WANG Y, LONG L, et al. A spatiotemporal release platform based on pH/ROS stimuli-responsive hydrogel in wound repairing. J Control Release. 2022;341:147-165. [44] NOH JW, LEE HY, LEE BC. Mangiferin Ameliorates Obesity-Associated Inflammation and Autophagy in High-Fat-Diet-Fed Mice: In Silico and In Vivo Approaches. Int J Mol Sci. 2022;23(23):15329. [45] LUM PT, SEKAR M, SEOW LJ, et al. Neuroprotective potency of mangiferin against 3-nitropropionic acid induced Huntington’s disease-like symptoms in rats: possible antioxidant and anti-inflammatory mechanisms. Front Pharmacol. 2023;14:1189957. [46] WANG Y, GUO X, FAN X, et al. The protective effect of mangiferin on osteoarthritis: An in vitro and in vivo study. Physiol Res. 2022;71(1): 135-145. [47] TSATURYAN V, POGHOSYAN A, TOCZYŁOWSKI M, et al. Evaluation of Malondialdehyde Levels, Oxidative Stress and Host-Bacteria Interactions: Escherichia coli and Salmonella Derby. Cells. 2022; 11(19):2989. |
| [1] | Zhao Jiyu, Wang Shaowei. Forkhead box transcription factor O1 signaling pathway in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1923-1930. |
| [2] | Yang Zhihang, Sun Zuyan, Huang Wenliang, Wan Yu, Chen Shida, Deng Jiang. Nerve growth factor promotes chondrogenic differentiation and inhibits hypertrophic differentiation of rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1336-1342. |
| [3] | Liu Qi, Li Linzhen, Li Yusheng, Jiao Hongzhuo, Yang Cheng, Zhang Juntao. Icariin-containing serum promotes chondrocyte proliferation and chondrogenic differentiation of stem cells in the co-culture system of three kinds of cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1371-1379. |
| [4] | Zhang Zhenyu, Liang Qiujian, Yang Jun, Wei Xiangyu, Jiang Jie, Huang Linke, Tan Zhen. Target of neohesperidin in treatment of osteoporosis and its effect on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1437-1447. |
| [5] | He Guanghui, Yuan Jie, Ke Yanqin, Qiu Xiaoting, Zhang Xiaoling. Hemin regulates mitochondrial pathway of oxidative stress in mouse chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1183-1191. |
| [6] | He Bo, Chen Wen, Ma Suilu, He Zhijun, Song Yuan, Li Jinpeng, Liu Tao, Wei Xiaotao, Wang Weiwei, Xie Jing . Pathogenesis and treatment progress of flap ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1230-1238. |
| [7] | Lu Ranran, Zhou Xu, Zhang Lijie, Yang Xinling. Dimethyl fumarate alleviates nerve damage in a mouse model of Parkinson’s disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 989-994. |
| [8] | Sun Xianjuan, Wang Qiuhua, Zhang Jinyi, Yang Yangyang, Wang Wenshuang, Zhang Xiaoqing. Adhesion, proliferation, and vascular smooth muscle differentiation of bone marrow mesenchymal stem cells on different electrospinning membranes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 661-669. |
| [9] | Ge Xiao, Zhao Zhuangzhuang, Guo Shuyu, Xu Rongyao. HOXA10 gene-modified bone marrow mesenchymal stem cells promote bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7701-7708. |
| [10] | Zhang Xiongjinfu, Chen Yida, Cheng Xinyi, Liu Daihui, Shi Qin . Exosomes derived from bone marrow mesenchymal stem cells of young rats to reverse senescence in aged rat bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7709-7718. |
| [11] | Sima Xinli, Liu Danping, Qi Hui. Effect and mechanism of metformin-modified bone marrow mesenchymal stem cell exosomes on regulating chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7728-7734. |
| [12] | Liu Chengyuan, Guo Qianping. Differential effects of kartogenin on chondrogenic and osteogenic differentiation of rat and rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7490-7498. |
| [13] | Liu Xuan, Ding Yuqing, Xia Ruohan, Wang Xianwang, Hu Shujuan. Exercise prevention and treatment of insulin resistance: role and molecular mechanism of Keap1/nuclear factor erythroid2-related factor 2 signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7578-7588. |
| [14] | Zhang Xiaoyu, Wei Shanwen, Fang Jiawei, Ni Li. Prussian blue nanoparticles restore mitochondrial function in nucleus pulposus cells through antioxidation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7318-7325. |
| [15] | Su Yongkun, Sun Hong, Liu Miao, Yang Hua, Li Qingsong. Development of novel antioxidants and antioxidant combination carried by nano-hydrogel systems in treatment of intervertebral disc degeneration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7376-7384. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||