Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (3): 599-607.doi: 10.12307/2025.118
Previous Articles Next Articles
A meta-analysis of clinical efficacy and safety of intravenous glucocorticoids before lower limb joint arthroplasty
Wang Jianlei1, He Peiliang2, Sun Yongjian1, 3
- 1Guangdong Medical University, Zhanjiang 524000, Guangdong Province, China; 2Department of Traumatology and Orthopedics, Guangzhou Red Cross Hospital Affiliated to Jinan University, Guangzhou 510000, Guangdong Province, China; 3Department of Pediatric Orthopedics, Third Affiliated Hospital of Southern Medical University, Guangzhou 510000, Guangdong Province, China
-
Online:2025-01-28Published:2024-06-04 -
Contact:Sun Yongjian, MD, Chief physician, Guangdong Medical University, Zhanjiang 524000, Guangdong Province, China; Department of Pediatric Orthopedics, Third Affiliated Hospital of Southern Medical University, Guangzhou 510000, Guangdong Province, China -
About author:Wang Jianlei, Master, Physician, Guangdong Medical University, Zhanjiang 524000, Guangdong Province, China
CLC Number:
Cite this article
Wang Jianlei, He Peiliang, Sun Yongjian. A meta-analysis of clinical efficacy and safety of intravenous glucocorticoids before lower limb joint arthroplasty[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(3): 599-607.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
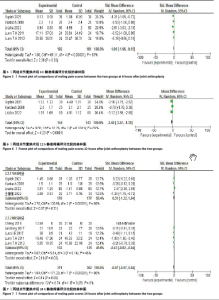
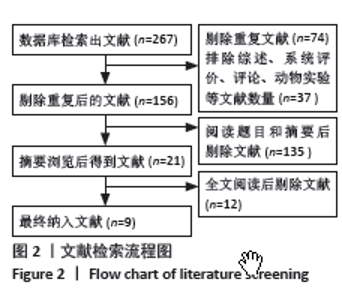
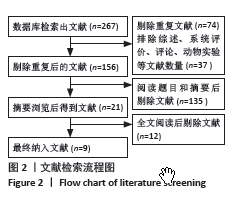
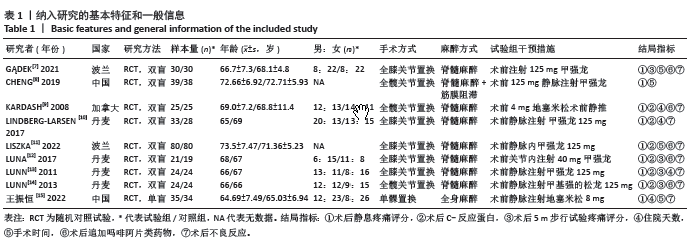
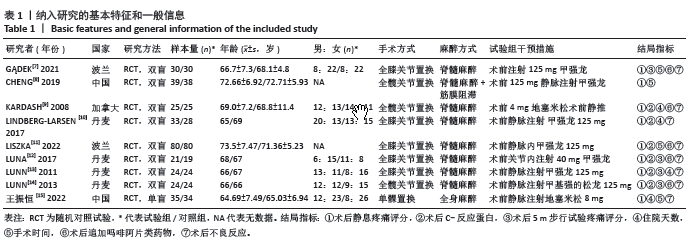
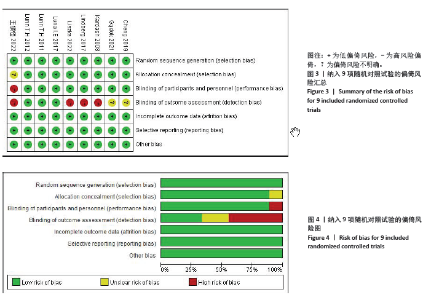
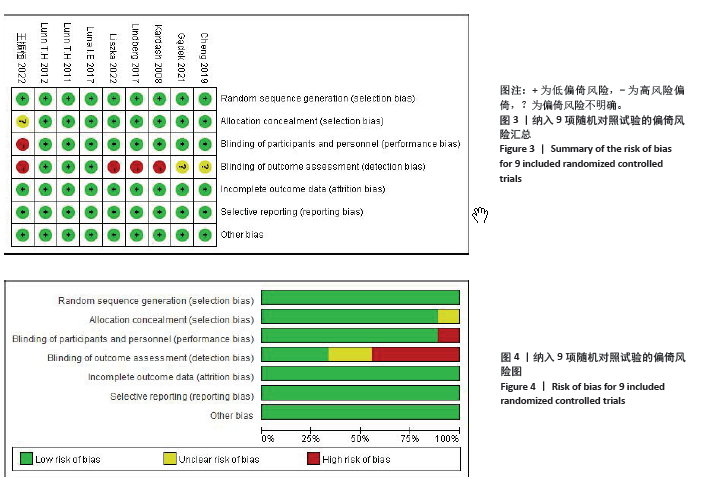
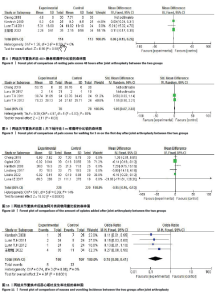
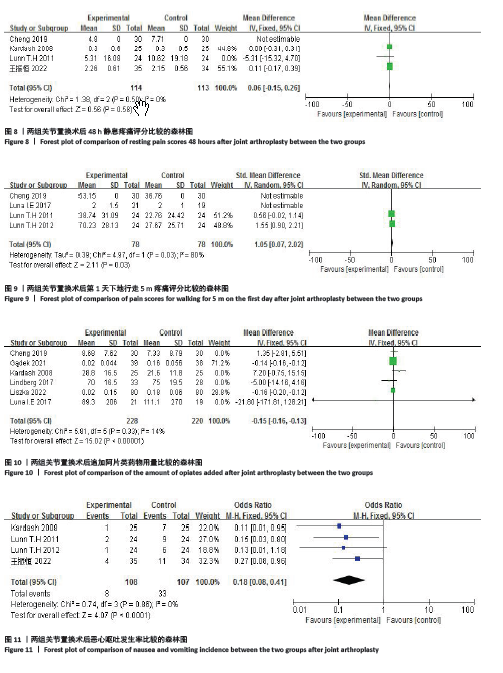
2.4 Meta分析结果 2.4.1 术后6,12,24,48 h静息疼痛评分 5项研究报告了两组下肢关节置换术后6 h的静息疼痛评分[7,9,11,13-14],纳入的研究之间存在很大的异质性(I2=93%,P < 0.000 01),故用随机效应模型进行分析。与对照组相比,糖皮质激素组患者术后6 h VAS评分明显降低,差异有显著性意义(SMD =-1.06,95%CI:-1.96至-0.15,P=0.02),见图5。 有3项研究报告了两组术后12 h 的静息时疼痛评分[7,9,11],纳入的研究之间存在较大异质性(I2= 89%,P=0.000 1)。与对照组相比,糖皮质激素患者组术后12 h VAS评分降低,差异有显著性意义(MD =-2.44,95%CI:-3.57至-1.31,P < 0.000 1),见图6。 9项研究报告了两组术后24 h的静息疼痛评分[7-15],其中4项使用VAS疼痛评分[7,9,11,15],5项使用NRS疼痛评分[8,10,12-14],因为VAS和NRS是2个疼痛评分系统,合并后异质性较大,所以按照不同评分分为VAS及NRS评分2个亚组,亚组分析显示,两组术后24 h疼痛评分差异无显著性意义(Z=0.14,P=0.89),见图7。 4项研究报告了两组术后48 h的静息时疼痛评分[8-9,13,15],纳入的研究无异质性(I2=0%,P=0.5)。与对照组相比,糖皮质激素组患者的术后48 h静息VAS疼痛评分无显著性差异(MD=0.06,95%CI:-0.15-0.26,P=0.58),见图8。 2.4.2 术后第1天下地行走5 m疼痛评分 4项研究报告了两组在术后第1天下地行走5 m疼痛评分[8,12-14], 纳入的研究有异质性(I2=80%, P=0.03)。与对照组相比,糖皮质激素组患者术后第1天下地行走5 m的疼痛评分相对降低(SMD=1.05,95%CI:0.07-2.02,P=0.03),见图9。 2.4.3 术后阿片类药物需要量 6项涉及448例患者的研究报告了术后追加阿片类药物的情况[7-12],经分析纳入的研究之间异质性较小(I2=14%,P=0.33),故使用固定效应模型用于汇集数据。两组之间差异有显著性意义,糖皮质激素组术后阿片类药物需要量减少(MD =-0.15,95%CI:-0.16至-0.13,P < 0.000 01), 见图10。 2.4.4 术后发生恶心呕吐反应 4项研究报告了患者出现恶心呕吐[9,13-15],纳入的研究无明显异质性(I2=0%,P=0.86)。因此,使用固定效应模型来汇集数据。与对照组相比,糖皮质激素组患者术后恶心呕吐发生率显著降低(7.4% vs. 30.8%,OR=0.18,95%CI:0.08-0.41,P < 0.000 1),见图11。"

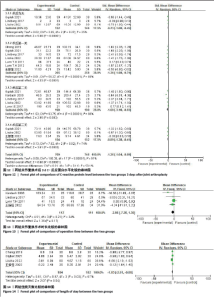
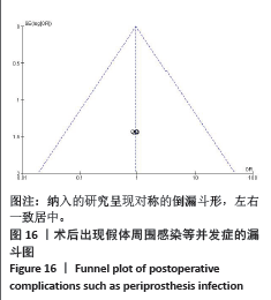
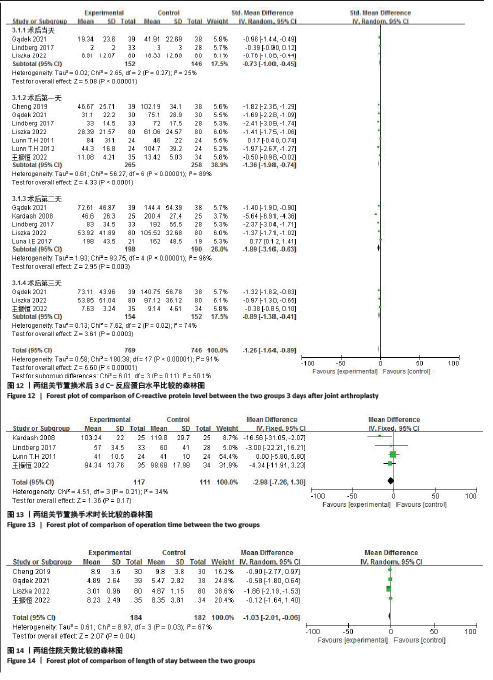
2.4.5 术后3 d的C-反应蛋白水平 按照术后不同时间段C-反应蛋白结果分为术后当日、术后第1,2,3天共4个亚组,亚组分析表明两组之间的异质性极强,达到高度异质,意味着术后不同时间C-反应蛋白值在很大程度上会影响Meta分析结果,其中亚组分析中组内异质性明显降低,亚组分析显示,糖皮质激素组术后3 d内C-反应蛋白水平较对照组明显降低,差异有显著性意义(P < 0.000 01),见图12。 2.4.6 手术时长 4项研究报告了患者手术时长[9-10,13,15],两组之间异质性较小(I2=34%,P=0.21)。糖皮质激素组与对照组的手术时长相比差异无显著性意义(MD=-2.98,95%CI:-7.26-1.30,P=0.17),见图13。 2.4.7 住院天数 4项研究报告了患者住院天数[7-8,11,15],两组之间存在一定异质性(I2=67%,P=0.03),故用随机效应模型进行分析。与对照组比较,糖皮质激素组住院天数缩短,差异有显著性意义(MD=-1.03,95%CI:-2.01至-0.06,P=0.04),见图14。 2.4.8 术后出现假体周围感染等并发症 7项研究报告患者术后出现假体周围感染等并发 症[8-10,12-15],无异质性(I2=0.0%,P=1.0)。糖皮质激素组与对照组感染发生率比较差异无显著性意义(OR=0.96,95%CI:0.33-2.79,P=0.94),见图15。"
| [1] KEHLET H. Glucocorticoids for peri-operative analgesia: how far are we from general recommendations? Acta Anaesthesiol Scand. 2007;51(9):1133-1135. [2] 张先龙, 王坤正. 关节外科的未来——数字骨科技术在关节外科的应用[J] . 中华骨科杂志,2021,41(8):525-531. [3] TERKAWI AS, MAVRIDIS D, SESSLER DI, et al. Pain management modalities after total knee arthroplasty: a network meta-analysis of 170 randomized controlled trials. Anesthesiology. 2017;126:923-937. [4] 齐诗园,恽惠方.加速康复外科理念下关节置换术疼痛管理方案——多模式超前镇痛[J].中国临床研究,2023,36(6): 948-951. [5] 张小青,徐懋.膝关节置换术后多模式镇痛的进展[J].中国微创外科杂志,2019, 19(6):552-555. [6] MAO L, WEI W, CHEN J. Biased regulation of glucocorticoid receptors signaling. Biomed Pharmacother. 2023;165:115145. [7] GĄDEK A, LISZKA H, ZAJĄC M. The effect of pre-operative high doses of methylprednisolone on pain management and convalescence after total hip replacement in elderly: a double-blind randomized study. Int Orthop. 2021;45(4): 857-863. [8] CHENG BLY, SO EHK, HUI GKM, et al. Pre-operative intravenous steroid improves pain and joint mobility after total knee arthroplasty in Chinese population: a double-blind randomized controlled trial. Eur J Orthop Surg Traumatol. 2019;29(7): 1473-1479. [9] KARDASH KJ, SARRAZIN F, TESSLER MJ, et al. Single-dose dexamethasone reduces dynamic pain after total hip arthroplasty. Anesth Analg. 2008;106(4):1253-1257. [10] LINDBERG-LARSEN V, BANDHOLM TQ, ZILMER CK, et al. Preoperative methylprednisolone does not reduce loss of knee-extension strength after total knee arthroplastyA randomized, double-blind, placebo-controlled trial of 61 patients. Acta Orthop. 2017;88(5):543-549. [11] LISZKA H, ZAJĄC M, GĄDEK A. Pre-emptive analgesia with methylprednisolone and gabapentin in total knee arthroplasty in the elderly. Sci Rep. 2022;12(1):2320. [12] LUNA IE, KEHLET H, JENSEN CM, et al. The Effect of Preoperative Intra-Articular Methylprednisolone on Pain After TKA: A Randomized Double-Blinded Placebo Controlled Trial in Patients With High-Pain Knee Osteoarthritis and Sensitization. J Pain. 2017;18(12): 1476-1487. [13] LUNN TH, KRISTENSEN BB, ANDERSEN LØ, et al. Effect of high-dose preoperative methylprednisolone on pain and recovery after total knee arthroplasty: a randomized, placebo-controlled trial. Br J Anaesth. 2011; 106(2):230-238. [14] LUNN TH, ANDERSEN LØ, KRISTENSEN BB, et al. Effect of high-dose preoperative methylprednisolone on recovery after total hip arthroplasty: a randomized, double-blind, placebo-controlled trial. Br J Anaesth. 2013;110(1):66-73. [15] 王振恒,王治栋,刘乃澄,等.单髁关节置换前静脉应用地塞米松对置换后患者疼痛及并发症的影响[J].中国组织工程研究,2022,26(33):5297-5302. [16] WANG X, JIANG W, HUANG Q, et al. Dexamethasone Attenuates the Perioperative Acute Phase Response for Simultaneous Bilateral Total Hip Arthroplasty. J Arthroplasty. 2022;37(5): 888-891. [17] DENG Z, LI Y, STORM GR, et al. The efficiency and safety of steroid addition to multimodal cocktail periarticular injection in knee joint arthroplasty: a meta-analysis of randomized controlled trials. Sci Rep. 2019;9(1):7031. [18] LI FL, LI BC, HUANG X, et al. Different dexamethasone doses in the perioperative period improve short-term outcomes of total hip arthroplasty: a randomized controlled trial. Eur Rev Med Pharmacol Sci. 2023;27(8):3438-3447. [19] CORCORAN TB, MARTIN C, O’LOUGHLIN E, et al. Dexamethasone and clinically significant postoperative nausea and vomiting: a prespecified substudy of the randomised perioperative administration of dexamethasone and infection (PADDI) trial. Br J Anaesth. 2022;129(3):327-335. [20] BOUGARNE N, MYLKA V, RATMAN D, et al. Mechanisms Underlying the Functional Cooperation Between PPARα and GRα to Attenuate Inflammatory Responses. Front Immunol. 2019;10:1769. [21] DE OLIVEIRA GS JR, CASTRO-ALVES LJ, AHMAD S, et al. Dexamethasone to prevent postoperative nausea and vomiting: an updated meta-analysis of randomized controlled trials. Anesth Analg. 2013;116(1):58-74. [22] GHAI B, JAFRA A, BHATIA N, et al. Opioid sparing strategies for perioperative pain management other than regional anaesthesia: A narrative review. J Anaesthesiol Clin Pharmacol. 2022;38(1):3-10. [23] FEO CV, SORTINI D, RAGAZZI R, et al. Randomized clinical trial of the effect of preoperative dexamethasone on nausea and vomiting after laparoscopic cholecystectomy. Br J Surg. 2006;93(3): 295-299. [24] LUNN TH, KEHLET H. Perioperative glucocorticoids in hip and knee surgery - benefit vs. harm? A review of randomized clinical trials. Acta Anaesthesiol Scand. 2013;57(7):823-834. [25] TONER AJ, GANESHANATHAN V, CHAN MT, et al. Safety of Perioperative Glucocorticoids in Elective Noncardiac Surgery: A Systematic Review and Meta-analysis. Anesthesiology. 2017;126(2):234-248. [26] RICHARDSON AB, BALA A, WELLMAN SS, et al. Perioperative Dexamethasone Administration Does Not Increase the Incidence of Postoperative Infection in Total Hip and Knee Arthroplasty: A Retrospective Analysis. J Arthroplasty. 2016;31(8):1784-1787. |
| [1] | Zhou Jinhai, Li Jiangwei, Wang Xuquan, Zhuang Ying, Zhao Ying, Yang Yuyong, Wang Jiajia, Yang Yang, Zhou Shilian. Three-dimensional finite element analysis of anterior femoral notching during total knee arthroplasty at different bone strengths [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1775-1782. |
| [2] | Zhang Xinxin, Gao Ke, Xie Shidong, Tuo Haowen, Jing Feiyue, Liu Weiguo. Network meta-analysis of non-surgical treatments for foot and ankle ability and dynamic balance in patients with chronic ankle instability [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1931-1944. |
| [3] | Sun Yundi, Cheng Lulu, Wan Haili, Chang Ying, Xiong Wenjuan, Xia Yuan. Effect of neuromuscular exercise for knee osteoarthritis pain and function: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1945-1952. |
| [4] | Liang Haobo, Wang Zeyu, Ma Wenlong, Liu Hao, Liu Youwen. Hot issues in the field of joint revision: infection, rehabilitation nursing, bone defect, and prosthesis loosening [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1963-1971. |
| [5] | Wang Yida, Liu Jun, Wang Xiaoling, Wang Liyan, Yang Chengru, Zhang Xuexiao. Effects of wearable electronic device-based interventions on physical activity and sedentary behavior in healthy adolescents: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1693-1704. |
| [6] | Zhang Zixian, Xu Youliang, Wu Shaokui, Wang Xiangying. Effects of blood flow restriction training combined with resistance training on muscle indicators in college athletes: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1705-1713. |
| [7] | Wang Juan, Wang Guanglan, Zuo Huiwu. Efficacy of exercise therapy in the treatment of anterior cruciate ligament reconstruction patients: #br# a network meta-analysis #br# [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1714-1726. |
| [8] | Zheng Huakun, Yin Mingyue, Liu Qian. Effects of interval and continuous training on the quality of life in physically inactive adults: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1727-1740. |
| [9] | Li Zhe, Li Ping, Zhang Chao, Guo Guangling. A network meta-analysis of efficacy of mesenchymal stem cells from different sources in treatment of premature ovarian failure animal models [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7898-7908. |
| [10] | Wang Feng, Cao Chunfeng, He Chao, Zhang Tao, Zhou Zixian, Zhu Fengchen. All-inside versus traditional techniques of anterior cruciate ligament reconstruction: meta-analysis of therapeutic efficacy and radiological outcomes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7629-7638. |
| [11] | Tian Jinxin, Zhao Yuxin, Hu Tong, Cui Tiantian, Ma Lihong. Effects of different transcranial magnetic stimulation modes on refractory depression in adults: a network meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7639-7648. |
| [12] | Wang Chen, Zhang Weinan, Shen Jining, Liu Fan, Yuan Jishan, Liu Yake. Inhibitory effect of ferroptosis inhibitor toxicity induced by cobalt nanoparticles through reactive oxygen species [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7310-7317. |
| [13] | Wang Kaigang, Hao Dongsheng, Ma Pei, Zhou Shuo, Li Ruimin. Comparison of efficacy of different biological scaffolds for pulp regeneration therapy in immature permanent teeth: a Bayesian network meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7447-7460. |
| [14] | Wang Rongqiang, Yang Liu, Wu Xiangkun, Shang Lilin. Analysis of factors associated with prognosis of osteoporosis patients after hip arthroplasty and construction of Nomogram prediction model [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(33): 7137-7142. |
| [15] | Abuduainijiang·Abulimiti, Alimu·Mamuti, Li Simi. Artificial femoral head replacement for femoral neck fracture in the elderly: validation of a risk prediction model for hip dysfunction [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(33): 7143-7149. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||