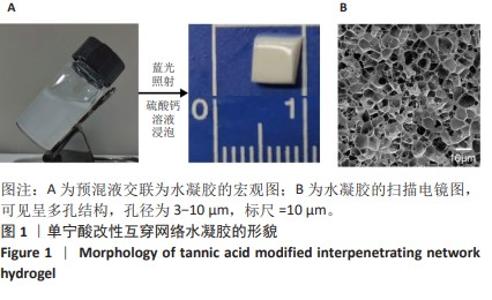
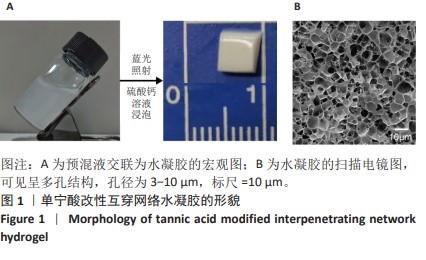
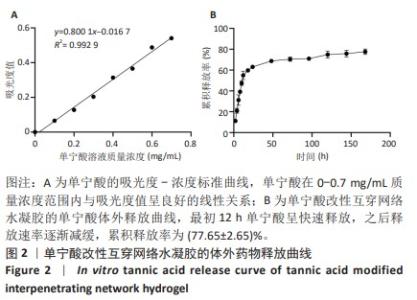
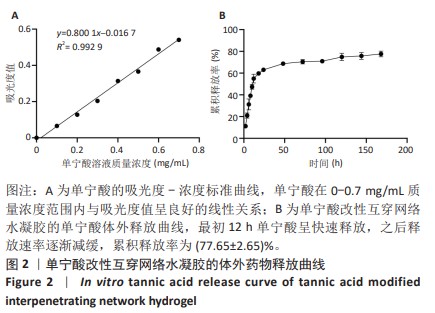
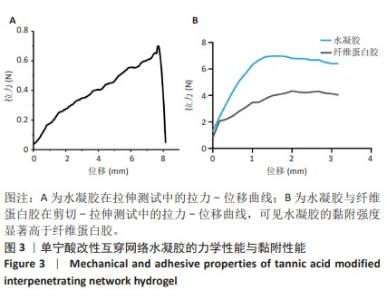
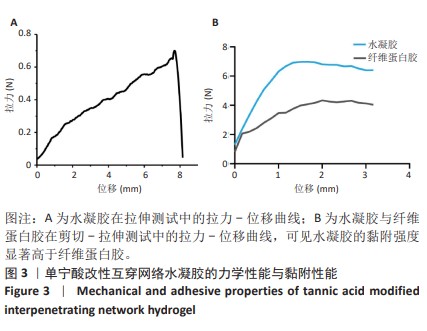
Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (4): 721-729.doi: 10.12307/2025.251
Previous Articles Next Articles
Tannic acid modified interpenetrating network hydrogel promotes tissue remodeling of ruptured Achilles tendon after surgery#br#
#br#
Zhang Bo, Zhang Zhen, Jiang Dong
- Department of Sports Medicine, Peking University Third Hospital, Beijing 100191, China
-
Received:2023-12-05Accepted:2024-01-20Online:2025-02-08Published:2024-05-30 -
Contact:Jiang Dong, MD, Professor, Chief physician, Doctoral supervisor, Department of Sports Medicine, Peking University Third Hospital, Beijing 100191, China -
About author:Zhang Bo, Doctoral candidate, Department of Sports Medicine, Peking University Third Hospital, Beijing 100191, China -
Supported by:National Natural Science Foundation of China, No. 82072428 (to JD); National Strategic Cooperation Foundation of Peking University, No. 2022ZLHB002 (to JD)
CLC Number:
Cite this article
Zhang Bo, Zhang Zhen, Jiang Dong. Tannic acid modified interpenetrating network hydrogel promotes tissue remodeling of ruptured Achilles tendon after surgery#br#
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
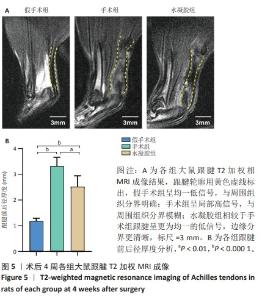
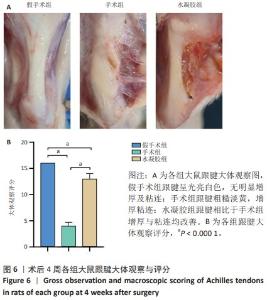
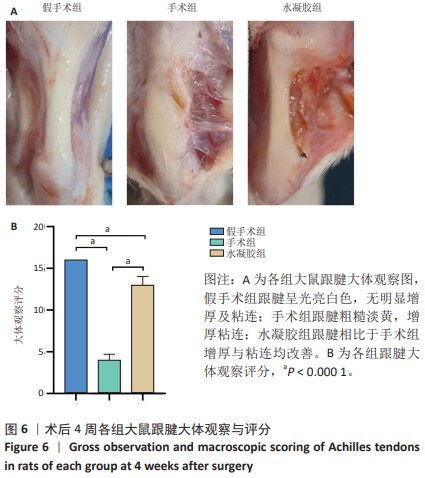
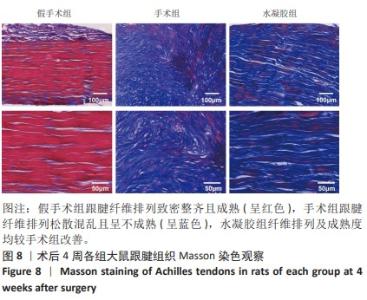
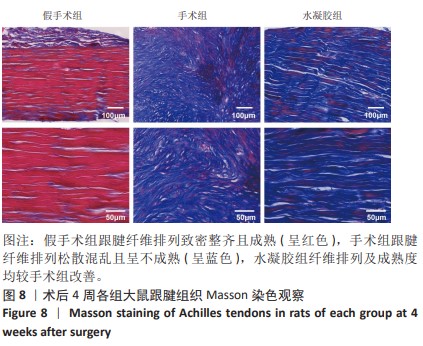
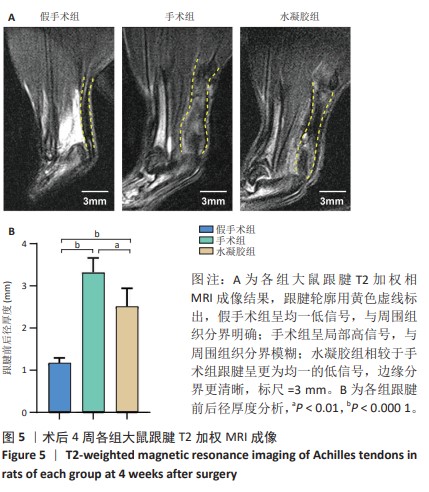
2.5 单宁酸改性互穿网络水凝胶促进术后跟腱修复 2.5.1 实验动物数量分析 30只大鼠全部进入结果分析。 2.5.2 各组大鼠跟腱MRI成像结果 术后4周各组大鼠跟腱T2加权相核磁共振成像结果,见图5A。假手术组大鼠跟腱可见均一低信号,与周围组织分界明确;手术组大鼠跟腱则呈现部分区域局部高信号,跟腱前后径厚度明显增加,与周围组织分界模糊不清;与手术组相比,水凝胶组大鼠跟腱显示出更为均一的低信号,跟腱前后径厚度减小,与周围组织分界清晰。假手术组、手术组、水凝胶组大鼠跟腱前后径厚度分别为(1.17±0.12),(3.32±0.34),(2.51±0.43) mm,3组间比较差异有显著性意义(F=55.96,P < 0.05),见图5B。"
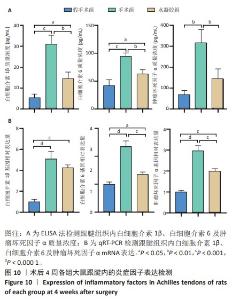
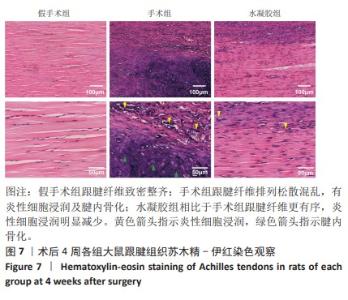
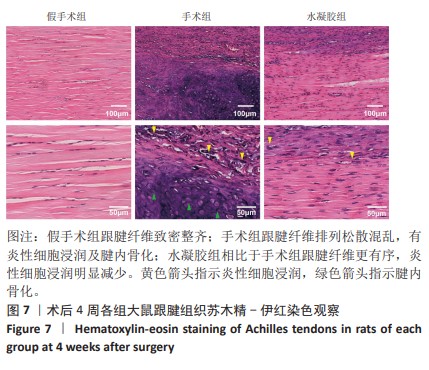
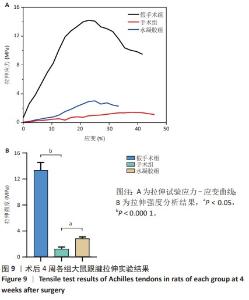
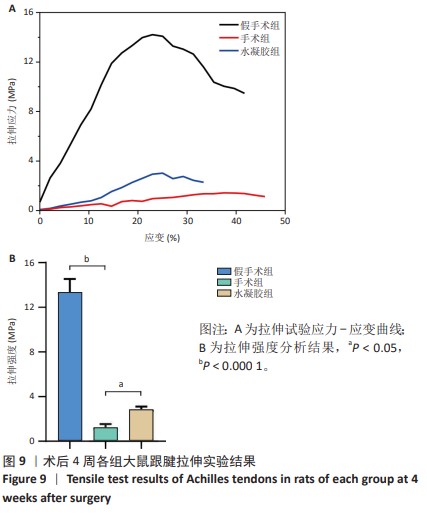
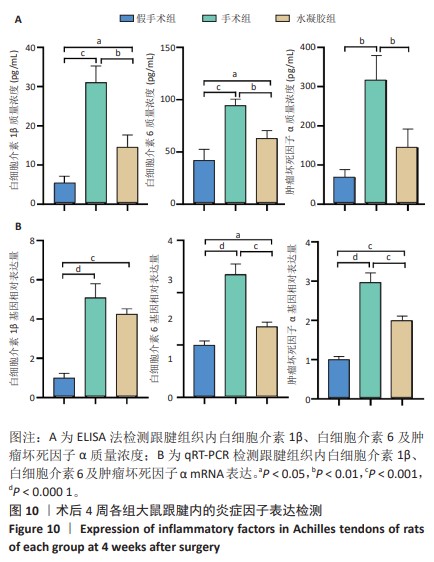
2.5.6 各组大鼠跟腱内的炎症反应 ELISA检测结果显示,3组大鼠跟腱内的白细胞介素1β、白细胞介素6及肿瘤坏死因子α水平比较差异均有显著性意义(F=50.17,F=30.00,F=22.78,P < 0.05)。与假手术组比较,手术组大鼠跟腱内的白细胞介素1β、白细胞介素6及肿瘤坏死因子α水平升高(P < 0.01,P < 0.001);与手术组比较,水凝胶组大鼠跟腱内的上述3种炎症因子水平降低(P < 0.01),见图10A。 qRT-PCR检测结果显示,3组大鼠跟腱内的白细胞介素1β、白细胞介素6及肿瘤坏死因子α mRNA表达比较差异有显著性意义(F=69.36,F=88.16,F=115.9,P < 0.05)。与假手术组比较,手术组大鼠跟腱内的白细胞介素1β、白细胞介素6和肿瘤坏死因子α mRNA表达均升高(P < 0.01,P < 0.001);与手术组比较,水凝胶组大鼠跟腱内的白细胞介素6和肿瘤坏死因子α mRNA表达均降低(P < 0.001),白细胞介素1β mRNA表达无明显变化(P > 0.05),见图10B。以上结果证实,单宁酸互穿网络水凝胶可有效抑制术后跟腱的炎症反应。"
| [1] MYHRVOLD SB, BROUWER EF, ANDRESEN TKM, et al. Nonoperative or Surgical Treatment of Acute Achilles’ Tendon Rupture. N Engl J Med. 2022;386(15):1409-1420. [2] HOLM C, KJAER M, ELIASSON P. Achilles tendon rupture--treatment and complications: a systematic review. Scand J Med Sci Sports. 2015;25(1):e1-10. [3] KULICK MI, BRAZLOW R, SMITH S, et al. Injectable Ibuprofen: Preliminary Evaluation of Its Ability to Decrease Peritendinous Adhesions. Ann Plast Surg. 1984;13(6):459. [4] YAN Z, MENG X, SU Y, et al. Double layer composite membrane for preventing tendon adhesion and promoting tendon healing. Mater Sci Eng C Mater Biol Appl. 2021;123:111941. [5] CHOI HJ, CHOI S, KIM JG, et al. Enhanced tendon restoration effects of anti-inflammatory, lactoferrin-immobilized, heparin-polymeric nanoparticles in an Achilles tendinitis rat model. Carbohydr Polym. 2020;241:116284. [6] TU T, SHEN Y, WANG X, et al. Tendon ECM modified bioactive electrospun fibers promote MSC tenogenic differentiation and tendon regeneration. Appl Mater Today. 2020;18:100495. [7] CAI C, ZHANG X, LI Y, et al. Self-Healing Hydrogel Embodied with Macrophage-Regulation and Responsive-Gene-Silencing Properties for Synergistic Prevention of Peritendinous Adhesion. Adv Mater. 2022;34(5):e2106564. [8] ZHANG Q, YANG Y, YILDIRIMER L, et al. Advanced technology-driven therapeutic interventions for prevention of tendon adhesion: Design, intrinsic and extrinsic factor considerations. Acta Biomater. 2021;124:15-32. [9] JAFARI H, GHAFFARI-BOHLOULI P, NIKNEZHAD SV, et al. Tannic acid: a versatile polyphenol for design of biomedical hydrogels. J Mater Chem B. 2022;10(31):5873-5912. [10] GUO S, YAO M, ZHANG D, et al. One-Step Synthesis of Multifunctional Chitosan Hydrogel for Full-Thickness Wound Closure and Healing. Adv Healthc Mater. 2022;11(4):2101808. [11] LI Q, XIAO W, ZHANG F, et al. Tannic acid-derived metal-phenolic networks facilitate PCL nanofiber mesh vascularization by promoting the adhesion and spreading of endothelial cells. J Mater Chem B. 2018; 6(18):2734-2738. [12] NAGESH PKB, CHOWDHURY P, HATAMI E, et al. Tannic acid inhibits lipid metabolism and induce ROS in prostate cancer cells. Sci Rep. 2020;10(1):980. [13] DHAND AP, GALARRAGA JH, BURDICK JA. Enhancing Biopolymer Hydrogel Functionality through Interpenetrating Networks. Trends Biotechnol. 2021;39(5):519-538. [14] HU M, YANG J, XU J. Structural and biological investigation of chitosan/hyaluronic acid with silanized-hydroxypropyl methylcellulose as an injectable reinforced interpenetrating network hydrogel for cartilage tissue engineering. Drug Deliv. 2021;28(1):607-619. [15] ZHANG Y, DOU X, ZHANG L, et al. Facile fabrication of a biocompatible composite gel with sustained release of aspirin for bone regeneration. Bioact Mater. 2022;11:130-139. [16] THANKAM FG, DIAZ C, CHANDRA I, et al. Hybrid interpenetrating hydrogel network favoring the bidirectional migration of tenocytes for rotator cuff tendon regeneration. J Biomed Mater Res B Appl Biomater. 2022;110(2):467-477. [17] FAN X, WANG S, FANG Y, et al. Tough polyacrylamide-tannic acid-kaolin adhesive hydrogels for quick hemostatic application. Mater Sci Eng C Mater Biol Appl. 2020;109:110649. [18] SMAJILAGIĆ A, ALJIČEVIĆ M, REDŽIĆ A, et al. Rat bone marrow stem cells isolation and culture as a bone formative experimental system. Bosn J Basic Med Sci. 2013;13(1):27-30. [19] YANG S, SHI X, LI X, et al. Oriented collagen fiber membranes formed through counter-rotating extrusion and their application in tendon regeneration. Biomaterials. 2019;207:61-75. [20] FREEDMAN BR, MOONEY DJ. Biomaterials to Mimic and Heal Connective Tissues. Adv Mater. 2019;31(19):e1806695. [21] LI J, FENG X, LIU B, et al. Polymer materials for prevention of postoperative adhesion. Acta Biomater. 2017;61:21-40. [22] ZHANG J, XIAO C, ZHANG X, et al. An oxidative stress-responsive electrospun polyester membrane capable of releasing anti-bacterial and anti-inflammatory agents for postoperative anti-adhesion. J Control Release. 2021;335:359-368. [23] EVROVA O, BÜRGISSER GM, EBNÖTHER C, et al. Elastic and surgeon friendly electrospun tubes delivering PDGF-BB positively impact tendon rupture healing in a rabbit Achilles tendon model. Biomaterials. 2020;232:119722. [24] LIU C, TIAN S, BAI J, et al. Regulation of ERK1/2 and SMAD2/3 Pathways by Using Multi-Layered Electrospun PCL-Amnion Nanofibrous Membranes for the Prevention of Post-Surgical Tendon Adhesion. Int J Nanomedicine. 2020;15:927-942. [25] YANG Y, XU T, ZHANG Q, et al. Biomimetic, Stiff, and Adhesive Periosteum with Osteogenic-Angiogenic Coupling Effect for Bone Regeneration. Small. 2021;17(14):e2006598. [26] COOMBES BK, BISSET L, VICENZINO B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet. 2010;376(9754):1751-1767. [27] GUO Z, XIE W, LU J, et al. Tannic acid-based metal phenolic networks for bio-applications: a review. J Mater Chem B. 2021;9(20):4098-4110. [28] GRACEY E, BURSSENS A, CAMBRÉ I, et al. Tendon and ligament mechanical loading in the pathogenesis of inflammatory arthritis. Nat Rev Rheumatol. 2020;16(4):193-207. [29] CHOI S, CHOI Y, KIM J. Anisotropic Hybrid Hydrogels with Superior Mechanical Properties Reminiscent of Tendons or Ligaments. Adv Funct Mater. 2019;29(38):1904342. [30] TANG Y, WANG Z, XIANG L, et al. Functional biomaterials for tendon/ligament repair and regeneration. Regen Biomater. 2022;9:rbac062. [31] CHEN C, YANG H, YANG X, et al. Tannic acid: a crosslinker leading to versatile functional polymeric networks: a review. RSC Adv. 2022; 12(13):7689-7711. [32] REN L, GONG P, GAO X, et al. Metal-phenolic networks acted as a novel bio-filler of a barrier membrane to improve guided bone regeneration via manipulating osteoimmunomodulation. J Mater Chem B. 2022;10(48):10128-10138. [33] WU F, NERLICH M, DOCHEVA D. Tendon injuries: Basic science and new repair proposals. EFORT Open Rev. 2017;2(7):332-342. [34] MILLAR NL, MURRELL GAC, MCINNES IB. Inflammatory mechanisms in tendinopathy - towards translation. Nat Rev Rheumatol. 2017;13(2): 110-122. [35] JUNEJA SC, SCHWARZ EM, O’KEEFE RJ, et al. Cellular and molecular factors in flexor tendon repair and adhesions: a histological and gene expression analysis. Connect Tissue Res. 2013;54(3):218-226. [36] VOLETI PB, BUCKLEY MR, SOSLOWSKY LJ. Tendon healing: repair and regeneration. Annu Rev Biomed Eng. 2012;14:47-71. [37] HOU J, YANG R, VUONG I, et al. Biomaterials strategies to balance inflammation and tenogenesis for tendon repair. Acta Biomater. 2021;130:1-16. [38] NICHOLS AEC, BEST KT, LOISELLE AE. The cellular basis of fibrotic tendon healing: challenges and opportunities. Transl Res. 2019;209: 156-168. [39] JING W, XIAOLAN C, YU C, et al. Pharmacological effects and mechanisms of tannic acid. Biomed Pharmacother. 2022;154:113561. [40] CHEN Y, TIAN L, YANG F, et al. Tannic Acid Accelerates Cutaneous Wound Healing in Rats Via Activation of the ERK 1/2 Signaling Pathways. Adv Wound Care. 2019;8(7):341-354. [41] XI Q, HOTH-HANNIG W, DENG S, et al. The effect of polyphenol-containing solutions on in situ biofilm formation on enamel and dentin. J Dent. 2020;102:103482. [42] HU F, ZHANG R, GUO W, et al. PEGylated-PLGA Nanoparticles Coated with pH-Responsive Tannic Acid–Fe(III) Complexes for Reduced Premature Doxorubicin Release and Enhanced Targeting in Breast Cancer. Mol Pharmaceutics. 2021;18(6):2161-2173. |
| [1] | Zhao Hongxia, Sun Zhengwei, Han Yang, Wu Xuechao , Han Jing. Osteogenic properties of platelet-rich fibrin combined with gelatin methacryloyl hydrogel [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 809-817. |
| [2] | Zhao Zengbo, Li Chenxi, Dou Chenlei, Ma Na, Zhou Guanjun. Anti-inflammatory and osteogenic effects of chitosan/sodium glycerophosphate/sodium alginate/leonurine hydrogel [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 678-685. |
| [3] | Dong Meilin, Du Haiyu, Liu Yuan. Quercetin-loaded carboxymethyl chitosan hydrogel promotes wound healing in diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 692-699. |
| [4] | Zhang Yu, Xu Ruian, Fang Lei, Li Longfei, Liu Shuyan, Ding Lingxue, Wang Yuexi, Guo Ziyan, Tian Feng, Xue Jiajia. Gradient artificial bone repair scaffold regulates skeletal system tissue repair and regeneration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 846-855. |
| [5] | He Rui, Li Chongyi, Wang Ruiyao, Zeng Dan, Fan Daidi. Application of MXene-based hydrogels in wound repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3486-3493. |
| [6] | Liu Zhongyu, Li Wenya, Fan Yonghong, Lyu Shuang, Pei Juan, Chen Yaqin, Liu Beiyu, Sun Hongyu. Methacrylated dermal extracellular matrix hydrogel promotes repair of abdominal wall defects [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(10): 2074-2082. |
| [7] | Zhao Wenqi, Yu Haichi, Song Yiru, Yuan Tianyang, Liu Qinyi. Platelet-rich plasma and hydrogel for spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(10): 2189-2200. |
| [8] | Zhou Pengfei, Lin Jing, Chen Yuying, Lin Minkui. Canine dental pulp stem cells-polyglycolic acid scaffold complex for canine periodontal tissue defect [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(34): 5526-5531. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||