Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (2): 302-311.doi: 10.12307/2025.245
Previous Articles Next Articles
Analysis of oxidative stress-related genes and immune infiltration in osteoarthritis
Wu Ao1, Yu Peng1, Teng Jiawen2, Kong Peng2, Bian Sishan2
- 1The First Clinical Medical College of Shandong University of Traditional Chinese Medicine, Jinan 250000, Shandong Province, China; 2Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan 250000, Shandong Province, China
-
Received:2024-01-17Accepted:2024-02-22Online:2025-01-18Published:2024-05-24 -
Contact:Bian Sishan, MD, Chief physician, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan 250000, Shandong Province, China -
About author:Wu Ao, Master candidate, The First Clinical Medical College of Shandong University of Traditional Chinese Medicine, Jinan 250000, Shandong Province, China
CLC Number:
Cite this article
Wu Ao, Yu Peng, Teng Jiawen, Kong Peng, Bian Sishan. Analysis of oxidative stress-related genes and immune infiltration in osteoarthritis[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(2): 302-311.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
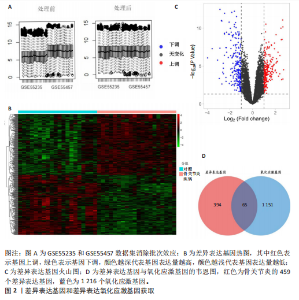
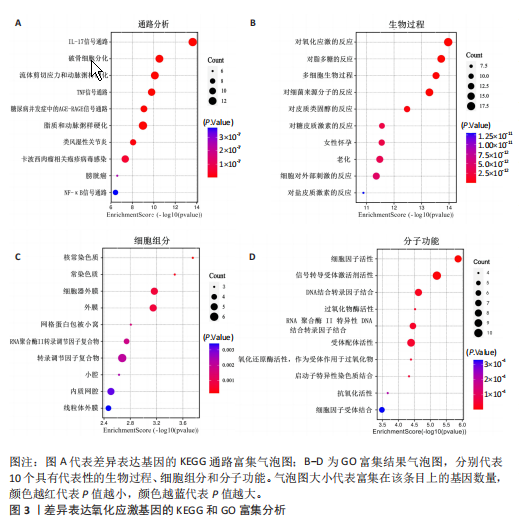
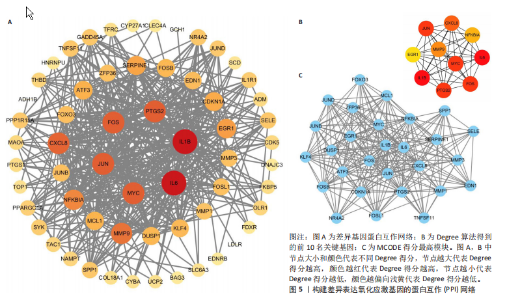
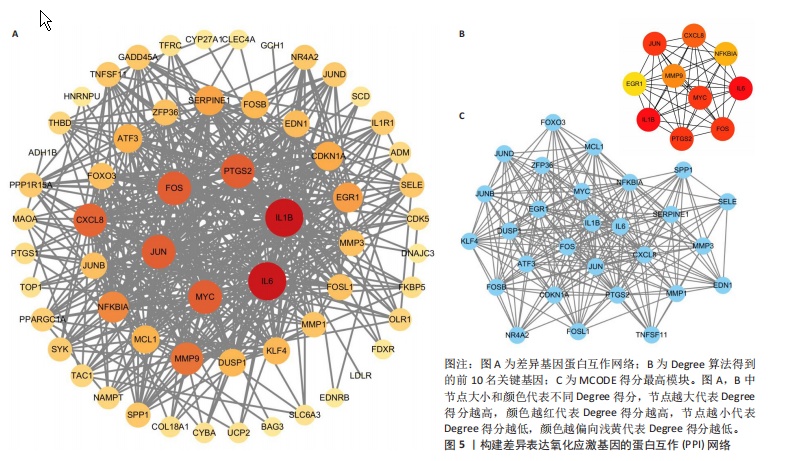
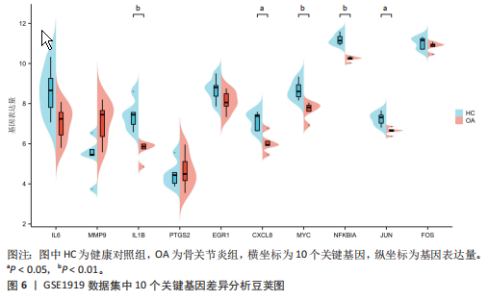
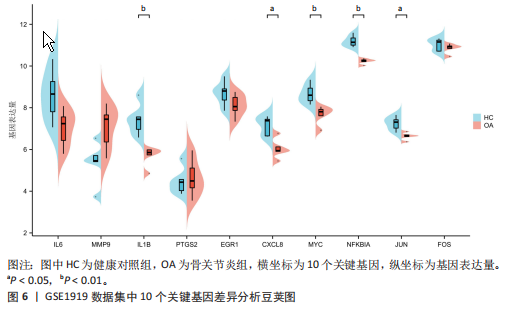
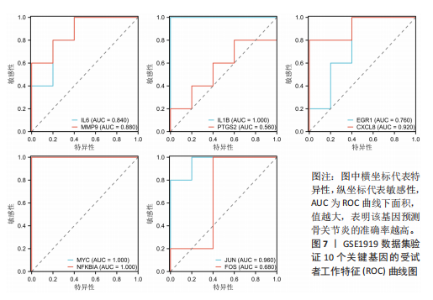
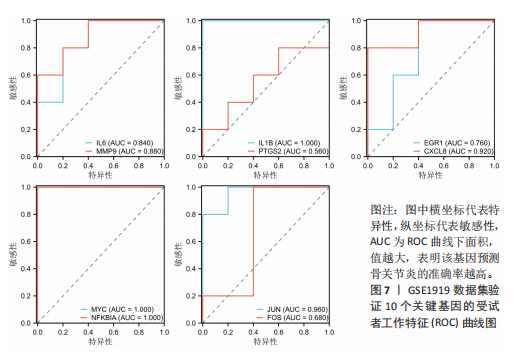
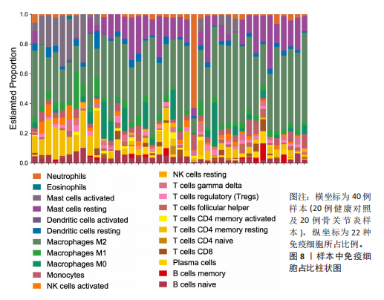
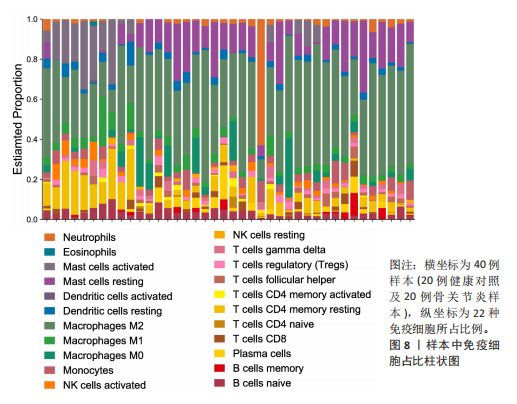
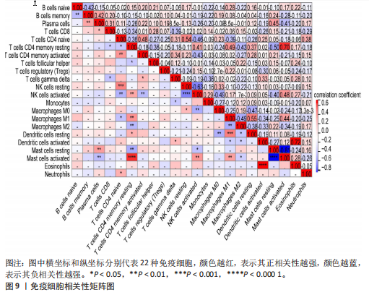
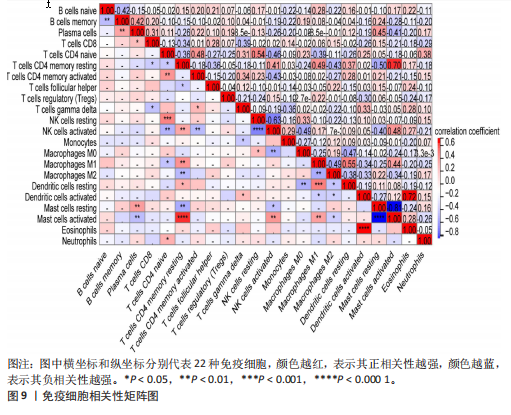
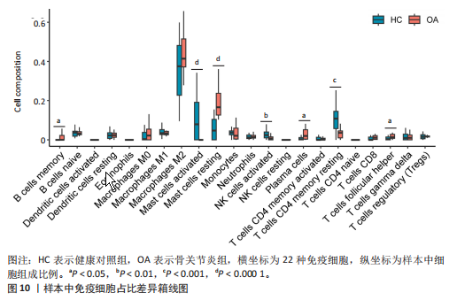
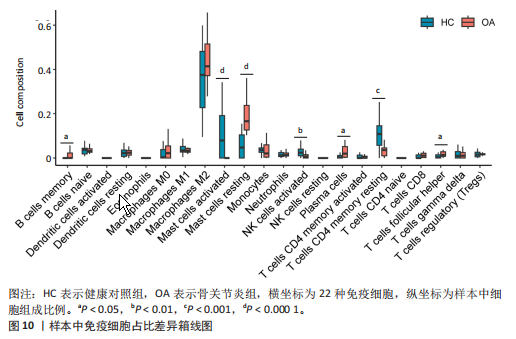
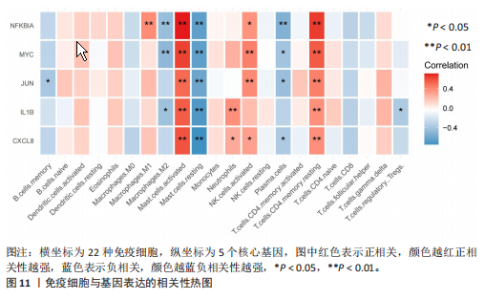
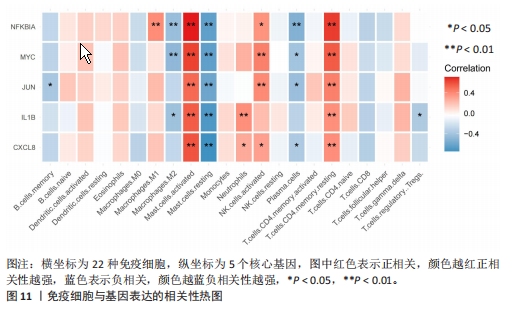
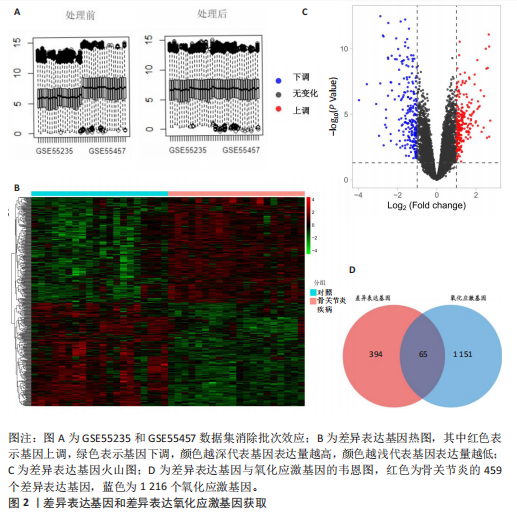
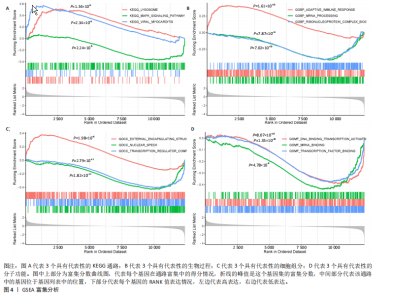
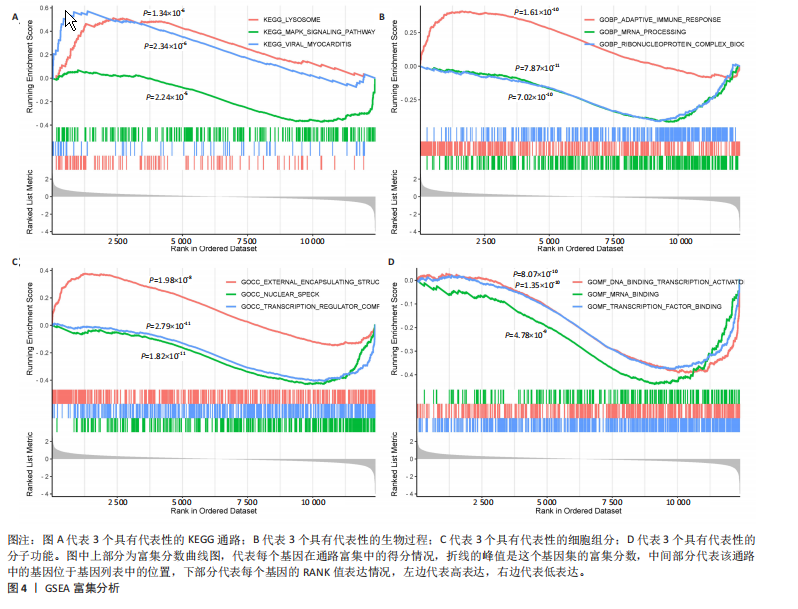
2.1 差异表达基因和差异表达氧化应激基因获取 对GSE55235和GSE55457数据集进行去除批次效应处理(图2A),成功筛选出459个差异表达基因,其中227个上调基因,232个下调基因,并用热图及火山图对差异表达基因进行可视化(图2B,C)。此外,根据Relevance score≥7,获取到了1 216个氧化应激基因,通过取交集得到65个差异表达氧化应激基因(图2D),其中19个上调基因,46个下调基因。 2.2 富集分析结果 为了确定差异表达氧化应激基因的生物学功能,进行KEGG和GO富集分析,KEGG分析发现(图3A),差异表达氧化应激基因在信号通路方面表现出显著的富集,包括白细胞介素17、破骨细胞分化、流体剪切应力和动脉粥样硬化、肿瘤坏死因子等信号通路;GO分析发现(图3B-D),差异表达氧化应激基因在对氧化应激、脂多糖、细菌来源分子、皮质类固醇、糖皮质激素的反应和老化等生物学过程方面呈现富集现象。它们还在核常染色质、常染色质、细胞器外膜等细胞学组分中高度存在。此外,它们还具有细胞因子、信号受体激活剂、DNA结合转录因子等多种分子功能。为了进一步探索与差异表达氧化应激相关的生物学途径,GSEA分析发现主要富集在溶酶体的自噬、MAPK信号通路、免疫反应等途径(图4)。 2.3 蛋白互作网络构建与关键基因的确定 将差异表达氧化应激基因导入STRING,过滤得到蛋白互作网络(图5A),由60个节点和453条边组成;使用Cytoscape软件中CytoHubba插件的Degree算法得到前10名为关键基因(图5B),包括IL6、MMP9、IL1B、PTGS2、EGR1、CXCL8、MYC、NFKBIA、JUN、FOS;将蛋白互作网络中的关键模块进行分析,采用MCODE插件,选取分数最高(Score=18.815)的模块(图5C),该模块由28个节点和254条边组成,模块中心部分由IL1B、CXCL8、MYC、NFKBIA、JUN等组成。 2.4 核心基因的鉴定 使用验证数据集GSE1919对关键基因进行差异分析及ROC曲线分析,设置P < 0.05;分析得到豆荚图(图6)及ROC曲线图(图7);具有差异性的基因(P < 0.05)包括IL1B、CXCL8、MYC、NFKBIA、JUN;其中IL1B、MYC和NFKBIA的 ROC曲线下面积为1.00,说明其预测骨关节炎的准确性接近完美,CXCL8和JUN的ROC曲线下面积皆 > 0.85,说明其预测骨关节炎的准确率也非常高。综合分析得到核心基因为IL1B、CXCL8、MYC、NFKBIA、JUN,显示它们对骨关节炎的敏感性和差异性显著,具备诊断骨关节炎的能力。 2.5 免疫浸润分析 设置P < 0.05,采用CIBERSORT对包含20例骨关节炎患者和20例健康对照样本的实验数据集进行免疫细胞组成分析,图8呈现出每个样本中22种免疫细胞的比例,通过柱状图展示。图9中的CIBERSORT分析结果显示,嗜酸性粒细胞与活化树突状细胞表现出最强的正相关性,而活化肥大细胞和静息肥大细胞则表现出最强的负相关性。同时,免疫细胞箱线图显示(图10):健康对照样本中的活化肥大细胞、活化NK细胞和静息记忆CD4 T细胞的数量相对较高,而骨关节炎患者的静息肥大细胞、记忆B细胞、浆细胞和滤泡辅助T细胞的数量相对较高。这些免疫细胞可能与骨关节炎的发展息息相关。 2.6 核心基因与免疫细胞的相关性 基于P < 0.05,探究核心基因(IL1B、CXCL8、MYC、NFKBIA、JUN)与免疫细胞的相关性。根据结果展示(图11),IL1B与活化肥大细胞、中性粒细胞、静息记忆CD4 T细胞之间存在正相关,而与巨噬细胞M2、静息肥大细胞、调节性T细胞之间呈现负相关关系。CXCL8与活化肥大细胞、活化NK细胞、静息记忆CD4 T细胞、中性粒细胞之间呈现正相关关系,然而与静息肥大细胞、浆细胞之间则呈现负相关关系。同样地,MYC与活化肥大细胞、活化NK细胞、静息记忆CD4 T细胞之间存在正相关,而与巨噬细胞M2、静息肥大细胞、浆细胞之间则存在负相关。此外,NFKBIA与巨噬细"
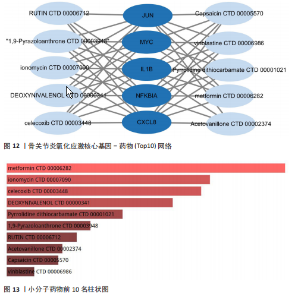
胞M1、活化肥大细胞、活化NK细胞、静息记忆CD4 T细胞之间存在正相关,然而与巨噬细胞M2、静息肥大细胞、浆细胞之间则存在负相关。进一步分析发现,JUN与静息记忆CD4 T细胞、活化NK细胞、活化肥大细胞之间存在正相关,然而与浆细胞、记忆B细胞、静息肥大细胞之间则存在负相关。 2.7 miRNA及小分子药物预测 通过TargetScan数据库,对核心基因进行miRNA预测,设置P < 0.05,得到hsa-miR-3937。从DSigDB数据库中获得了约1 400种小分子药物,按照P值筛选出前10名小分子药物,依次为二甲双胍(metformin)、离子霉素(ionomycin)、塞来昔布(celecoxib)、呕吐毒素(DEOXYNIVALENOL)、吡咯烷二硫代甲酸铵(Pyrrolidine dithiocarbamate)、 吡唑蒽酮(1,9-Pyrazoloanthrone)、芸香苷(RUTIN)、香草乙酮(Acetovanillone)、 辣椒素(Capsaicin)和长春花碱 (vinblastine),与核心基因相关性见图12,柱状图见图13,详细信息见表1。 "
| [1] HASEEB A, HAQQI TM. Immunopathogenesis of osteoarthritis. Clin Immunol. 2013; 146(3):185-196. [2] MOLNAR V, MATIŠIĆ V, KODVANJ I, et al. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int J Mol Sci. 2021;22(17):9208. [3] SIDDIQ MAB, CLEGG D, JANSEN TL, et al. Emerging and New Treatment Options for Knee Osteoarthritis. Curr Rheumatol Rev. 2022;18(1):20-32. [4] ZHU X, SANG L, WU D, et al. Effectiveness and safety of glucosamine and chondroitin for the treatment of osteoarthritis: a meta-analysis of randomized controlled trials. J Orthop Surg Res. 2018;13(1):170. [5] MARTEL-PELLETIER J, BARR AJ, CICUTTINI FM, et al. Osteoarthritis. Nat Rev Dis Primers. 2016;2:16072. [6] ASTRIKE-DAVIS EM, CORYELL P, LOESER RF. Targeting cellular senescence as a novel treatment for osteoarthritis. Curr Opin Pharmacol. 2022;64:102213. [7] FOIS AG, PALIOGIANNIS P, SOTGIA S, et al. Evaluation of oxidative stress biomarkers in idiopathic pulmonary fibrosis and therapeutic applications: a systematic review. Respir Res. 2018;19(1):51. [8] DAENEN K, ANDRIES A, MEKAHLI D, et al. Oxidative stress in chronic kidney disease. Pediatr Nephrol. 2019;34(6):975-991. [9] LIU L, LUO P, YANG M, et al. The role of oxidative stress in the development of knee osteoarthritis: A comprehensive research review. Front Mol Biosci. 2022;9:1001212. [10] BLANCO FJ, VALDES AM, REGO-PÉREZ I. Mitochondrial DNA variation and the pathogenesis of osteoarthritis phenotypes. Nat Rev Rheumatol. 2018;14(6):327-340. [11] RAJAGOPALAN S, MENG XP, RAMASAMY S, et al. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest. 1996;98(11):2572-2579. [12] PETERSEN SV, OURY TD, OSTERGAARD L, et al. Extracellular superoxide dismutase (EC-SOD) binds to type i collagen and protects against oxidative fragmentation. J Biol Chem. 2004;279(14):13705-13710. [13] SCOTT JL, GABRIELIDES C, DAVIDSON RK, et al. Superoxide dismutase downregulation in osteoarthritis progression and end-stage disease. Ann Rheum Dis. 2010;69(8):1502-1510. [14] NEDUNCHEZHIYAN U, VARUGHESE I, SUN AR, et al. Obesity, Inflammation, and Immune System in Osteoarthritis. Front Immunol. 2022;13:907750. [15] LINDBLAD S, HEDFORS E. Arthroscopic and immunohistologic characterization of knee joint synovitis in osteoarthritis. Arthritis Rheum. 1987;30(10):1081-1088. [16] SAITO I, KOSHINO T, NAKASHIMA K, et al. Increased cellular infiltrate in inflammatory synovia of osteoarthritic knees. Osteoarthritis Cartilage. 2002;10(2): 156-162. [17] LUAN T, YANG X, KUANG G, et al. Identification and Analysis of Neutrophil Extracellular Trap-Related Genes in Osteoarthritis by Bioinformatics and Experimental Verification. J Inflamm Res. 2023;16:3837-3852. [18] DUAN ZX, LI YS, TU C, et al. Identification of a potential gene target for osteoarthritis based on bioinformatics analyses. J Orthop Surg Res. 2020;15(1):228. [19] 徐义峰,柯诗文,李可可,等.氧化应激和免疫浸润在特发性肺纤维化中的作用及中药防治研究[J/OL].中国免疫学杂志,1-10[2024-02-16]. https://link.cnki.net/urlid/22.1126.R.20231208.1514.004 [20] LIBERZON A, BIRGER C, THORVALDSDÓTTIR H, et al. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1(6):417-425. [21] GU X, LAI D, LIU S, et al. Hub Genes, Diagnostic Model, and Predicted Drugs Related to Iron Metabolism in Alzheimer’s Disease. Front Aging Neurosci. 2022;14:949083. [22] KULESHOV MV, JONES MR, ROUILLARD AD, et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44(W1):W90-97. [23] AGARWAL V, BELL GW, NAM JW, et al. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4: e05005. [24] YOO M, SHIN J, KIM J, et al. DSigDB: drug signatures database for gene set analysis. Bioinformatics. 2015;31(18):3069-3071. [25] YUNUS MHM, NORDIN A, KAMAL H. Pathophysiological Perspective of Osteoarthritis. Medicina (Kaunas). 2020; 56(11):614. [26] SACITHARAN PK. Ageing and Osteoarthritis. Subcell Biochem. 2019;91:123-159. [27] HARMAN D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298-300. [28] BOLDUC JA, COLLINS JA, LOESER RF. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic Biol Med. 2019;132:73-82. [29] KIMBALL JS, JOHNSON JP, CARLSON DA. Oxidative Stress and Osteoporosis. J Bone Joint Surg Am. 2021;103(15):1451-1461. [30] KAPOOR M, MARTEL-PELLETIER J, LAJEUNESSE D, et al. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33-42. [31] STANNUS O, JONES G, CICUTTINI F, et al. Circulating levels of IL-6 and TNF-α are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthritis Cartilage. 2010;18(11):1441-1447. [32] GREENE MA, LOESER RF. Aging-related inflammation in osteoarthritis. Osteoarthritis Cartilage. 2015;23(11): 1966-1971. [33] XIAO J, ZHANG P, CAI FL, et al. IL-17 in osteoarthritis: A narrative review. Open Life Sci. 2023;18(1):20220747. [34] ROBERT M, MIOSSEC P. IL-17 in Rheumatoid Arthritis and Precision Medicine: From Synovitis Expression to Circulating Bioactive Levels. Front Med (Lausanne). 2019;5:364. [35] TSUKAZAKI H, KAITO T. The Role of the IL-23/IL-17 Pathway in the Pathogenesis of Spondyloarthritis. Int J Mol Sci. 2020; 21(17):6401. [36] BLAUVELT A, CHIRICOZZI A. The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis. Clin Rev Allergy Immunol. 2018;55(3):379-390. [37] ZHANG X, YUAN Y, PAN Z, et al. Elevated circulating IL-17 level is associated with inflammatory arthritis and disease activity: A meta-analysis. Clin Chim Acta. 2019;496: 76-83. [38] MIMPEN JY, BALDWIN MJ, CRIBBS AP, et al. Interleukin-17A Causes Osteoarthritis-Like Transcriptional Changes in Human Osteoarthritis-Derived Chondrocytes and Synovial Fibroblasts In Vitro. Front Immunol. 2021;12:676173. [39] WANG K, XU J, CAI J, et al. Serum levels of resistin and interleukin-17 are associated with increased cartilage defects and bone marrow lesions in patients with knee osteoarthritis. Mod Rheumatol. 2017; 27(2):339-344. [40] 胡光亮,马振华,徐迈,等.骨关节炎患者TNF-α、IL-17、COMP、ADAMTS-7及mRNA表达水平研究[J].中国医学前沿杂志(电子版),2018,10(12):100-103. [41] XIN R, XU Y, LONG D, et al. Mitochonic Acid-5 Inhibits Reactive Oxygen Species Production and Improves Human Chondrocyte Survival by Upregulating SIRT3-Mediated, Parkin-dependent Mitophagy. Front Pharmacol. 2022;13:911716. [42] BENT R, MOLL L, GRABBE S, et al. Interleukin-1 Beta-A Friend or Foe in Malignancies? Int J Mol Sci. 2018;19(8): 2155. [43] DI FRANCESCO M, FRAGASSI A, PANNUZZO M, et al. Management of osteoarthritis: From drug molecules to nano/micromedicines. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022; 14(3):e1780. [44] GONG Z, WANG Y, LI L, et al. Cardamonin alleviates chondrocytes inflammation and cartilage degradation of osteoarthritis by inhibiting ferroptosis via p53 pathway. Food Chem Toxicol. 2023;174:113644. [45] XU W, ZHANG B, XI C, et al. Ferroptosis Plays a Role in Human Chondrocyte of Osteoarthritis Induced by IL-1β In Vitro. Cartilage. 2023;14(4):455-466. [46] LIU L, ZHANG W, LIU T, et al. The physiological metabolite α-ketoglutarate ameliorates osteoarthritis by regulating mitophagy and oxidative stress. Redox Biol. 2023;62:102663. [47] RUSSO RC, GARCIA CC, TEIXEIRA MM, et al. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev Clin Immunol. 2014;10(5):593-619. [48] BAGGIOLINI M. CXCL8 - The First Chemokine. Front Immunol. 2015;6:285. [49] CAMBIER S, GOUWY M, PROOST P. The chemokines CXCL8 and CXCL12: molecular and functional properties, role in disease and efforts towards pharmacological intervention. Cell Mol Immunol. 2023; 20(3):217-251. [50] YANG P, TAN J, YUAN Z, et al. Expression profile of cytokines and chemokines in osteoarthritis patients: Proinflammatory roles for CXCL8 and CXCL11 to chondrocytes. Int Immunopharmacol. 2016;40:16-23. [51] 杨鹏.骨关节炎患者细胞因子/趋化因子表达谱分析以及CXCL8和CXCL11对软骨细胞的调节作用[D].西安:第四军医大学,2018. [52] GE X, SHI R, MA X. The secreted protein WNT5A regulates condylar chondrocyte proliferation, hypertrophy and migration. Arch Oral Biol. 2017;82:171-179. [53] WU YH, LIU W, ZHANG L, et al. Effects of microRNA-24 targeting C-myc on apoptosis, proliferation, and cytokine expressions in chondrocytes of rats with osteoarthritis via MAPK signaling pathway. J Cell Biochem. 2018;119(10):7944-7958. [54] ZOU J, LI XL, SHI ZM, et al. Effects of C-myc gene silencing on interleukin-1β-induced rat chondrocyte cell proliferation, apoptosis and cytokine expression. J Bone Miner Metab. 2018;36(3):286-296. [55] TANG H, CHENG Z, MA W, et al. TLR10 and NFKBIA contributed to the risk of hip osteoarthritis: systematic evaluation based on Han Chinese population. Sci Rep. 2018;8(1):10243. [56] CAI P, JIANG T, LI B, et al. Comparison of rheumatoid arthritis (RA) and osteoarthritis (OA) based on microarray profiles of human joint fibroblast-like synoviocytes. Cell Biochem Funct. 2019;37(1):31-41. [57] LU H, HOU G, ZHANG Y, et al. c-Jun transactivates Puma gene expression to promote osteoarthritis. Mol Med Rep. 2014;9(5):1606-1612. [58] YE Z, CHEN Y, ZHANG R, et al. c-Jun N-terminal kinase - c-Jun pathway transactivates Bim to promote osteoarthritis. Can J Physiol Pharmacol. 2014;92(2):132-139. [59] DE LANGE-BROKAAR BJ, KLOPPENBURG M, ANDERSEN SN, et al. Characterization of synovial mast cells in knee osteoarthritis: association with clinical parameters. Osteoarthritis Cartilage. 2016;24(4):664-671. [60] FARINELLI L, AQUILI A, MATTIOLI-BELMONTE M, et al. Synovial mast cells from knee and hip osteoarthritis: histological study and clinical correlations. J Exp Orthop. 2022;9(1):13. [61] KULKARNI P, HARSULKAR A, MÄRTSON AG, et al. Mast Cells Differentiated in Synovial Fluid and Resident in Osteophytes Exalt the Inflammatory Pathology of Osteoarthritis. Int J Mol Sci. 2022;23(1):541. [62] DOSS F, MENARD J, HAUSCHILD M, et al. Elevated IL-6 levels in the synovial fluid of osteoarthritis patients stem from plasma cells. Scand J Rheumatol. 2007;36(2): 136-139. [63] QIAO D, GU C, WANG W, et al. Tumor-Originated Exosomal hsa-miR-3937 as a Minimally Invasive Early Biomarker for Liquid Biopsy of Colorectal Cancer. J Oncol. 2022;2022:6990955. [64] SANCHEZ-RANGEL E, INZUCCHI SE. Metformin: clinical use in type 2 diabetes. Diabetologia. 2017;60(9):1586-1593. [65] HE M, LU B, OPOKU M, et al. Metformin Prevents or Delays the Development and Progression of Osteoarthritis: New Insight and Mechanism of Action. Cells. 2022;11(19):3012. [66] TERKELTAUB R, YANG B, LOTZ M, et al. Chondrocyte AMP-activated protein kinase activity suppresses matrix degradation responses to proinflammatory cytokines interleukin-1β and tumor necrosis factor α. Arthritis Rheum. 2011;63(7):1928-1937. [67] SONG Y, WU Z, ZHAO P. The effects of metformin in the treatment of osteoarthritis: Current perspectives. Front Pharmacol. 2022;13:952560. [68] PARK MJ, MOON SJ, BAEK JA, et al Metformin Augments Anti-Inflammatory and Chondroprotective Properties of Mesenchymal Stem Cells in Experimental Osteoarthritis. J Immunol. 2019;203(1): 127-136. [69] WANG C, YAO Z, ZHANG Y, et al. Metformin Mitigates Cartilage Degradation by Activating AMPK/SIRT1-Mediated Autophagy in a Mouse Osteoarthritis Model. Front Pharmacol. 2020;11:1114. [70] NA HS, KWON JY, LEE SY, et al. Metformin Attenuates Monosodium-Iodoacetate-Induced Osteoarthritis via Regulation of Pain Mediators and the Autophagy-Lysosomal Pathway. Cells. 2021;10(3):681. [71] SCHADLER P, LOHBERGER B, STÜNDL N, et al. The Effect of Body Mass Index and Metformin on Matrix Gene Expression in Arthritic Primary Human Chondrocytes. Cartilage. 2021;13(2_suppl):1004S-1018S. [72] BENIGNI G, DIMITROVA P, ANTONANGELI F, et al. CXCR3/CXCL10 Axis Regulates Neutrophil-NK Cell Cross-Talk Determining the Severity of Experimental Osteoarthritis. J Immunol. 2017;198(5):2115-2124. [73] ROMERA-CÁRDENAS G, THOMAS LM, LOPEZ-COBO S, et al. Ionomycin Treatment Renders NK Cells Hyporesponsive. PLoS One. 2016;11(3):e0150998. [74] GREGORI D, GIACOVELLI G, MINTO C, et al. Association of Pharmacological Treatments With Long-term Pain Control in Patients With Knee Osteoarthritis: A Systematic Review and Meta-analysis. JAMA. 2018; 320(24):2564-2579. |
| [1] | Ma Chi, Wang Ning, Chen Yong, Wei Zhihan, Liu Fengji, Piao Chengzhe. Application of 3D-printing patient-specific instruments combined with customized locking plate in opening wedge high tibial osteotomy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1863-1869. |
| [2] | Yu Shuai, Liu Jiawei, Zhu Bin, Pan Tan, Li Xinglong, Sun Guangfeng, Yu Haiyang, Ding Ya, Wang Hongliang. Hot issues and application prospects of small molecule drugs in treatment of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1913-1922. |
| [3] | Zhao Jiyu, Wang Shaowei. Forkhead box transcription factor O1 signaling pathway in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1923-1930. |
| [4] | Sun Yundi, Cheng Lulu, Wan Haili, Chang Ying, Xiong Wenjuan, Xia Yuan. Effect of neuromuscular exercise for knee osteoarthritis pain and function: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1945-1952. |
| [5] | Deng Keqi, Li Guangdi, Goswami Ashutosh, Liu Xingyu, He Xiaoyong. Screening and validation of Hub genes for iron overload in osteoarthritis based on bioinformatics [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1972-1980. |
| [6] | Liu Lin, Liu Shixuan, Lu Xinyue, Wang Kan. Metabolomic analysis of urine in a rat model of chronic myofascial trigger points [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1585-1592. |
| [7] | Wang Qiuyue, Jin Pan, Pu Rui . Exercise intervention and the role of pyroptosis in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1667-1675. |
| [8] | Zhao Jiacheng, Ren Shiqi, Zhu Qin, Liu Jiajia, Zhu Xiang, Yang Yang. Bioinformatics analysis of potential biomarkers for primary osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1741-1750. |
| [9] | Chen Yueping, Chen Feng, Peng Qinglin, Chen Huiyi, Dong Panfeng . Based on UHPLC-QE-MS, network pharmacology, and molecular dynamics simulation to explore the mechanism of Panax notoginseng in treating osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1751-1760. |
| [10] | Yin Lu, Jiang Chuanfeng, Chen Junjie, Yi Ming, Wang Zihe, Shi Houyin, Wang Guoyou, Shen Huarui. Effect of Complanatoside A on the apoptosis of articular chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1541-1547. |
| [11] | Wang Peiguang, Zhang Xiaowen, Mai Meisi, Li Luqian, Huang Hao. Generalized equation estimation of the therapeutic effect of floating needle therapy combined with acupoint embedding on different stages of human knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1565-1571. |
| [12] | De Ji, Suo Langda, Wei Yuchen, Wang Bin, Awangcuoji, Renqingcuomu, Cui Jiuzeng, Zhang Lei, Ba Gui. Comprehensive analysis of genes related to endometrial receptivity and alternative splicing events in northwest Tibetan cashmere goats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1429-1436. |
| [13] | Zhang Haojun, Li Hongyi, Zhang Hui, Chen Haoran, Zhang Lizhong, Geng Jie, Hou Chuandong, Yu Qi, He Peifeng, Jia Jinpeng, Lu Xuechun. Identification and drug sensitivity analysis of key molecular markers in mesenchymal cell-derived osteosarcoma [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1448-1456. |
| [14] | Weng Zongqin, Zhao Hailong. Mechanism of exosomal miRNA involved in tumor chemotherapy resistance [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1504-1511. |
| [15] | Cao Yue, Ye Xinjian, Li Biyao, Zhang Yining, Feng Jianying. Effect of extracellular vesicles for diagnosis and therapy of oral squamous cell carcinoma [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1523-1530. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||