Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (1): 175-184.doi: 10.12307/2024.728
Previous Articles Next Articles
Long non-coding RNA directly or indirectly affects osteoporosis through p38MAPK signaling pathway
Qin Hao1, Kang Teng1, Liu Gang2
- 1Clinical College of Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China; 2Department of Orthopedics, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China
-
Received:2023-10-17Accepted:2023-11-21Online:2025-01-08Published:2024-05-20 -
Contact:Liu Gang, MD, Chief physician, Department of Orthopedics, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China -
About author:Qin Hao, Master candidate, Clinical College of Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China -
Supported by:2022 Doctoral Research Starting Fund of Affiliated Hospital of Guizhou Medical University, No. gyfybsky-2022-43 (to LG)
CLC Number:
Cite this article
Qin Hao, Kang Teng, Liu Gang. Long non-coding RNA directly or indirectly affects osteoporosis through p38MAPK signaling pathway[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(1): 175-184.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
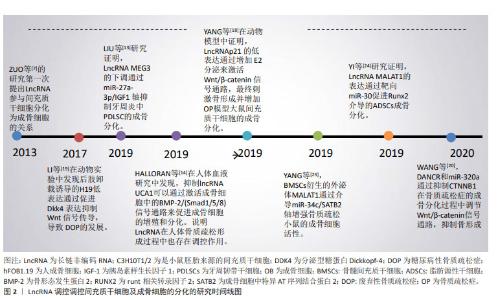
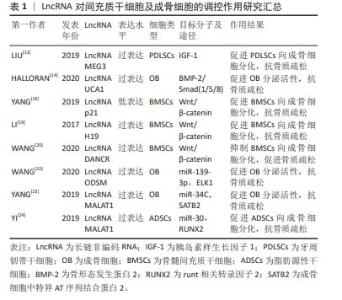
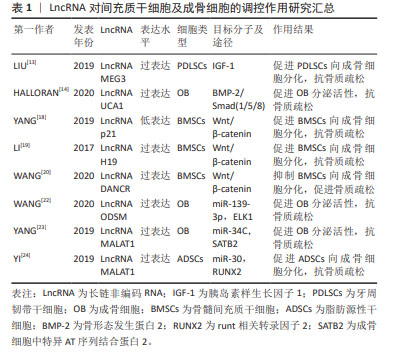
2.1 LncRNA调控间充质干细胞及成骨细胞分化参与骨质疏松症的发生和发展 2.1.1 LncRNA调控骨髓间充质干细胞及成骨细胞在骨质疏松症中的作用 间充质干细胞是具有分化成各种细胞的潜在能力和自我更新能力且类似于成纤维细胞的干细胞,包括骨髓间充质干细胞、脂肪间充质干细胞及胚胎干细胞等,可用于组织生物技术和再生医学的研究。间充质干细胞在特定的诱导条件及微环境下可分化为成骨细胞、脂肪细胞和软骨细胞等。近年来,大量的研究表明LncRNA可在转录水平、选择性剪接和翻译后调控等多个水平调控基因表达,在促进间充质干细胞向成骨细胞分化以及成骨细胞细胞的增殖、分化和凋亡中都发挥重要的作用[11-24],部分研究成果见图2及表1。 "
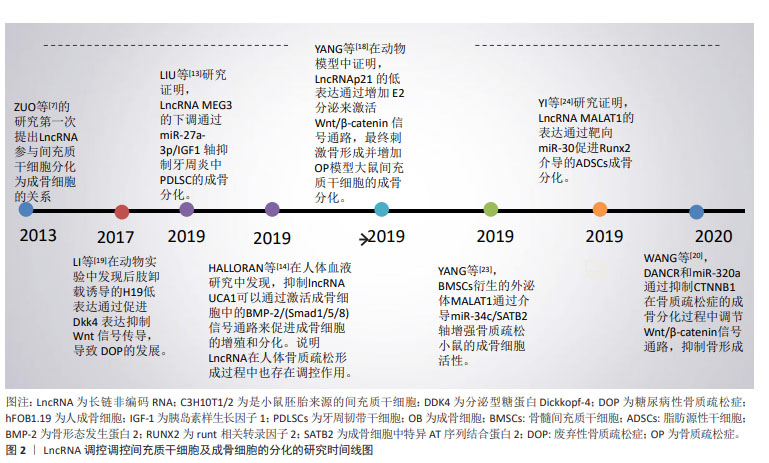
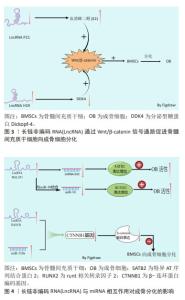
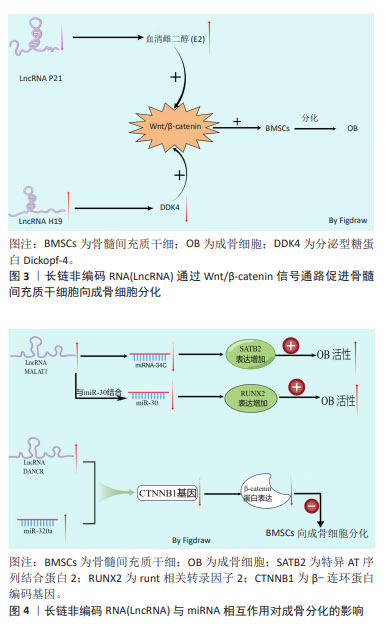
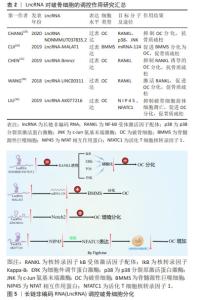
成骨细胞主要由内外骨膜和间充质干细胞分化而来,成骨细胞是骨形成和重建的主要功能细胞,负责骨基质的合成、分泌和矿化[11]。成骨细胞的减少和骨质疏松症的发生密切相关,因此,促进间充质干细胞向成骨细胞分化可有效阻止骨质疏松症的进展。骨髓间充质干细胞具有多能性,而细胞多能性的维持或多能细胞向其他细胞进行分化的过程都需要通过表观遗传、转录和转录后调控等来协调成百上千的基因的表达[12]。 近年来研究证实,LncRNA 已成为间充质干细胞成骨过程中有前途的新型调节因子,其可以介导众多细胞因子和骨代谢通路参与调控间充质干细胞的增殖和分化。胰岛素样生长因子1(insulin-like growth factor-1,IGF-1)是一种功能和结构与胰岛素相似的多功能肽,参与骨髓间充质干细胞的增殖、生长和成骨分化。过表达长链非编码RNA母系表达基因3(maternally expressed gene 3,MEG3)可通过增强胰岛素样生长因子1的表达,从而促进牙周韧带干细胞向成骨细胞分化[13]。促进经典的成骨信号通路的表达,是促进成骨细胞分化的主要途径。骨形态发生蛋白(bone morphogenetic protein,BMP)是转化生长因子β(transforming growth factor-β,TGF-β)超家族的多功能生长因子中的一员,BMP与其直接下游信号分子Smad1/5/8组成BMP/Smads信号通路,在骨形成过程中发挥重要作用[14]。ZHANG等[15]研究发现,LncRNA尿路上皮癌相关1(urothelial carcinoma associated 1 ,UCA1)可通过激活BMP-2/Smad (1/5/8)信号通路,来促进成骨细胞的增殖和分化。Wnt/β-catenin信号通路是调节骨稳态的关键信号通路之一,通过促进骨的形成来控制骨量[16-17]。该信号通路通过促进成骨细胞的分化和成骨细胞的活性等多个方面调节骨量,该信号通路可促进骨髓间充质干细胞向成骨细胞分化,促进骨生成,进而延缓骨质疏松症的进展。 雌激素是治疗和预防骨质疏松症的主要药物之一,雌激素可通过抑制破骨细胞活性,减少骨吸收,以及通过激活Wnt/β-catenin信号通路促进成骨细胞分化等来抑制骨质疏松症的进展。使用LncRNA p21抑制剂导致LncRNA p21的低表达可通过增加血清雌二醇(E2)分泌,从而激活Wnt/β-catenin信号通路,刺激骨的形成以及促进骨髓间充质干细胞向成骨细胞分化[18]。分泌型糖蛋白Dickkopf-4(DDK4)作为骨质疏松的重要生物标志,是Wnt信号通路的拮抗剂,上调DDK4的表达可通过抑制Wnt信号通路,进而抑制细胞的成骨分化功能。LncRNA H19可靶向调控DDK4,解除DDK4对Wnt/β-catenin信号通路的抑制作用而促进成骨分化[19]。编码β-连环蛋白(β-catenin)的基因CTNNB1的异常表达是Wnt信号通路异常改变的常见原因。WANG等[20]的研究发现,过表达LncRNA 分化拮抗非编码 RNA(differentiation antagonizing non-coding RNA,DANCR)会导致β-连环蛋白编码基因CTNNB1的低表达,抑制Wnt/β-catenin信号通路,进而抑制骨髓间充质干细胞向成骨细胞的分化,促进骨质疏松症的发展。 MicroRNAs(miRNAs)作为一种18-24个核苷酸的高度保守的小分子RNA,已被证实可反向调控靶基因的转录或翻译,与细胞分化、生长、迁移和肿瘤侵袭等多种生物学有关[21]。LncRNA在参与转录后基因调控过程中,除可直接调节成骨信号通路外,LncRNA还可作为竞争性内源性RNA(competing endogenous RNAs,ceRNA)与miRNA结合,通过上调miRNA的靶基因的表达来削弱miRNA的活性,解除miRNA对成骨信号通路中相应mRNA表达的抑制作用,进而促进成骨信号通路蛋白的表达,促进成骨。有研究表明,LncRNA成骨细胞分化相关LncRNA(the Long noncoding RNA osteoblast differentiation-related LncRNA under simulated microgravity,LncRNA ODSM)表达水平增加能通过与miR-139-3p的相互作用,调控其靶基因ELK1的表达,进而促进成骨细胞的分化和抑制成骨细胞的凋亡[22]。骨髓间充质干细胞来源的外泌体相关肺腺癌转录本1(metastasis-associated lung adenocarcinoma transcript 1,MALAT1)可通过介导miR-34C/SATB2轴增强骨质疏松小鼠的成骨细胞活性,促进成骨细胞中特异AT序列结合蛋白2(SATB2)的表达,从而促进成骨细胞增殖,增加成骨细胞中碱性磷酸酶(alkaline phosphatase,ALP)的活性和矿化结节的数量[23]。Runt相关转录因子2(Runt-related transcription factor 2,RUNX2)是一种特异性转录因子,在促进成骨过程中发挥重要作用。YI等[24]发现,miR-30作为LncRNA 相关MALAT1的潜在靶点,通过靶向作用于miR-30,促进RUNX2的表达而促进脂肪源性干细胞的成骨分化。由此可见,LncRNA可通过促进胰岛素样生长因子1、Wnt/β-catenin信号通路、BMP/Smads信号通路等影响成骨细胞分化,影响骨组织的生成,进而影响骨质疏松症的发生和发展。 LncRNA通过Wnt/β-catenin信号通路促进骨髓间充质干细胞向成骨细胞分化的机制见图3。LncRNA 与miRNA相互作用对成骨分化的影响机制,见图4。"
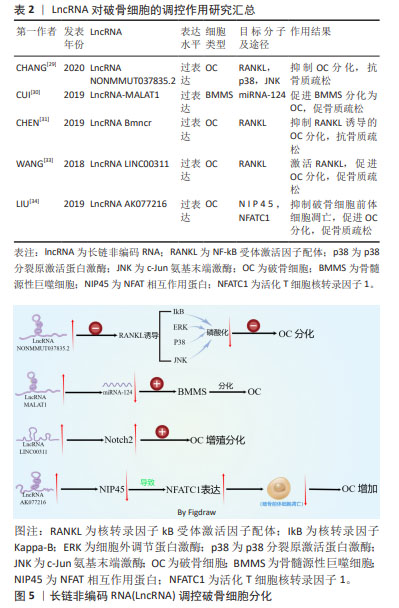
2.1.2 LncRNA调控破骨细胞在骨质疏松症中的作用 破骨细胞来自单核/巨噬细胞造血细胞系的巨大多核细胞,破骨细胞是骨吸收的主要功能细胞,在骨重塑、修复和维持骨稳态过程中发挥至关重要的作用[25]。 破骨细胞增多及其活性增强导致骨吸收过量是骨质疏松症发生的关键因素,抑制破骨细胞的分化和细胞活性可抑制骨质疏松症的进展。 破骨细胞的分化受多种细胞因子的调节,核转录因子kB受体激活因子配体(receptor activator of nuclear factor-κB ligand,RANKL)由成骨细胞系和骨髓基质细胞产生,通过与单核/巨噬细胞系的巨大多核细胞表面表达的核转录因子kB受体激活因子(RANK)结合,形成RANKL/RANK信号通路。该信号通路是破骨细胞增殖、分化和成熟的关键信号通路,可促进IkB激酶复合物(IKK)、磷脂酰肌醇-3激酶(PI3K)/AKt信号通路以及丝裂原活化激酶(mitogen-activated protein kinase,MAPKs)信号通路来调节破骨细胞的形成、活化及存活[26]。骨保护素(osteoprotegerin,OPG)或其相应的信号转导因子可组成RANKL/RANK/OPG信号通路,骨保护素通过与RANKL结合,以此来抑制RANKL/RANK信号通路,在调节骨的吸收和骨重塑过程中发挥重要作用[27]。LIU等[28]用RANKL和巨噬细胞集落刺激因子(macrophage colony stimulating factor,M-CSF)诱导单核巨噬细胞向破骨细胞的分化,与未分化细胞相比,破骨细胞中差异表达的LncRNA有1 117个,证实LncRNA在破骨细胞的分化过程中发挥重要作用。抑制RANKL信号通路,可有效抑制破骨细胞的分化。LncRNA NONMMUT037835.2过表达可抑制RANKL介导的P38分裂原激活蛋白激酶(p38MAPK)和c-Jun氨基末端激酶(c-jun N-terminal kinase,JNK)的磷酸化,还抑制破骨细胞分化后关键的破骨细胞特异性标志物的表达,从而抑制破骨细胞的分化[29]。过表达miRNA-124可抑制内皮祖细胞来源的外泌体诱导小鼠骨髓源性巨噬细胞的迁移和向破骨细胞分化,而LncRNA-MALAT1可负向调控miRNA-124的活性,促进内皮祖细胞来源的外泌体诱导小鼠骨髓源性巨噬细胞表达向破骨细胞分化相关的mRNA表达,进而促进小鼠骨髓源性巨噬细胞向破骨细胞分化[30]。CHEN等[31]研究表明,过表达LncRNA Bmncr可抑制RANKL诱导的破骨细胞分化过程。因此,抑制促进破骨细胞分化的LncRNA,破骨细胞分化后特异标志物也相应减少,破骨细胞的骨吸收能力受到抑制,可抑制骨质疏松症的进展。Notch信号通路在细胞分化和功能中发挥重要作用,Notch1抑制破骨细胞的生成,Notch2通过直接和间接机制促进破骨细胞的分化和功能,Notch3诱导成骨细胞和骨细胞表达RANKL,从而诱导破骨细胞分化[32]。LncRNA LINC00311过表达可使细胞中Notch2表达水平升高,诱导破骨细胞增殖,降低破骨细胞的凋亡率[33]。LncRNA AK077216过表达可抑制NFAT相互作用蛋白(NIP45)的表达,促进活化T细胞核转录因子1(activating T-cell nuclear factor 1 ,NFATC1)的表达,减少破骨细胞前体细胞的凋亡,促进破骨细胞的形成[34]。因此,抑制促进破骨细胞分化和活性的LncRNA或过表达抑制破骨细胞分化活性相关的LncRNA,可延缓骨质疏松症的进展。 LncRNA参与破骨细胞分化过程,见表2。LncRNA调控破骨细胞分化的机制,见图5。 "
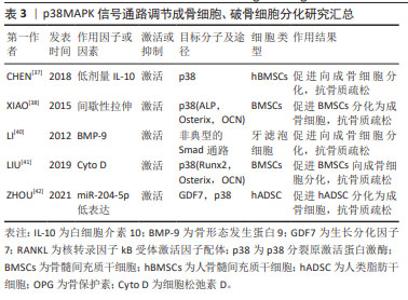
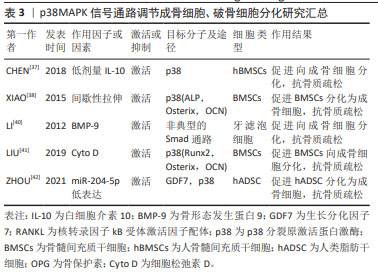
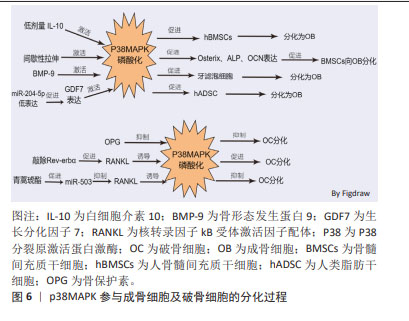
2.2 p38MAPK信号通路参与成骨细胞和破骨细胞分化在骨质疏松症中的作用 MAPKs是一种丝氨酸/苏氨酸蛋白激酶,能将信号转导到细胞质及细胞核。MAPKs的亚群主要包括细胞外信号调节激酶(extracellular regulating kinase,ERK)信号通路、JNK信号通路和p38信号通路[35]。p38MAPK作为MAPKs家族中的主要亚群之一,由MAPK14(p38α),MAPK11(p38β),MAPK12(p38γ)及MAPK13(p38δ)共4种异构体组成,是调节炎症、细胞分化、细胞生长和死亡过程中必不可少的调节因子[36]。p38信号通路参与成骨细胞和破骨细胞分化过程[37-42],见表3。 2.2.1 p38MAPK信号通路调节成骨细胞在骨质疏松症中的作用 已有许多研究证实,p38MAPK信号通路在促进骨髓间充质干细胞向成骨细胞分化过程中发挥重要作用。如CHEN等[37]发现,白细胞介素10通过激活p38MAPK信号通路在人骨髓间充质干细胞的成骨分化过程中具有调控作用。间歇性拉伸可通过p38MAPK通路促进小鼠骨髓间充质干细胞中成骨相关转录基因(Osterix),碱性磷酸酶、骨钙素的表达来促进小鼠骨髓间充质干细胞向成骨细胞分化[38]。以上研究表明,激活间充质干细胞中的p38MAPK信号通路可促进间充质干细胞分化为成骨细胞,进而促进骨生成,抑制骨质疏松症的进展。骨形态发生蛋白(bone morphogenetic protein,BMP)/Smads信号通路在骨骼发育、骨形成和骨稳态过程中对成骨细胞和破骨细胞的分化发挥重要作用。BMP/Smads的信号转导可特异的通过p38MAPK信号通路来促进成骨细胞的分化[39]。如BMP-9可通过非典型的Smad依赖信号通路,激活p38MAPK,促进牙滤泡细胞成骨分化[40]。此外,还有多种因素都可通过激活p38MAPK信号通路促进成骨,如细胞松弛素D(Cyto D)可上调RUNX2、骨钙素和成骨相关转录基因的表达,并激活p38MAPK信号通路促进骨髓间充质干细胞的成骨分化[41]。ZHOU等[42]的研究发现,过表达miR-204-5p可抑制人类脂肪干细胞成骨分化,而FOXC1可通过降低miR-204-5p的表达和促进生长分化因子7(GDF7)的表达来激活AKT和p38MAPK信号通路来诱导人脂肪干细胞的成骨分化。"
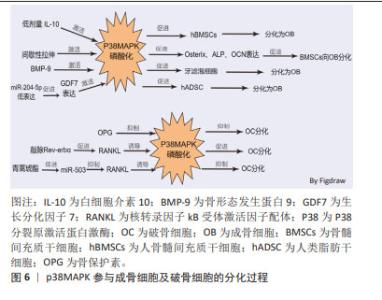
2.2.2 p38MAPK信号通路调节破骨细胞分化在骨质疏松症中的作用 p38MAPK、JNK和ERK1/2是破骨细胞发生过程中的重要因子。成骨细胞和骨髓基质细胞产生的RANKL与破骨前体细胞表达的RANK受体结合后,通过激活p38MAPK、JNK、ERK促进破骨细胞的分化。在M-CSF和RANKL刺激小鼠破骨前细胞向破骨细胞分化的过程中,p38MAPK、JNK和ERK的磷酸化水平增加,加入骨保护素竞争抑制RANKL与RANK受体结合后,p38MAPK、JNK和ERK磷酸化水平降低,即MAPK信号通路参与了M-CSF和RANKL诱导的破骨细胞的分化和活化,而骨保护素则通过抑制MAPK信号通路,进而抑制了破骨细胞的分化和活化[43-44]。核受体Reverbs,包括Rev-erbα和Rev-erbβ,在调节成骨细胞和破骨细胞分化中发挥关键作用,敲除Rev-erbα在破骨前体细胞中的表达可增强RANKL诱导p38MAPK和JNK的磷酸化,促进破骨细胞的形成[45]。微小RNA(miRNA)也可通过p38MAPK信号通路调控破骨细胞的分化过程。如青蒿琥酯可通过提高miR-503的表达来抑制RANKL诱导的p38MAPK的磷酸化而抑制破骨细胞的发生[46]。以上种种研究证明,p38MAPK信号通路在破骨细胞的发生过程中存在正向调控的作用,抑制破骨细胞分化路径的p38MAPK信号通路,可以延缓破骨细胞对骨组织的溶解吸收作用,延缓骨量的丢失,进而延缓骨质疏松症的进展。p38MAPK参与成骨细胞及破骨细胞的分化过程见图6。 "
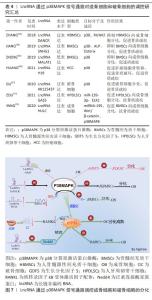
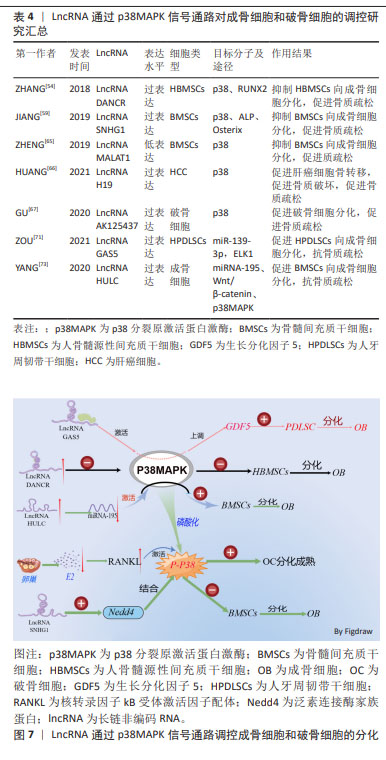
2.3 LncRNA通过调控p38MAPK信号通路影响骨质疏松症 骨形成是成骨细胞和破骨细胞之间的动态平衡过程[47]。体内成熟的成骨细胞不能分裂,只能辅助间充质干细胞不断增殖和成骨分化来增加成骨细胞的数量[48]。p38MAPK是一类丝氨酸/苏氨酸丝裂原活化蛋白激酶,作为细胞信号转导的枢纽,它们将细胞外信号与细胞内机制联系起来,以调节过多的细胞生物过程[49]。p38MAPK与JNK一起,被称为应激活化蛋白激酶 (stress-activated protein kinase,SAPK),被各种环境应激和细胞因子激活以诱导炎症,参与机体的炎症反应[50]。但是,大量的研究表明p38MAPK作用不仅限于炎症表达,p38MAPK信号通路参与细胞的周期调节、细胞死亡的诱导、分化和衰老。LncRNA以多种方式参与细胞生命周期活动,且可通过调节相应信号通路,影响细胞的生长、增殖及分化过程。LncRNA可通过调节p38MAPK信号通路,进而调控成骨细胞和破骨细胞分化过程,参与骨质疏松症的发生及发展。 p38激酶促进成骨的潜力与其磷酸化和增加一些关键成骨转录因子的活性能力有关。p38MAPK催化活性取决于活化环中磷酸化的苏氨酸或酪氨酸残基的数量。RUNX2是成骨细胞分化的必需转录因子,通过机械应力激活以促进成骨细胞功能[51]。例如,颅骨发育不良(CCD)是由RUNX2突变引起的[52]。p38MAPK对早期成骨具有重要意义,TGF-β活化激酶1(TAK1)是成骨细胞中p38上游的关键激活剂,而激活后的p38MAPK可促进Runx2在Ser31、Ser254和 Ser319.21处磷酸化,还增加RUNX2与p300/环AMP反应元件结合蛋白(CBP)的结合,导致Runx2转录活性增加,促进成骨细胞的分泌活性来促进骨形成[53]。 LncRNA参与控制炎症反应、细胞增殖、血管生成和组织重塑等过程,LncRNA可以通过调节染色质结构、转录、剪接、mRNA稳定性、mRNA可及性和翻译后改变来调节遗传信息。LncRNA可通过调控p38MAPK信号通路来增加成骨细胞的数量和抑制破骨细胞的形成,促进骨基质的形成,进而抑制或延缓骨质疏松症的发生。如ZHANG等[54]发现,在人骨髓源性间充质干细胞的成骨分化过程中,LncRNA分化拮抗非编码RNA (differentiation antagonizing non-coding RNA,DANCR)的表达水平显著降低,且还证实了LncRNA DANCR通过p38MAPK信号通路的失活来抑制Runx2基因的表达,进而抑制人骨髓源性间充质干细胞的增殖和成骨分化。 成骨细胞特异基因(Osterix,OSX)是另一类受p38MAPK调节的骨特异性转录因子。OSX的作用依赖于其调节许多成骨细胞标志物表达的能力,例如骨桥蛋白、骨钙素、Dkk1 和Ⅰ型胶原蛋白。OSX包含一个富含脯氨酸和丝氨酸的反式激活结构域,该结构域位于蛋白质的N末端部分,与Sp1/Kruppel转录因子家族相似的同源锌指结构[55]。OSX可以通过BMP依赖性方式被p38MAPK在Ser73和Ser77处激活和磷酸化,这是将OSX和p300/CBP/RNA聚合酶Ⅱ复合物募集到整合素结合唾液酸蛋白(IBSP)的启动子区域所必需的纤维调节蛋白。Osterix与靶基因启动子上的Sp1序列结合,且其被p38磷酸化可增强共激活因子的募集以形成转录活性复合物[56]。 泛素是一种由76个氨基酸组成的蛋白质,是泛素化的必要成分,泛素化是用泛素对蛋白质进行翻译后修饰,负责通过改变蛋白质活性、稳定性和定位来调节蛋白质特性,导致底物蛋白的单泛素链或多泛素链的形成[57]。泛素化是一种重要的调控机制,由泛素激活酶(E1)、泛素结合酶(E2)和泛素连接酶(E3)介导,该过程参与细胞周期、增殖、凋亡、分化、转移、基因表达、转录调节和炎症免疫等几乎所有生命活动的调节。Nedd4作为泛素连接酶家族的一员,在肿瘤、神经发育、T细胞功能以及癌症等多种疾病中都发挥重要作用[58]。 LncRNA小核仁RNA宿主基因1(small nucleolar RNA host gene 1,SNHG1)在骨质疏松小鼠中表达增高,LncRNA SNHG1可促进Nedd4与磷酸化的p38(p-p38)的相互作用,加速p-p38的降解,从而通过抑制碱性磷酸酶及Oserix的表达,抑制骨髓间充质干细胞的成骨分化,进而促进骨质疏松症的进展[59]。通过干扰或低表达抑制骨髓间充质干细胞向成骨细胞分化的相应LncRNA,可促进骨髓间充质干细胞中p38MAPK信号通路的激活,促进成骨相关的RUNX2、碱性磷酸酶及成骨细胞特异基因的表达,促进骨髓间充质干细胞向成骨细胞分化,促进骨生成而抑制骨质疏松症的进展。 p38MAPK信号通路也可促进破骨细胞的分化,导致破骨细胞骨吸收增加而导致骨质疏松症的发生。RANKL在破骨细胞的分化、功能及存活过程中发挥重要作用,RANKL通过与破骨细胞及其前体中的核转录因子kB受体激活剂RANK结合来发挥作用。结合 RANKL后,RANK激活6种主要信号通路:NFATc1、核转录因子kB,Akt/PKB,JNK,ERK和p38,这些通路在破骨细胞分化、功能和存活中发挥独特作用。前体破骨细胞和成熟破骨细胞中均表达p38MAPK,在小鼠来源的RAW264.7体外细胞实验中,证明了p38α参与破骨细胞生成的过程[60]。p38α在RANKL刺激后在RANK下游发挥作用,从而刺激破骨细胞的形成、成熟和骨吸收[61]。RANK在其细胞内结构域中缺乏内在的酶活性,需要通过募集衔接分子[如TNF受体相关因子(tumour necrosis factor receptor-associated factor,TRAF)蛋白家族]来转导信号。TRAF6可作为将RANK与破骨细胞分化和功能联系起来的主要衔接分子,RANKL与RANK结合诱导 TRAF6的三聚体化,从而导致核转录因子κB和MAPK信号转导的激活[62]。 p38MAPK通路通过磷酸化Ser65上的p536 核转录因子κB亚基从而增加核转录因子κB转录活性来促进RANKL诱导的破骨细胞形成。RANKL在很大程度上通过增加核转录因子κB转录活性和随后的破骨细胞特异性基因NFATC1表达来诱导破骨细胞生成[63]。p38MAPK信号通路是NFATC1活化的主要调节因子,抑制p38MAPK可抑制NFATC1的表达及扩增进而抑制破骨细胞的分化[64]。 LncRNA MALAT1在骨质疏松小鼠中低表达,LncRNA MALAT1的低表达可激活骨质疏松症相关的MAPK信号通路,抑制骨髓间充质干细胞的体外成骨分化,进而抑制骨的生成[65]。 骨是癌症转移的主要器官之一,癌症的骨转移伴随破骨细胞的过度激活,导致骨吸收增加,骨质破坏进而导致骨质疏松,容易导致骨质疏松性骨折的发生。HUANG等[66]的研究发现,过表达LncRNA H19通过与蛋白磷酸酶1催化亚基α (PPP1CA)结合,抑制骨保护素的表达,从而使p38去磷酸化,促进肝细胞癌的骨转移及破骨细胞生成,导致骨质破坏增加,进而出现骨质疏松。此外,该研究还发现,SB-203580 (Adezmapimod是一种选择性的、ATP竞争性的 p38MAPK 抑制剂)介导的p38MAPK失活逆转了体内H19敲低引起的HCC骨转移的下调。GPR173属于G蛋白偶联受体的一个亚家族,也称为脑中表达的超保守受体(SREB),GPR173的选择性激动剂phoenixin 20激活可诱导多种成骨细胞特征基因的表达,而p38MAPK抑制剂SB-203580抑制p38MAPK 信号通路后,会削弱phoenixin 20介导的 RUNX-2、碱性磷酸酶和基质矿化的诱导[67]。 动脉粥样硬化的主要原因是动脉钙化,这是由于血管平滑肌向成骨细胞表型转变所致。LncRNA H19过表达显著上调 p-p38和P-ERK1/2的表达,以Runx2依赖性方式通过MAPK通路促进β-甘油磷酸诱导的血管平滑肌钙化[68]。绝经期的女性在雌激素分泌不足的情况下,会刺激骨组织中分泌的RANKL与破骨前细胞表面的RANK受体结合,磷酸化p38MAPK,形成p-p38MAPK,从而激活破骨细胞分化相关的p38MAPK 信号通路,促使未分化成熟的破骨前细胞分化为成熟的破骨细胞,体内的破骨细胞增加,骨吸收能力增强,促进骨质疏松症的进展。LncRNA AK125437在绝经后的骨质疏松小鼠模型中高表达,并通过激活p38MAPK信号通路而促进破骨细胞分化,加速骨的吸收来影响绝经后骨质疏松大鼠的骨密度[69]。 TGF-β/BMP信号转导参与绝大多数细胞过程,并且在细胞生命活动周期中至关重要。TGF-β/BMPs的信号转导是通过典型的Smad依赖性通路(TGF-β/BMP配体、受体和Smads)和非典型的Smad非依赖性信号转导通路(例如P38丝裂原活化蛋白激酶通路)进行的,TGF-β/BMP 诱导后,Smad和P38 MAPK 通路均汇聚在Runx2基因上,以控制间充质前体细胞分化[70]。在典型的Smad依赖性通路中,TGF-β信号转导首先通过形成特异性Ⅰ型和Ⅱ型丝氨酸/苏氨酸激酶受体的异聚复合物在质膜上传递信号。Ⅰ型受体在特异性Ⅱ型受体激活后被磷酸化,激活的Ⅰ型受体通过磷酸化特定的Smad蛋白R-Smads来启动细胞内信号传导。激活的R-Smad与co-Smad和Smad4形成复合物,然后转位到细胞核中以直接转录反应[71]。 在非典型的Smad信号通路中,TGF-β信号转导通过选择性激活p38MAPK和Smad2/3通路,促使Smad和p38 MAPK通路都汇聚在Runx2基因上,以控制间充质前体细胞分化为成骨细胞[72]。生长分化因子5(growth differentiation factor 5,GDF5)作为BMP家族的一员,在组织分化以及骨修复中发挥重要作用,YANG等[73]发现,LncRNA 生长停滞特异性转录本5(growth arrest specific transcript 5,GAS5)可以通过激活p38和JNK信号通路来上调GDF5的水平,从而促进人牙周韧带干细胞的成骨分化。LncRNA除直接激活成骨细胞和破骨细胞分化的信号通路外,还可通过与微小RNA(miRNA)、环状RNA(cicrRNA)相互作用,促进成骨细胞和破骨细胞分化过程中相应mRNAs的表达参与骨的代谢调节过程[74]。过表达miRNA-195会抑制p38MAPK的磷酸化,抑制间充质干细胞的成骨分化,而LncRNA HULC的过表达可下调miRNA-195,增强了Wnt/β-catenin和p38MAPK信号通路的激活,促进骨髓间充质干细胞的增殖和成骨分化,促进骨形成,进而间接抑制骨质疏松症的发生、发展[75]。综上所述,相应LncRNA的过表达或低表达会通过p38MAPK信号通路来影响成骨细胞和破骨细胞的增殖或分化,调节骨重塑过程,进而影响骨质疏松症的发生、发展。这些研究结果表示,LncRNA和p38MAPK信号通路或许可以成为骨质疏松症治疗中的潜在应用和临床转化价值,见表4和图7。 相应的LncRNA过表达或低表达慢病毒、转染质粒,相应的p38MAPK信号通路抑制剂等在体外细胞实验及动物模型中都被证实有靶向调控作用,因此,通过靶向调控LncRNA和p38MAPK信号通路来调节骨髓间充质干细胞的分化和功能,或通过LncRNA和p38MAPK信号通路来抑制破骨细胞的增殖分化,这或许能提供一种创新的治疗策略,可以延缓骨质疏松症的进展。 "
| 1] 马远征,王以朋,刘强,等.中国老年骨质疏松诊疗指南(2018)[J].中国老年学杂志,2019,39(11):2557-2575. [2] NOH J Y, YANG Y, JUNG H. Molecular mechanisms and emerging therapeutics for osteoporosis. Int J Mol Sci. 2020. doi: 10.3390/ijms21207623. [3] GAO Y, PATIL S, JIA J. The development of molecular biology of osteoporosis. Int J Mol Sci. 2021;22(15):8182. [4] REID IR, BILLINGTON EO. Drug therapy for osteoporosis in older adults. Lancet. 2022;399(10329):1080-1092. [5] 王红,李明升,陈海翎,等.髋部骨质疏松性骨折患者骨质疏松药物治疗现状调查[J].中国组织工程研究,2012,16(35):6636-6640. [6] BRIDGES MC, DAULAGALA AC, KOURTIDIS A. LNCcation: lncRNA localization and function. J Cell Biol. 2021;220(2):e202009045. [7] ZUO C, WANG Z, LU H, et al. Expression profiling of lncRNAs in C3H10T1/2 mesenchymal stem cells undergoing early osteoblast differentiation. Mol Med Rep. 2013;8(2):463-467. [8] GUO B, ZHU X, LI X, et al. The roles of LncRNAs in osteogenesis, adipogenesis and osteoporosis. Curr Pharm Des. 2021;27(1):91-104. [9] LIN B, XU P, ZHENG J, et al. Effects and mechanisms of natural alkaloids for prevention and treatment of osteoporosis. Front Pharmacol. 2022;13: 1014173. [10] LI R, SHI TT, WANG Q, et al. Elevated lncRNA MIAT in peripheral blood mononuclear cells contributes to post-menopausal osteoporosis. Aging (Albany NY). 2022;14(7):3143-3154. [11] MIZOGUCHI T, ONO N. The diverse origin of bone-forming osteoblasts. J Bone Miner Res. 2021;36(8):1432-1447. [12] FU X, LIU G, HALIM A, et al. Mesenchymal stem cell migration and tissue repair. Cells. 2019;8(8):784. [13] LIU Y, LIU C, ZHANG A, et al. Down-regulation of long non-coding RNA MEG3 suppresses osteogenic differentiation of periodontal ligament stem cells (PDLSCs) through miR-27a-3p/IGF1 axis in periodontitis. Aging (Albany NY). 2019; 11(15):5334-5350. [14] HALLORAN D, DURBANO HW, NOHE A. Bone morphogenetic protein-2 in development and bone homeostasis. J Dev Biol. 2020;8(3):19. [15] ZHANG RF, LIU JW, YU SP, et al. LncRNA UCA1 affects osteoblast proliferation and differentiation by regulating BMP-2 expression. Eur Rev Med Pharmacol Sci. 2019;23(16):6774-6782. [16] 杨洲,高静媛,田发明.Wnt信号通路在骨稳态中的作用[J].中国骨质疏松杂志,2022,28(1):109-113. [17] 廖紫芮, 陈建权. Wnt蛋白生成抑制剂2干预破骨细胞的分化[J].中国组织工程研究,2023,27(31):4990-4995. [18] YANG K, TIAN N, LIU H, et al. LncRNAp21 promotes osteogenic differentiation of mesenchymal stem cells in the rat model of osteoporosis by the Wnt/beta-catenin signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(10):4303-4309. [19] LI B, LIU J, ZHAO J, et al. LncRNA-H19 modulates wnt/beta-catenin signaling by targeting Dkk4 in hindlimb unloaded rat. Orthop Surg. 2017;9(3): 319-327. [20] WANG CG, HU YH, SU SL, et al. LncRNA DANCR and miR-320a suppressed osteogenic differentiation in osteoporosis by directly inhibiting the Wnt/beta-catenin signaling pathway. Exp Mol Med. 2020;52(8):1310-1325. [21] SALIMINEJAD K, KHORRAM KH, SOLEMANI FS, et al. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J Cell Physiol. 2019;234(5): 5451-5465. [22] WANG Y, WANG K, ZHANG L, et al. Targeted overexpression of the long noncoding RNA ODSM can regulate osteoblast function in vitro and in vivo. Cell Death Dis. 2020;11(2):133. [23] YANG X, YANG J, LEI P, et al. LncRNA MALAT1 shuttled by bone marrow-derived mesenchymal stem cells-secreted exosomes alleviates osteoporosis through mediating microRNA-34c/SATB2 axis. Aging (Albany NY). 2019; 11(20):8777-8791. [24] YI J, LIU D, XIAO J. LncRNA MALAT1 sponges miR-30 to promote osteoblast differentiation of adipose-derived mesenchymal stem cells by promotion of Runx2 expression. Cell Tissue Res. 2019;376(1):113-121. [25] SUN Y, LI J, XIE X, et al. Macrophage-osteoclast associations: origin, polarization, and subgroups. Front Immunol. 2021;12:778078. [26] UDAGAWA N, KOIDE M, NAKAMURA M, et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J Bone Miner Metab. 2021; 39(1):19-26. [27] MATSUMOTO T, ENDO I. RANKL as a target for the treatment of osteoporosis. J Bone Miner Metab. 2021;39(1):91-105. [28] LIU W, LI Z, CAI Z, et al. LncRNA-mRNA expression profiles and functional networks in osteoclast differentiation. J Cell Mol Med. 2020;24(17):9786-9797. [29] CHANG Y, YU D, CHU W, et al. LncRNA expression profiles and the negative regulation of lncRNA-NOMMUT037835.2 in osteoclastogenesis. Bone. 2020;130:115072. [30] CUI Y, FU S, SUN D, et al. EPC-derived exosomes promote osteoclastogenesis through LncRNA-MALAT1. J Cell Mol Med. 2019;23(6):3843-3854. [31] CHEN RS, ZHANG XB, ZHU XT, et al. LncRNA Bmncr alleviates the progression of osteoporosis by inhibiting RANML-induced osteoclast differentiation. Eur Rev Med Pharmacol Sci. 2019;23(21):9199-9206. [32] YU J, CANALIS E. Notch and the regulation of osteoclast differentiation and function. Bone. 2020;138:115474. [33] WANG Y, LUO T B, LIU L, et al. LncRNA LINC00311 Promotes the proliferation and differentiation of osteoclasts in osteoporotic rats through the notch signaling pathway by targeting DLL3. Cell Physiol Biochem. 2018;47(6):2291-2306. [34] LIU C, CAO Z, BAI Y, et al. LncRNA AK077216 promotes RANKL-induced osteoclastogenesis and bone resorption via NFATc1 by inhibition of NIP45. J Cell Physiol. 2019;234(2):1606-1617. [35] SUN Y, LIU WZ, LIU T, et al. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 2015;35(6):600-604. [36] MAIK-RACHLINE G, LIFSHITS L, SEGER R. Nuclear P38: roles in physiological and pathological processes and regulation of nuclear translocation. Int J Mol Sci. 2020;21(17):6102. [37] CHEN E, LIU G, ZHOU X, et al. Concentration-dependent, dual roles of IL-10 in the osteogenesis of human BMSCs via P38/MAPK and NF-kappaB signaling pathways. FASEB J. 2018;32(9):4917-4929. [38] XIAO WL, ZHANG DZ, FAN CH, et al. Intermittent stretching and osteogenic differentiation of bone marrow derived mesenchymal stem cells via the p38MAPK-osterix signaling pathway. Cell Physiol Biochem. 2015;36(3):1015-1025. [39] ZOU ML, CHEN ZH, TENG YY, et al. The smad dependent TGF-beta and BMP signaling pathway in bone remodeling and therapies. Front Mol Biosci. 2021;8:593310. [40] LI C, YANG X, HE Y, et al. Bone morphogenetic protein-9 induces osteogenic differentiation of rat dental follicle stem cells in P38 and ERK1/2 MAPK dependent manner. Int J Med Sci. 2012;9(10):862-871. [41] LIU Q, ZHUANG Y, OUYANG N, et al. Cytochalasin d promotes osteogenic differentiation of MC3T3-E1 cells via p38-MAPK signaling pathway. Curr Mol Med. 2019;20(1):79-88. [42] ZHOU Y, LIU S, WANG W, et al. The miR-204-5p/FOXC1/GDF7 axis regulates the osteogenic differentiation of human adipose-derived stem cells via the AKT and p38 signalling pathways. Stem Cell Res Ther. 2021;12(1):64. [43] FU Y, GU J, WANG Y, et al. Involvement of the mitogenactivated protein kinase signaling pathway in osteoprotegerininduced inhibition of osteoclast differentiation and maturation. Mol Med Rep. 2015;12(5):6939-6945. [44] HAN SY, KIM YK. Yukmijihwang-tang suppresses receptor activator of nuclear factor Kappa-B ligand (RANKL)-induced osteoclast differentiation and prevents ovariectomy (OVX)-mediated bone loss. Molecules. 2021; 26(24):7579. [45] KIM K, KIM JH, KIM I, et al. Rev-erbalpha negatively regulates osteoclast and osteoblast differentiation through p38 MAPK signaling pathway. Mol Cells. 2020;43(1):34-47. [46] HUANG MZ, ZHUANG Y, NING X, et al. Artesunate inhibits osteoclastogenesis through the miR-503/RANK axis. Biosci Rep. 2020;40(7):BSR20194387. [47] KIM JM, LIN C, STAVRE Z, et al. Osteoblast-osteoclast communication and bone homeostasis. Cells. 2020;9(9):2073. [48] PONZETTI M, RUCCI N. Osteoblast differentiation and signaling: established concepts and emerging topics. Int J Mol Sci. 2021;22(13):6651. [49] COULTHARD LR, WHITE DE, JONES DL, et al. p38(MAPK): stress responses from molecular mechanisms to therapeutics. Trends Mol Med. 2009;15(8): 369-379. [50] WANG XX, ZHANG B, XIA R, et al. Inflammation, apoptosis and autophagy as critical players in vascular dementia. Eur Rev Med Pharmacol Sci. 2020; 24(18): 9601-9614. [51] KOMORI T. Molecular mechanism of runx2-dependent bone development. Mol Cells. 2020;43(2):168-175. [52] KUTILEK S, MACHYTKA R, MUNZAR P. Cleidocranial dysplasia. Sudan J Paediatr. 2019;19(2):165-168. [53] GREENBLATT MB, SHIM JH, ZOU W, et al. The p38 MAPK pathway is essential for skeletogenesis and bone homeostasis in mice. J Clin Invest. 2010;120(7):2457-2473. [54] ZHANG J, TAO Z, WANG Y. Long noncoding RNA DANCR regulates the proliferation and osteogenic differentiation of human bone-derived marrow mesenchymal stem cells via the p38 MAPK pathway. Int J Mol Med. 2018;41(1):213-219. [55] ORTUNO MJ, RUIZ-GASPA S, RODRIGUEZ-CARBALLO E, et al. p38 regulates expression of osteoblast-specific genes by phosphorylation of osterix. J Biol Chem. 2010;285(42):31985-31994. [56] KANG MA, LEE J, PARK SH. Cannabidiol induces osteoblast differentiation via angiopoietin1 and p38 MAPK. Environ Toxicol. 2020;35(12):1318-1325. [57] PARK HB, BAEK KH. E3 ligases and deubiquitinating enzymes regulating the MAPK signaling pathway in cancers. Biochim Biophys Acta Rev Cancer. 2022;1877(3):188736. [58] BOASE NA, KUMAR S. NEDD4: The founding member of a family of ubiquitin-protein ligases. Gene. 2015;557(2):113-122. [59] JIANG Y, WU W, JIAO G, et al. LncRNA SNHG1 modulates p38 MAPK pathway through Nedd4 and thus inhibits osteogenic differentiation of bone marrow mesenchymal stem cells. Life Sci. 2019;228:208-214. [60] BOHM C, HAYER S, KILIAN A, et al. The alpha-isoform of p38 MAPK specifically regulates arthritic bone loss. J Immunol. 2009;183(9):5938-5947. [61] WANG F, LONG S, ZHANG J. Moringa oleifera Lam. leaf extract safely inhibits periodontitis by regulating the expression of p38alpha/MAPK14-OPG/RANKL. Arch Oral Biol. 2021;132:105280. [62] FANG C, HE M, LI D, et al. YTHDF2 mediates LPS-induced osteoclastogenesis and inflammatory response via the NF-kappaB and MAPK signaling pathways. Cell Signal. 2021;85:110060. [63] WU L, LUO Z, LIU Y, et al. Aspirin inhibits RANKL-induced osteoclast differentiation in dendritic cells by suppressing NF-kappaB and NFATc1 activation. Stem Cell Res Ther. 2019;10(1):375. [64] WEI L, CHEN W, HUANG L, et al. Alpinetin ameliorates bone loss in LPS-induced inflammation osteolysis via ROS mediated P38/PI3K signaling pathway. Pharmacol Res. 2022;184:106400. [65] ZHENG S, WANG YB, YANG YL, et al. LncRNA MALAT1 inhibits osteogenic differentiation of mesenchymal stem cells in osteoporosis rats through MAPK signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(11): 4609-4617. [66] HUANG Z, CHU L, LIANG J, et al. H19 promotes HCC bone metastasis through reducing osteoprotegerin expression in a protein phosphatase 1 catalytic subunit alpha/p38 mitogen-activated protein kinase-dependent manner and sponging microRNA 200b-3p. Hepatology (Baltimore, Md.). 2021;74(1):214-232. [67] GU Z, XIE D, DING R, et al. GPR173 agonist phoenixin 20 promotes osteoblastic differentiation of MC3T3-E1 cells. Aging (Albany NY). 2020; 13(4):4976-4985. [68] LIU F, YANG XC, CHEN ML, et al. LncRNA H19/Runx2 axis promotes VSMCs transition via MAPK pathway. Am J Transl Res. 2020;12(4):1338-1347. [69] WANG H, LI YK, CUI M, et al. Effect of lncRNA AK125437 on postmenopausal osteoporosis rats via MAPK pathway. Eur Rev Med Pharmacol Sci. 2020; 24(5):2173-2180. [70] CHEN G, DENG C, LI YP. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8(2):272-288. [71] ZOU ML, CHEN ZH, TENG YY, et al. The smad dependent TGF-beta and BMP signaling pathway in bone remodeling and therapies. Front Mol Biosci. 2021;8:593310. [72] WU M, CHEN G, LI YP. TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016;4:16009. [73] YANG Q, HAN Y, LIU P, et al. Long noncoding RNA GAS5 promotes osteogenic differentiation of human periodontal ligament stem cells by regulating GDF5 and p38/JNK signaling pathway. Front Pharmacol. 2020;11:701. [74] YANG Y, YUJIAO W, FANG W, et al. The roles of miRNA, lncRNA and circRNA in the development of osteoporosis. Biol Res. 2020;53(1):40. [75] JIANG XR, GUO N, LI XQ, et al. Long non-coding RNA HULC promotes proliferation and osteogenic differentiation of bone mesenchymal stem cells via down-regulation of miR-195. Eur Rev Med Pharmacol Sci. 2018; 22(10):2954-2965. [76] MURACA M, CAPPARIELLO A. The role of extracellular vesicles (EVs) in the epigenetic regulation of bone metabolism and osteoporosis. Int J Mol Sci. 2020; 21(22):6102. |
| [1] | Han Haihui, Ran Lei, Meng Xiaohui, Xin Pengfei, Xiang Zheng, Bian Yanqin, Shi Qi, Xiao Lianbo. Targeting fibroblast growth factor receptor 1 signaling to improve bone destruction in rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1905-1912. |
| [2] | Zhao Jiyu, Wang Shaowei. Forkhead box transcription factor O1 signaling pathway in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1923-1930. |
| [3] | Zhou Jinhai, Li Jiangwei, Wang Xuquan, Zhuang Ying, Zhao Ying, Yang Yuyong, Wang Jiajia, Yang Yang, Zhou Shilian. Three-dimensional finite element analysis of anterior femoral notching during total knee arthroplasty at different bone strengths [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1775-1782. |
| [4] | Zhu Hanmin, Wang Song, Xiao Wenlin, Zhang Wenjing, Zhou Xi, He Ye, Li Wei, . Mitophagy regulates bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1676-1683. |
| [5] | Zhao Jiacheng, Ren Shiqi, Zhu Qin, Liu Jiajia, Zhu Xiang, Yang Yang. Bioinformatics analysis of potential biomarkers for primary osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1741-1750. |
| [6] | Yin Lu, Jiang Chuanfeng, Chen Junjie, Yi Ming, Wang Zihe, Shi Houyin, Wang Guoyou, Shen Huarui. Effect of Complanatoside A on the apoptosis of articular chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1541-1547. |
| [7] | Wang Wentao, Hou Zhenyang, Wang Yijun, Xu Yaozeng. Apelin-13 alleviates systemic inflammatory bone loss by inhibiting macrophage M1 polarization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1548-1555. |
| [8] | Chen Shuai, Jin Jie, Han Huawei, Tian Ningsheng, Li Zhiwei . Causal relationship between circulating inflammatory cytokines and bone mineral density based on two-sample Mendelian randomization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1556-1564. |
| [9] | Hu Taotao, Liu Bing, Chen Cheng, Yin Zongyin, Kan Daohong, Ni Jie, Ye Lingxiao, Zheng Xiangbing, Yan Min, Zou Yong. Human amniotic mesenchymal stem cells overexpressing neuregulin-1 promote skin wound healing in mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1343-1349. |
| [10] | Jin Kai, Tang Ting, Li Meile, Xie Yuan. Effects of conditioned medium and exosomes of human umbilical cord mesenchymal stem cells on proliferation, migration, invasion, and apoptosis of hepatocellular carcinoma cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1350-1355. |
| [11] | Li Dijun, Jiu Jingwei, Liu Haifeng, Yan Lei, Li Songyan, Wang Bin. Three-dimensional gelatin microspheres loaded human umbilical cord mesenchymal stem cells for chronic tendinopathy repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1356-1362. |
| [12] | Liu Qi, Li Linzhen, Li Yusheng, Jiao Hongzhuo, Yang Cheng, Zhang Juntao. Icariin-containing serum promotes chondrocyte proliferation and chondrogenic differentiation of stem cells in the co-culture system of three kinds of cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1371-1379. |
| [13] | Aikepaer · Aierken, Chen Xiaotao, Wufanbieke · Baheti. Osteogenesis-induced exosomes derived from human periodontal ligament stem cells promote osteogenic differentiation of human periodontal ligament stem cells in an inflammatory microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1388-1394. |
| [14] | Zhang Zhenyu, Liang Qiujian, Yang Jun, Wei Xiangyu, Jiang Jie, Huang Linke, Tan Zhen. Target of neohesperidin in treatment of osteoporosis and its effect on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1437-1447. |
| [15] | Zhang Haojun, Li Hongyi, Zhang Hui, Chen Haoran, Zhang Lizhong, Geng Jie, Hou Chuandong, Yu Qi, He Peifeng, Jia Jinpeng, Lu Xuechun. Identification and drug sensitivity analysis of key molecular markers in mesenchymal cell-derived osteosarcoma [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1448-1456. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||