Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (16): 2590-2595.doi: 10.12307/2022.262
Previous Articles Next Articles
Application of self-healing hydrogels in bone tissue engineering
Fu Zheng1, Li Runze1, Luo Haotian1, Chen Jun2, Wang Weicai1
- 1Guanghua School of Stomatology, Hospital of Stomatology, Sun Yat-sen University, Guangdong Provincial Key Laboratory of Stomatology, Guangzhou 510055, Guangdong Province, China; 2Guangdong Provincial Key Laboratory of Orthopedics and Traumatology, First Affiliated Hospital of Sun Yat-sen University, Guangzhou 510080, Guangdong Province, China
-
Received:2020-12-07Revised:2020-12-11Accepted:2021-01-27Online:2022-06-08Published:2021-12-23 -
Contact:Wang Weicai, MD, Attending physician, Guanghua School of Stomatology, Hospital of Stomatology, Sun Yat-sen University, Guangdong Provincial Key Laboratory of Stomatology, Guangzhou 510055, Guangdong Province, China -
About author:Fu Zheng, Guanghua School of Stomatology, Hospital of Stomatology, Sun Yat-sen University, Guangdong Provincial Key Laboratory of Stomatology, Guangzhou 510055, Guangdong Province, China -
Supported by:National Natural Science Foundation of China, No. 82001005 (to WWC); Natural Science Foundation of Guangdong Province, No. 2018A030310278 (to WWC); Science and Technology Program of Guangzhou, No. 201804010459 (to WWC)
CLC Number:
Cite this article
Fu Zheng, Li Runze, Luo Haotian, Chen Jun, Wang Weicai. Application of self-healing hydrogels in bone tissue engineering[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(16): 2590-2595.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
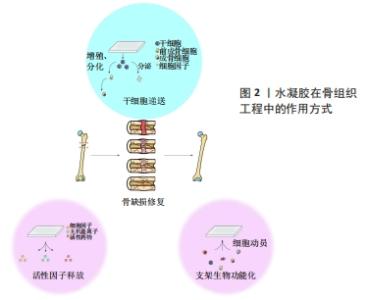
2.1 自修复水凝胶的凝胶化机制 由于传统水凝胶的共价键在解离后难以重建,因此微小裂缝不断积累极易导致水凝胶断裂。许多天然组织如骨骼、软骨、皮肤等,都具有在轻微损伤后自修复的特性,这使其能够承受多次损伤,维持生物学功能。受这一自然现象的启发,赋予人工水凝胶自修复特性是研发智能水凝胶的一大步。下文将分别讨论基于动态共价键、超分子键和多机制交联的自修复水凝胶的凝胶化机制。 2.1.1 动态共价键 与常规共价键不同的是,动态共价键处于可逆状态,具体来说,平衡的正向或逆向移动可使共价键在较温和的条件下经历形成、破坏或重新形成的状态。理论上,平衡的动态性主要由平衡常数决定,一般情况下,平衡常数在10-7-107范围内表示反应具有可逆性,大于107或小于10-7则表示不可逆[20]。与超分子键相比,动态共价键键能较高、更稳定,因此动态共价键既能赋予水凝胶常规共价键的稳定性,又能赋予其超分子键的可逆性,呈现出“自修复”的特性。希夫碱键(包括亚胺、腙和肟)是指分别由醛与伯胺、酰肼和氨基经缩合反应形成的化学键[21]。JIANG等[22]利用壳聚糖盐酸盐和氧化葡聚糖制备了一种具有良好自修复能力的水凝胶,壳聚糖盐酸盐的氨基和氧化葡聚糖的醛基之间通过轻微的席夫碱反应形成亚胺键,使水凝胶在中性条件下具有自愈能力;该水凝胶被切割成两半后,室温下30 min内可以修复为一个整体。此外,由于自身特殊的化学结构,希夫碱键表现出pH值响应性,即与生理微环境相比,酸性微环境中水凝胶的降解速率更高、内部孔隙更大,提高了药物释放速率。 除了希夫碱键外,许多具有类似“动态”特性的共价键也可用于制备自修复水凝胶,比如狄尔斯-阿尔德反应[23]、硼酸酯化学键[24]、二硫键[25]、克诺维纳盖尔缩合反应等[26]。 2.1.2 超分子键 非共价键主要来自于分子之间的弱连接,因此也被称为超分子键。超分子键可以在解离后迅速重建,使系统具有“自修复”性质[20]。当大环的宿主部分插入到客体部分中时,就会发生主-客体相互作用,形成独特的包含结构[27]。HIGHLEY等[28]报道了一种由主-客体相互作用交联的自修复水凝胶,这种超分子水凝胶以透明质酸为基础,经金刚烷或β-环糊精(金刚烷-透明质酸和β-环糊精-透明质酸)修饰,并且在金刚烷-透明质酸和β-环糊精-透明质酸通过分子间主客-宿主键(金刚烷和β-环糊精部分之间)混合后迅速形成超分子组合;金刚烷和β-环糊精之间的主-客体相互作用可以在解离后迅速重建,使水凝胶具有良好的自修复性,是理想的3D打印材料。此外,该超分子水凝胶的性质可以通过调节水凝胶中的物质浓度以及最终凝胶中客体与主体部分的比例来改变。 除了主-客体相互作用之外,还有许多超分子键,比如氢键[29]、疏水相互作用[30]、离子键[31]、金属配位键和π-π共轭堆积等[32-33],都已成功地被运用于自修复水凝胶的设计和制备。 2.1.3 多机制交联 虽然近年来自修复水凝胶的研发取得了较大进展,但是仅具备单一动态键的水凝胶仍然有局限性,比如:尽管大多数超分子键都能在温和的条件下迅速结合,但是由于其键能较弱,单一超分子键很难赋予水凝胶理想的机械强度;动态共价键键能一般比超分子键强,但其中很多不能在生理条件下发生(如希夫碱或硼酯键)或需要很长时间来形成或重建(如狄尔斯-阿尔德反应)[10]。运用多个动态键或者联合运用动态键和共价键是改善水凝胶性能最常用的方法[23,30-31]。WANG等[34]通过在同一体系中加入主-客体相互作用和共价键,研制出了具有高机械强度(杨氏模量可达9.13 MPa)和高抗疲劳/抗切片性能的自修复水凝胶,其一是主体(丙烯酸异氰酸根合乙酯改性的β-环糊精)和客体[2-(2-(2-(2-(金刚烷基-1-氧基)乙氧基)乙氧基)乙氧基)乙醇丙烯酸酯]之间的主-客体相互作用形成“三臂”主客体超分子;其二是主客体超分子之间的共价键合(通过紫外线引发的聚合反应),从而在水凝胶中形成牢固的交联键。主-客体相互作用提供了一种能量耗散机制来缓冲受力,从而延缓或避免断裂,使水凝胶具有快速自修复的特性;共价键易由UV引发聚合,可以将水凝胶互连成一个强大的网络,使其坚固。 2.2 自修复水凝胶的组成成分 自修复水凝胶的成分会影响其力学性能和生物学性能,例如:天然聚合物通常具有更强的生物相容性;而合成聚合物通常以降低生物亲和性为代价生成更强、更有弹性的水凝胶;将纳米复合材料加入到自修复水凝胶中可赋予该体系独特的性能[35]。该文将根据自修复水凝胶的主要成分将其分为三类——天然水凝胶、合成水凝胶和纳米复合水凝胶,并进一步讨论不同成分的自修复水凝胶的特点。 天然聚合物如藻脘酸盐[36]、胶原[37]、明胶[38]、透明质酸[39],由于具有易获得和廉价的优点已被广泛用于研发水凝胶[10]。与合成水凝胶相比,天然自修复水凝胶与天然细胞外基质有更大的相似性,可赋予水凝胶较好的生物相容性、生物降解性及一些特殊性能。WU等[40]研发的抗坏血碱-铜离子交联体系具有生物安全性,保证了水凝胶的生物相容性。GA?ANIN等[15]开发了一种通过DNA杂交交联的蛋白质-DNA混合水凝胶,通过DNA上的配体将所需的生物活性分子组装成蛋白质-DNA杂化水凝胶,避免了使用活性有机试剂或催化剂,提高了水凝胶生物相容性。同时,利用DNA酶可以实现从复合凝胶中释放时空可控的生物活性因子。然而,由于天然水凝胶在机械强度和稳定性方面性能不佳,其应用受到了限制。 由于具有化学惰性、强弹性模量、灵活性并易于定制修改,合成聚合物也是研发自修复水凝胶的一种选择。YU等[18]用香豆素衍生物合成了聚丙烯酰胺基水凝胶,研究人员对合成的水凝胶进行了光二聚和光裂解反应,由于香豆素分子内[2+2]光环加成反应,该水凝胶在365 nm紫外线照射下显示出非凡的自愈能力,同时聚丙烯酰胺交联剂的存在显著增强了水凝胶的细胞附着性能。然而,与天然水凝胶相比,合成水凝胶的生物相容性、生物降解性较差。 纳米复合水凝胶是指通过动态键将水凝胶网络和纳米材料增强物连接在一起的水凝胶。将纳米材料引入自修复水凝胶有两个基本目标,一是增强水凝胶的物理性能,如机械强度、流变性能、膨胀度等。MU等[41]将自体富血小板纤维蛋白与明胶纳米颗粒结合研制出双网络水凝胶,研究显示此两组分之间的亲合力(如静电和疏水相互作用)非常高,促进了双网络的形成。理论上,复合凝胶中的自体富血小板纤维蛋白网络通过初级共价网络提供了维持水凝胶完整性的基础,而明胶纳米颗粒形成的次级胶体网络则通过牺牲键来耗散能量,维持自身结构和功能的完整性。二是赋予水凝胶优越的生物特性,例如SHALUMON等[42]开发了凝胶基水凝胶,并研究了纳米羟基磷灰石对支架性能的影响。与大粒径的羟基磷灰石颗粒相比,纳米羟基磷灰石能增强细胞与材料之间的相互作用,提高材料的生物活性[43]。同时,纳米羟基磷灰石可改善蛋白黏附、细胞黏附和增殖[44]。 2.3 自修复水凝胶在骨组织工程中的应用 2.3.1 骨组织工程基础 骨是一种坚硬致密的组织,支撑着全身的肌肉、韧带、腺体、皮肤层和皮下结构[45]。骨组织具有一定程度的自修复能力,可以在小的骨折后自然愈合[46]。了解骨组织的结构和自然愈合过程,可以为正确修复骨缺损提供指导。宏观来看,骨组织结构主要包括皮质骨和松质骨;微观上讲,骨组织中含有机质、无机物和骨相关细胞。骨损伤的愈合可分为5个相互重叠的阶段[47],一是炎症反应阶段,骨折后短时间内血液在骨折端和周围凝固形成血肿,招募多种炎症细胞并以特定的顺序分泌不同的生物分子,启动修复级联反应;二是血管重建和血管生成阶段;三是软骨痂形成阶段;四是软骨痂矿化和再吸收阶段;五是骨重塑阶段,该过程在损伤后的三四周开始,并可持续数年。 近年来,天然/合成聚合物在骨再生领域引起了广泛关注。水凝胶可以提供自然亲水的三维环境,有利于细胞存活,在修复骨缺损方面有很大的潜力。在研发骨组织工程应用的水凝胶过程中,有以下几个方面因素需要考虑。 首先,从自修复性的角度考虑,研发生物应用的水凝胶需要考虑2个因素。一是水凝胶的自修复动力学,包括修复时间和效率。理想的自修复水凝胶能在损伤后短时间内完全恢复。HUANG等[48]利用端苯甲醛四臂聚乙二醇的醛基与羧甲基壳聚糖主链上的胺基之间的希夫碱键,研发了一种快速自修复水凝胶。自修复实验表明,室温下5 min内,在没有外界干预的情况下被分为两半的水凝胶可在分界处形成一个完整的水凝胶膜;梁形应变压缩实验表明,该水凝胶在生理温度下12 h后自修复率为(94.0±9.8)%。二是水凝胶的自修复条件。部分动态交联只有在特定的条件下(如一定的pH值、温度或特定的介质存在时)才表现出可逆性,例如希夫碱键在酸性环境中不稳定。DING等[49]的研究表明,由丙烯酰胺修饰的几丁质和海藻酸二醛之间的希夫碱键连接形成的水凝胶,只能在碱性或中性环境下自修复。 从骨组织工程应用的角度考虑,水凝胶需要满足特定的物理和生化要求。在物理方面,水凝胶的物理性质(如机械强度、孔径大小和形态)会影响骨修复的最终效果。第一,细胞可以感知水凝胶的硬度并改变自身形态、体积和谱系承受力[50];同时,一些骨组织有承重需求,需要具有一定的韧性[46],因此在设计骨再生水凝胶时需要考虑合适的机械强度。第二,水凝胶的孔径大小影响细胞的生物行为。有报道称,孔径大于300 μm的水凝胶在骨再生方面具有优势,孔径小于100 μm的水凝胶对骨再生可能存在不利影响[51]。第三,水凝胶的整体和表面形态也会影响细胞的生物行为。许多研究报道了具有非纤维状形态的水凝胶对特定蛋白质的吸附增强,可能有利于细胞黏附,从而提高促进成骨的潜力[52]。亦有研究表明,利用纳米涂层、喷砂、酸蚀刻、激光蚀刻和等离子体处理等方法改变材料的表面形貌,可以极大地提高细胞的增殖、黏附和谱系定向,从而加速骨再生过程[53]。在生物化学方面,用于骨再生最佳的水凝胶需要满足以下要求:①非细胞毒性和非免疫原性:避免对细胞造成损伤或慢性炎症;②可生物降解能力:酶或水解引起的水凝胶降解应与新骨长入同步,并最终被新形成的骨组织所取代;③骨诱导、骨传导、成骨和骨相容性:增强骨再生。 2.3.2 自修复水凝胶在骨组织工程中的作用方式 自修复水凝胶可以通过不同的方式促进骨再生,现有的研究主要分为两个方向,见图2。"
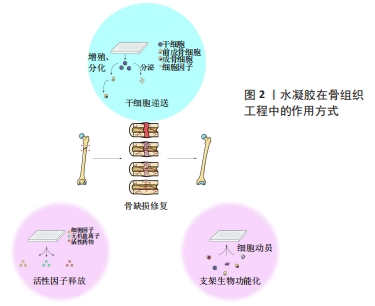

其一,含细胞水凝胶可直接作为干细胞的载体促进骨再生;其二,无细胞水凝胶可通过生物活性药物传递或支架生物功能化来促进细胞动员和细胞活性,进而促进骨再生。该节将分别讨论自修复水凝胶在骨再生中干细胞递送和支架修饰的两种作用方式。 (1)干细胞递送:如上所述,骨再生过程主要依赖于充足的细胞数量、调控生长因子的释放、维持稳定的支架及刺激血管生成[54]。骨髓间充质干细胞具有自我更新、分化潜能、易获得和免疫调节等特性,因此干细胞递送疗法已在骨再生中被广泛应用。干细胞递送主要是将特异性干细胞移植到损伤部位促进愈合[55],一方面,移植入体内的干细胞可以在体内分化为目标组织(如骨组织)细胞,促进损伤部位的组织再生和修复;另一方面,这些干细胞在体内可以稳定地分泌相关生长因子,并且生长因子的功效随着干细胞的增殖和分化而增强[56]。然而,由于病变区域内的不良环境及干细胞移植后有限的余量和生物活性,干细胞直接注入是不可行的[57],因此具有出色生物相容性和生物功能性的水凝胶被认为是干细胞递送入体的良好载体[6]。水凝胶不仅为干细胞附着提供了良好支架,在递送过程中保存细胞活性,而且可促进干细胞的增殖和分化,更好地保证了干细胞疗效[58]。 MU等[41]合成了一种多功能地塞米松-脲基嘧啶酮聚合物,结果显示,包裹在水凝胶中的软骨细胞和骨髓干细胞在培养2周后均保持了较高的生存能力,这表明该聚合物具有良好的细胞相容性,可作为细胞递送系统促进骨组织工程。在细胞治疗中,自修复水凝胶比常规水凝胶在用作细胞递送载体方面具有更好的表现,例如:自修复水凝胶的剪切稀变性可以保护封装细胞在注射入体过程中免受剪切力的损害,同时更高效地完成营养/废物交换,为细胞的增殖分化提供更良好的环境[59]。亦有报道显示,自修复水凝胶封装的干细胞具有更高的迁移率与长期生存能力,但具体原因尚不清楚[60]。 (2)支架修饰:上述干细胞治疗方法虽已有运用,但仍具有一定局限性,如有限的自体细胞、时间/成本高的细胞扩张程序、相对较低的细胞存活率和免疫排斥的高风险。利用可募集和激活体内内源性细胞的无细胞水凝胶,可以解决这些问题[61]。该节将分别讨论无细胞自修复水凝胶在活性因子(生长因子、无机离子、小分子药物)递送和支架生物功能化方面促进骨再生的潜力。 活性因子递送:活性因子如生长因子、无机离子和小分子药物,可通过募集或/及激活内源性细胞来实现无细胞水凝胶促进骨再生。然而,活性因子直接导入缺损区是不可行的,首先,骨愈合所需活性物质的量常高于生理剂量,而生理环境对活性物质的量有严格要求,直接注入易造成严重不良反应,例如局部Mg2+水平过高可能导致骨丢失[62];其次,部分活性物质可扩散到其他部位或被酶分解,导致局部残留量有限,不能满足骨再生的需求;同时,活性物质的扩散可出现外周不良反应,例如生长因子扩散可导致囊肿样骨形成和明显的软组织肿胀[63]。利用自修复水凝胶作为递送系统并完成各种活性物质的缓释,是解决上述问题的一种方法。与常规水凝胶相比,剪切稀变性和可注射性使得自修复水凝胶更容易注入到特定区域,侵入量最小。 生长因子在骨形成过程中发挥重要作用,如骨形成间充质干细胞的募集、增殖和分化[64]。最近,许多研究表明引入骨形态发生蛋白[12]、基质细胞源性因子1a[65]、成纤维细胞生长因子[66]、注射富血小板纤维蛋白和转化生长因子β的自修复水凝胶在骨再生中均有显著作用[41,67]。PHIPPS等[12]利用自修复多肽水凝胶RADA-16作为骨形态发生蛋白2的药物递送载体,通过水凝胶中释放的骨形态发生蛋白2上清液处理猪骨髓基质细胞,证明了从RADA-16中释放的骨形态发生蛋白2保持了其原有的生物活性并刺激了骨髓间充质干细胞的生长。 无机盐离子(如Ca2+和Mg2+)在骨再生过程中发挥重要作用。钙的摄入与沉积是骨骼生理发育和维持骨量不可缺少的环境因素。镁离子不仅可以调节细胞的黏附和分化等行 为[68],还可以促进局部骨形成和愈合[69]。ZHANG等[13]提出了一种基于透明质酸和自组装双膦酸盐镁纳米颗粒的复合水凝胶。由于自组装双膦酸盐与镁离子之间存在较强的相互作用,材料中Mg2+的释放速率较慢;同时,钙离子(Ca2+)可以通过与双膦酸盐的竞争性结合加速该水凝胶中镁离子的释放,这表明该水凝胶在促进钙化的同时仍可维持镁离子释放的能力,从而可以促进人骨髓间充质干细胞的体外分化,促进骨的原位再生。 将促进成骨细胞生物活性的药物引入自修复水凝胶中可促进骨再生。SEHGAL等[14]开发了一种骨诱导药物—地塞米松磷酸二钠的缓释系统——壳聚糖纳米颗粒。另一方面,抑制破骨细胞生物活性的药物也可以被封装和缓释。GA?ANIN等[15]开发了一种由化学修饰的人血清白蛋白和经设计的DNA连接物组成的蛋白质-DNA混合水凝胶,并用其封装和缓释rho-inhibiting C3毒素,通过对RAW 264.7细胞进行培养发现,时空调控C3毒素释放可特异性地抑制破骨细胞形成和骨吸收,同时不影响成骨细胞分化和矿化。 此外,部分自修复水凝胶表现出多重刺激响应性。在不同pH值条件下,透明质酸水凝胶对重组人骨形态发生蛋白2释放速率的调节作用已被研究,酸性水凝胶对重组人骨形态发生蛋白2的持续释放超过28 d,而中性水凝胶对重组人骨形态发生蛋白2的释放呈现先爆发后持续平缓过程[13]。 支架生物功能化:除了释放活性因子外,自修复水凝胶的特殊结构和生物功能化也可通过促进细胞补充、细胞黏附及细胞动员和活性,进而促进骨再生。 部分水凝胶通过自身的化学结构促进细胞黏附。ANDO等[16]开发了一种人工自愈肽SPG-178,研究发现该聚合物通过其中带正电荷的胺与细胞负膜相互作用来促进细胞黏附和动员,从而促进移植物骨与宿主骨的附着;同时,该水凝胶具有剪切稀变性和可注射的特点,可用于填充形状复杂的缺陷。TAKEUCHI等[17]研究了自组装肽纳米纤维水凝胶(2.5%RADA16)促进骨再生的作用,随着时间的推移,2.5%RADA-16组中大鼠的牙周膜细胞增殖明显高于基质凝胶组,与此同时,增殖细胞核抗原阳性细胞、血管内皮生长因子和骨桥蛋白的表达水平也明显高于未填充组和基质胶组;第2,4周时,2.5%RADA-16组的骨体积分数均高于其他组。以上结果表明该水凝胶通过自身结构促进细胞黏附,从而促进骨再生。部分水凝胶通过引入细胞黏附基序来促进细胞黏附。YU等[18]将精氨酸-甘氨酸-天冬氨酸细胞黏附基序引入弹性蛋白样重组子,并用该支架培养骨髓来源的人间充质干细胞。 W?ODARCZYK-BIEGUN等[19]在用pastoris酵母毕赤酵母分泌蛋白研发的自修复水凝胶中引入了精氨酸-甘氨酸-天冬氨酸和赖氨酸-精氨酸-丝氨酸-精氨酸细胞黏附序列,并使用该支架对MG-63成骨细胞进行二维培养,研究结果表明这两种细胞黏附序列均可促进细胞黏附,并有协同作用。总而言之,包含细胞黏附序列的水凝胶可促进细胞附着,因此提供了一种更相似于细胞外基质且具有骨传导性的环境,有利于骨再生应用。"

| [1] RAMASAMY SK, KUSUMBE AP, WANG L, et al. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. 2014;507(7492): 376-380. [2] HAYASHI K, OCHIAI-SHINO H, SHIGA T, et al. Transplantation of human-induced pluripotent stem cells carried by self-assembling peptide nanofiber hydrogel improves bone regeneration in rat calvarial bone defects. BDJ Open. 2016;2:15007. [3] BAI X, GAO M, SYED S, et al. Bioactive hydrogels for bone regeneration. Bioact Mater. 2018;3(4):401-417. [4] WU G, FENG C, QUAN J, et al. In situ controlled release of stromal cell-derived factor-1α and antimiR-138 for on-demand cranial bone regeneration. Carbohydr Polym. 2018;182:215-224. [5] LI J, MOONEY DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater. 2016;1(12):16071. [6] HUANG Q, ZOU Y, ARNO MC, et al. Hydrogel scaffolds for differentiation of adipose-derived stem cells. Chem Soc Rev. 2017;46(20):6255-6275. [7] CULVER HR, CLEGG JR, PEPPAS NA. Analyte-Responsive Hydrogels: Intelligent Materials for Biosensing and Drug Delivery. Acc Chem Res. 2017;50(2):170-178. [8] YANG J, ZHANG YS, YUE K, et al. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017;57:1-25. [9] SLAUGHTER BV, KHURSHID SS, FISHER OZ, et al. Hydrogels in regenerative medicine. Adv Mater. 2009;21(32–33):3307-3329. [10] TALEBIAN S, MEHRALI M, TAEBNIA N, et al. Self‐Healing Hydrogels: The Next Paradigm Shift in Tissue Engineering? Adv Sci (Weinh). 2019;6(16): 1801664. [11] HOU S, WANG X, PARK S, et al. Rapid Self-Integrating, Injectable Hydrogel for Tissue Complex Regeneration. Adv Healthc Mater. 2015;4(10):1491-1495,1423. [12] PHIPPS MC, MONTE F, MEHTA M, et al. Intraosseous Delivery of Bone Morphogenic Protein-2 Using a Self-Assembling Peptide Hydrogel. Biomacromolecules. 2016;17(7):2329-2336. [13] ZHANG K, LIN S, FENG Q, et al. Nanocomposite hydrogels stabilized by self-assembled multivalent bisphosphonate-magnesium nanoparticles mediate sustained release of magnesium ion and promote in-situ bone regeneration. Acta Biomater. 2017; 64: 389-400. [14] SEHGAL RR, ROOHANI-ESFAHANI SI, ZREIQAT H, et al. Nanostructured gellan and xanthan hydrogel depot integrated within a baghdadite scaffold augments bone regeneration. J Tissue Eng Regen Med. 2017;11(4):1195-1211. [15] GAČANIN J, KOVTUN A, FISCHER S, et al. Spatiotemporally Controlled Release of Rho-Inhibiting C3 Toxin from a Protein-DNA Hybrid Hydrogel for Targeted Inhibition of Osteoclast Formation and Activity. Adv Healthc Mater. 2017;6(21). doi:10.1002/adhm.201700392. [16] ANDO K, IMAGAMA S, KOBAYASHI K, et al. Feasibility and effects of a self-assembling peptide as a scaffold in bone healing: An in vivo study in rabbit lumbar posterolateral fusion and tibial intramedullary models. J Orthop Res. 2018;36(12):3285-3293. [17] TAKEUCHI T, BIZENJIMA T, ISHII Y, et al. Enhanced healing of surgical periodontal defects in rats following application of a self-assembling peptide nanofibre hydrogel. J Clin Periodontol. 2016;43(3):279-288. [18] Yu L, Xu K, Ge L, et al. Cytocompatible, Photoreversible, and Self-Healing Hydrogels for Regulating Bone Marrow Stromal Cell Differentiation. Macromol Biosci. 2016;16(9):1381-1390. [19] WŁODARCZYK-BIEGUN MK, WERTEN MWT, POSADOWSKA U, et al. Nanofibrillar hydrogel scaffolds from recombinant protein-based polymers with integrin- and proteoglycan-binding domains. J Biomed Mater Res A. 2016;104(12):3082-3092. [20] TENG L, CHEN Y, JIA YG, et al. Supramolecular and dynamic covalent hydrogel scaffolds: from gelation chemistry to enhanced cell retention and cartilage regeneration. J Mater Chem B. 2019;7(43):6705-6736. [21] XU J, LIU Y, HSU S. Hydrogels Based on Schiff Base Linkages for Biomedical Applications. Molecules. 2019;24(16):3005. [22] JIANG F, TANG Z, ZHANG Y, et al. Enhanced proliferation and differentiation of retinal progenitor cells through a self-healing injectable hydrogel. Biomater Sci. 2019;7(6):2335-2347. [23] BI B, MA M, LV S, et al. In-situ forming thermosensitive hydroxypropyl chitin-based hydrogel crosslinked by Diels-Alder reaction for three dimensional cell culture. Carbohydr Polym. 2019;212:368-377. [24] SHI W, HASS B, KUSS MA, et al. Fabrication of versatile dynamic hyaluronic acid-based hydrogels. Carbohydr Polym. 2020;233:115803. [25] BANERJEE SL, BHATTACHARYA K, SAMANTA S, et al. Self-Healable Antifouling Zwitterionic Hydrogel Based on Synergistic Phototriggered Dynamic Disulfide Metathesis Reaction and Ionic Interaction. ACS Appl Mater Interfaces. 2018;10(32):27391-27406. [26] JIAO C, GAO L, ZHANG H, et al. Dynamic Covalent C-C Bond, Cross-Linked, Injectable, and Self-Healable Hydrogels via Knoevenagel Condensation. Biomacromolecules. 2020;21(3):1234-1242. [27] YU G, CHEN X. Host-Guest Chemistry in Supramolecular Theranostics. Theranostics. 2019;9(11):3041-3074. [28] HIGHLEY CB, RODELL CB, BURDICK JA. Direct 3D Printing of Shear-Thinning Hydrogels into Self-Healing Hydrogels. Adv Mater. 2015;27(34):5075-5079. [29] FENG Q, XU J, ZHANG K, et al. Dynamic and Cell-Infiltratable Hydrogels as Injectable Carrier of Therapeutic Cells and Drugs for Treating Challenging Bone Defects. ACS Cent Sci. 2019;5(3):440-450. [30] WANG S, LIU M, GAO L, et al. Optimized Association of Short Alkyl Side Chains Enables Stiff, Self-Recoverable, and Durable Shape-Memory Hydrogel. ACS Appl Mater Interfaces. 2019;11(21):19554-19564. [31] GHANIAN MH, MIRZADEH H, BAHARVAND H. In Situ Forming, Cytocompatible, and Self-Recoverable Tough Hydrogels Based on Dual Ionic and Click Cross-Linked Alginate. Biomacromolecules. 2018;19(5):1646-1662. [32] SHI L, ZHAO Y, XIE Q, et al. Moldable Hyaluronan Hydrogel Enabled by Dynamic Metal-Bisphosphonate Coordination Chemistry for Wound Healing. Adv Healthc Mater. 2018;7(5):1700973. [33] BORRÉ E, BELLEMIN‐LAPONNAZ S, MAURO M. Amphiphilic Metallopolymers for Photoswitchable Supramolecular Hydrogels. Chemistry. 2016;22(52): 18718-18721. [34] WANG Z, REN Y, ZHU Y, et al. A Rapidly Self-Healing Host–Guest Supramolecular Hydrogel with High Mechanical Strength and Excellent Biocompatibility. Angew Chem Int Ed Engl. 2018; 57(29): 9008-9012. [35] NAAHIDI S, JAFARI M, LOGAN M, et al. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol Adv. 2017;35(5): 530-544. [36] RASTOGI P, KANDASUBRAMANIAN B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication. 2019;11(4): 042001. [37] ANTOINE EE, VLACHOS PP, RYLANDER MN. Review of collagen I hydrogels for bioengineered tissue microenvironments: characterization of mechanics, structure, and transport. Tissue Eng Part B Rev. 2014;20(6):683-696. [38] KLOTZ BJ, GAWLITTA D, ROSENBERG AJWP, et al. Gelatin-Methacryloyl Hydrogels: Towards Biofabrication-Based Tissue Repair. Trends Biotechnol. 2016;34(5):394-407. [39] AHMADIAN E, EFTEKHARI A, DIZAJ SM, et al. The effect of hyaluronic acid hydrogels on dental pulp stem cells behavior. Int J Biol Macromol. 2019; 140:245-254. [40] WU Y, LI C, BOLDT F, et al. Programmable protein-DNA hybrid hydrogels for the immobilization and release of functional proteins. Chem Commun (Camb). 2014;50(93):14620-14622. [41] MU Z, CHEN K, YUAN S, et al. Gelatin Nanoparticle-Injectable Platelet-Rich Fibrin Double Network Hydrogels with Local Adaptability and Bioactivity for Enhanced Osteogenesis. Adv Healthc Mater. 2020;9(5):e1901469. [42] SHALUMON KT, LIAO HT, KUO CY, et al. Rational design of gelatin/nanohydroxyapatite cryogel scaffolds for bone regeneration by introducing chemical and physical cues to enhance osteogenesis of bone marrow mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl. 2019;104:109855. [43] ZHOU H, LEE J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011;7(7):2769-2781. [44] MEIRELLES L, ARVIDSSON A, ANDERSSON M, et al. Nano hydroxyapatite structures influence early bone formation. J Biomed Mater Res A. 2008; 87(2):299-307. [45] ZAIDI M, YUEN T, SUN L, et al. Regulation of Skeletal Homeostasis. Endocr Rev. 2018;39(5):701-718. [46] LOPES D, MARTINS-CRUZ C, OLIVEIRA MB, et al. Bone physiology as inspiration for tissue regenerative therapies. Biomaterials. 2018;185:240-275. [47] KEAVENY TM, MORGAN EF, NIEBUR GL, et al. Biomechanics of trabecular bone. Annu Rev Biomed Eng. 2001;3:307-333. [48] HUANG W, WANG Y, CHEN Y, et al. Strong and Rapidly Self-Healing Hydrogels: Potential Hemostatic Materials. Adv Healthc Mater. 2016;5(21):2813-2822. [49] DING F, WU S, WANG S, et al. A dynamic and self-crosslinked polysaccharide hydrogel with autonomous self-healing ability. Soft Matter. 2015;11(20): 3971-3976. [50] MURPHY CM, O’BRIEN FJ, LITTLE DG, et al. Cell-scaffold interactions in the bone tissue engineering triad. Eur Cell Mater. 2013;26:120-132. [51] LOH QL, CHOONG C. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng Part B Rev. 2013; 19(6):485-502. [52] XIA Y, SUN J, ZHAO L, et al. Magnetic field and nano-scaffolds with stem cells to enhance bone regeneration. Biomaterials. 2018;183:151-170. [53] RUPP F, LIANG L, GEIS-GERSTORFER J, et al. Surface characteristics of dental implants: A review. Dent Mater. 2018;34(1):40-57. [54] WANG X, WANG Y, GOU W, et al. Role of mesenchymal stem cells in bone regeneration and fracture repair: a review. Int Orthop. 2013;37(12):2491-2498. [55] NGUYEN PK, RHEE JW, WU JC. Adult Stem Cell Therapy and Heart Failure, 2000 to 2016: A Systematic Review. JAMA Cardiol. 2016;1(7):831-841. [56] HAO Z, WANG S, ZHANG X, et al. Stem cell therapy: a promising biological strategy for tendon–bone healing after anterior cruciate ligament reconstruction. Cell Prolif. 2016;49(2):154-162. [57] MENASCHÉ P. Cell therapy trials for heart regeneration - lessons learned and future directions. Nat Rev Cardiol. 2018;15(11):659-671. [58] CAI L, DEWI RE, HEILSHORN SC. Injectable Hydrogels with In Situ Double Network Formation Enhance Retention of Transplanted Stem Cells. Adv Funct Mater. 2015;25(9):1344-1351. [59] LI F, TRUONG VX, FISCH P, et al. Cartilage tissue formation through assembly of microgels containing mesenchymal stem cells. Acta Biomater. 2018;77:48-62. [60] LI F, TRUONG VX, THISSEN H, et al. Microfluidic Encapsulation of Human Mesenchymal Stem Cells for Articular Cartilage Tissue Regeneration. ACS Appl Mater Interfaces. 2017;9(10):8589-8601. [61] LI L, LU H, ZHAO Y, et al. Functionalized cell-free scaffolds for bone defect repair inspired by self-healing of bone fractures: A review and new perspectives. Mater Sci Eng C Mater Biol Appl. 2019;98:1241-1251. [62] MOE SM. Disorders involving calcium, phosphorus, and magnesium. Primary Care. 2008;35(2):215-237,v–vi. [63] ZARA JN, SIU RK, ZHANG X, et al. High Doses of Bone Morphogenetic Protein 2 Induce Structurally Abnormal Bone and Inflammation In Vivo. Tissue Eng Part A. 2011;17(9-10):1389-1399. [64] HANKENSON KD, GAGNE K, SHAUGHNESSY M. Extracellular signaling molecules to promote fracture healing and bone regeneration. Adv Drug Deliv Rev. 2015;94:3-12. [65] TAN J, ZHANG M, HAI Z, et al. Sustained Release of Two Bioactive Factors from Supramolecular Hydrogel Promotes Periodontal Bone Regeneration. ACS nano. 2019;13(5):5616-5622. [66] MOMOSE T, MIYAJI H, KATO A, et al. Collagen Hydrogel Scaffold and Fibroblast Growth Factor-2 Accelerate Periodontal Healing of Class II Furcation Defects in Dog. Open Dent J. 2016;10:347-359. [67] ZHOU A, CHEN S, HE B, et al. Controlled release of TGF-beta 1 from RADA self-assembling peptide hydrogel scaffolds. Drug Des Devel Ther. 2016;10: 3043-3051. [68] YOSHIZAWA S, BROWN A, BARCHOWSKY A, et al. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014; 10(6):2834-2842. [69] VENKATRAMAN SK, SWAMIAPPAN S. Review on calcium- and magnesium-based silicates for bone tissue engineering applications. J Biomed Mater Res A. 2020;108(7):1546-1562. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Wu Weiyue, Guo Xiaodong, Bao Chongyun. Application of engineered exosomes in bone repair and regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1102-1106. |
| [3] | Zhou Hongqin, Wu Dandan, Yang Kun, Liu Qi. Exosomes that deliver specific miRNAs can regulate osteogenesis and promote angiogenesis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1107-1112. |
| [4] | Huang Chuanjun, Zou Yu, Zhou Xiaoting, Zhu Yangqing, Qian Wei, Zhang Wei, Liu Xing. Transplantation of umbilical cord mesenchymal stem cells encapsulated in RADA16-BDNF hydrogel promotes neurological recovery in an intracerebral hemorrhage rat model [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 510-515. |
| [5] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [6] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [7] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [8] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [9] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [10] | Liu Yingsong, Guo Xiaopeng, Wei Mingzhu. Transforming growth factor beta 3 and alginate hydrogel complex on the repair of articular cartilage defects of the knee [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(16): 2504-2509. |
| [11] | Yuan Yihang, Xu Menghan, Niu Xufeng. Gelatin collagen composite hydrogel and inducible factor regulate differentiation of rat bone marrow mesenchymal stem cells into hepatocyte-like cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(16): 2510-2515. |
| [12] | Li Mingxin, Li Jun, Wang Wenchao, Song Ping, Lei Haoyuan, Gui Xingyu, Zhang Chengyun, Zhou Changchun, Liu Lei. Cell-carrying porous methacrylate anhydride gelatin three-dimensional scaffolds and their effects on cell behavior [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(16): 2532-2539. |
| [13] | Ma Ziyu, Zhang Yuntao, Ma Xiangrui, Qiao Luhui, Guo Haoyu, Hou Yudong. Surface treatment of iron oxide nanoparticles in bone defect repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(16): 2570-2575. |
| [14] | Liu Xiaolin, Liu Shutai, Han Xiaoqian, Mu Xinyue. Topical simvastatin administration in the treatment of periodontitis: effect of loading on sustained drug release system or biomaterial scaffold system [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(16): 2596-2601. |
| [15] | Huang Bo, Chen Mingxue, Peng Liqing, Luo Xujiang, Li Huo, Wang Hao, Tian Qinyu, Lu Xiaobo, Liu Shuyun, Guo Quanyi . Fabrication and biocompatibility of injectable gelatin-methacryloyl/cartilage-derived matrix particles composite hydrogel scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(16): 2480-2486. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||