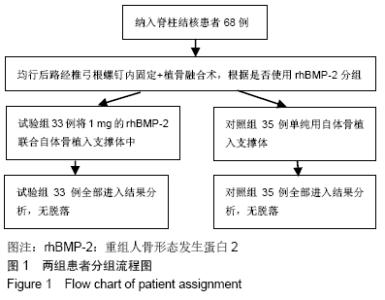
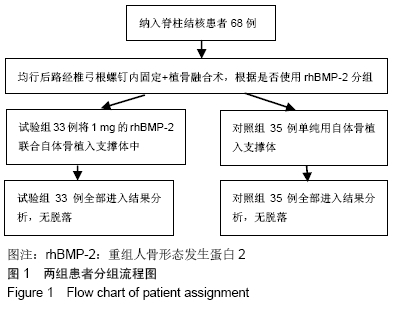
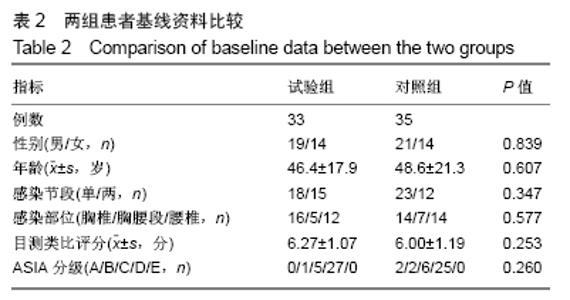
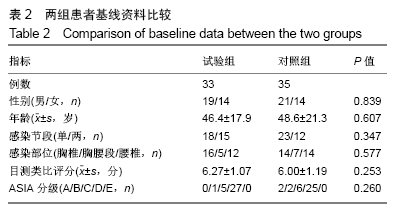
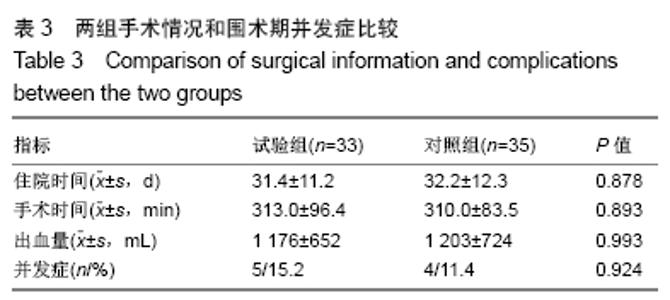
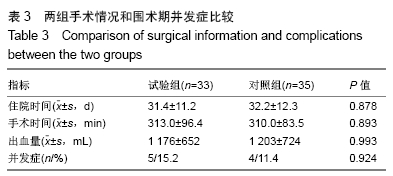
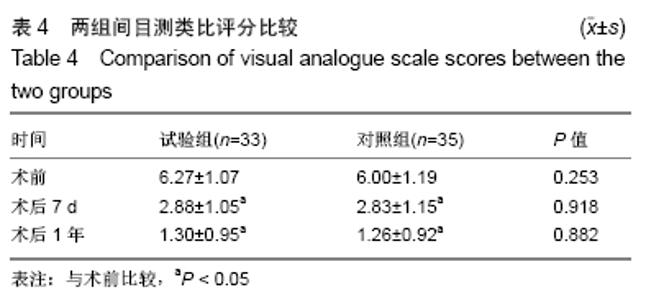
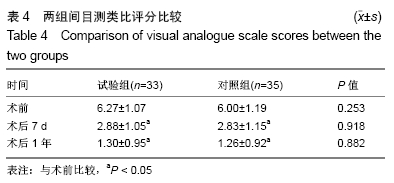
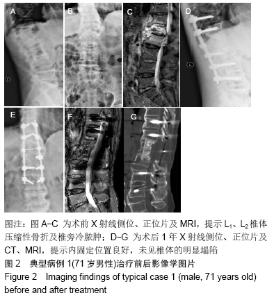
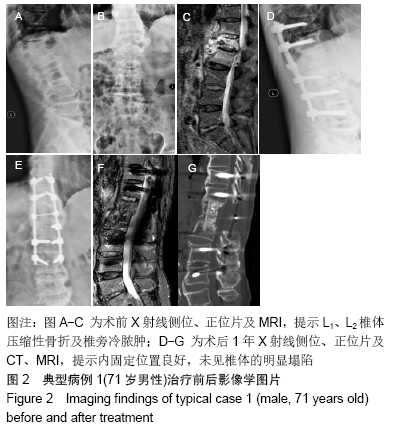
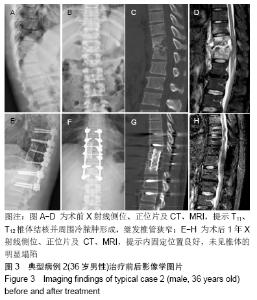
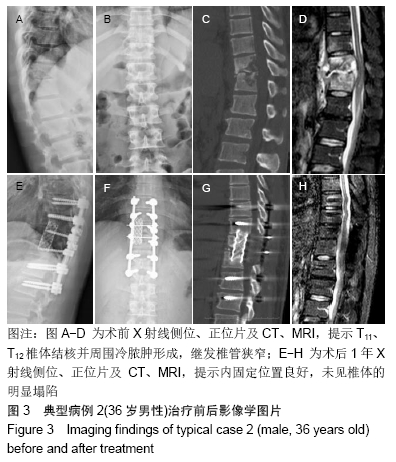
[1] 甘锋平,谭海涛,江建中,等.微创侧路病灶清除融合内固定治疗腰椎结核[J].中国微创外科杂志,2015,15(7): 624-627.
[2] 李兆鹏.后路病灶清除仿生植骨联合内固定术治疗胸腰椎结核的疗效[J].中国继续医学教育,2019,11(3): 78-80.
[3] 王健.人重组骨形态发生蛋白-2在脊柱融合中的作用[J].生物骨科材料与临床研究,2016,13(5):62-66.
[4] POUNTOS I, GEORGOULI T, HENSHAW K, et al.The effect of bone morphogenetic protein-2, bone morphogenetic protein-7, parathyroid hormone, and platelet-derived growth factor on the proliferation and osteogenic differentiation of mesenchymal stem cells derived from osteoporotic bone.J Orthop Trauma. 2010;24(9):552-556.
[5] NOURIAN A A, HARRINGTON J, PULIDO PA, et al. Fusion rates of lateral lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Global Spine J.2019;9(4): 398-402.
[6] VINCENTELLI AF, SZADKOWSKI M, VARDON D, et al. rhBMP-2 (Recombinant Human Bone Morphogenetic Protein-2) In real world spine surgery. A phase IV, National, multicentre, retrospective study collecting data from patient medical files in French spinal centres.Orthop Traumatol Surg Res.2019; 105(6):1157-1163.
[7] 唐勇,叶记超,胡旭民,等. 基因重组人骨形态发生蛋白-2联合自体骨椎间打压植骨在腰椎融合术中的应用研究[J].中华骨与关节外科杂志,2018, 11(9):664-667.
[8] 胡庆柱,代国,余铃,等. 重组人骨形态发生蛋白-2联合后椎弓根螺钉系统固定加椎间植骨融合治疗退变性腰椎滑脱的效果[J].中国医药导报,2018, 15(12):69-72.
[9] SUK SI, LEE CK, KIM WJ, et al. Adding posterior lumbar interbody fusion to pediclescrew fixation and posterolateral fusion after decompression in spondylolytic spondyiolisthesis.Spine.1997;22(2): 210-229.
[10] 山富彦. 不同植骨材料联合钉棒系统在胸腰椎结核植骨融合术的应用和比较[J].实用医学杂志,2012, 28(14):2416-2418.
[11] 印飞,孙振中,殷渠东,等.短节段椎弓根钉固定联合重组人BMP-2和同种异体骨植骨治疗胸腰椎爆裂骨折[J].中国修复重建外科杂志,2017,31(9): 1080-1085.
[12] FUNG SL, WU X, MACEREN JP, et al. In vitro evaluation of recombinant bone morphogenetic protein-2 activity for regenerative medicine. Tissue Eng Part C Methods.2019;25(9):553-559.
[13] ONG KL, VILLARRAGA ML, LAU E, et al.Off-label use of bone morphogenetic proteins in the united states using administrative data. Spine.2010;35(19):1794-1800.
[14] BURKUS JK, HEIM SE, GORNET MF, et al.Is INFUSE bone graft superior to autograft bone? An integrated analysis of clinical trials using the LT-CAGE lumbar tapered fusion device. J Spinal Disord Tech. 2003;16(2):113-122.
[15] 王展,张军,王登峰,等.重组人骨形态发生蛋白2复合骨在腰椎融合中的效果[J].中国组织工程研究, 2015, 19(25):3957-3961.
[16] KHAN TR, PEARCE KR, MCANANY SJ, et al. Comparison of transforaminal lumbar interbody fusion outcomes in patients receiving rhBMP-2 versus autograft. Spine J. 2018;18(3):439-446.
[17] MANZUR M, VIRK SS, JIVANELLI B, et al. The rate of fusion for stand-alone anterior lumbar interbody fusion: a systematic review. Spine J. 2019;19(7):1294-1301.
[18] CRAWFORD CH, CARREON LY, MCGINNIS MD, et al. Perioperative complications of recombinant human bone morphogenetic protein-2 on an absorbable collagen sponge versus iliac crest bone graft for posterior cervical arthrodesis. Spine. 2009;34(13):1390-1394.
[19] RIHN JA, PATEL R, MAKDA J, et al. Complications associated with single-level transforaminal lumbar interbody fusion. Spine J. 2009;9(8): 623-629.
[20] LOW J, ROSS JS, RITCHIE JD, et al. Comparison of two independent systematic reviews of trials of recombinant human bone morphogenetic protein-2 (rhBMP-2): the Yale Open Data Access Medtronic Project. Syst Rev. 2017;6(1): 28.
[21] DMITRIEV AE, LEHMAN RA, SYMES AJ, et al. Bone morphogenetic protein-2 andspinal arthrodesis: the basic science perspective on protein interaction with the nervous system. Spine J.2011;11(6): 500-505.
|