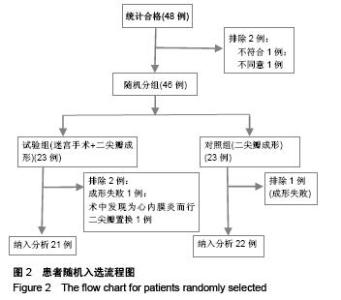
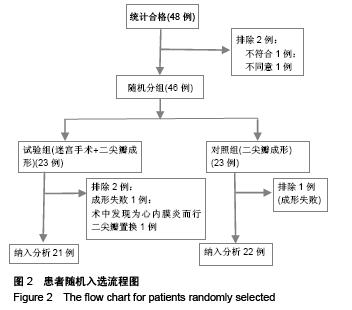
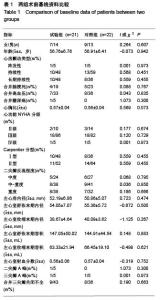
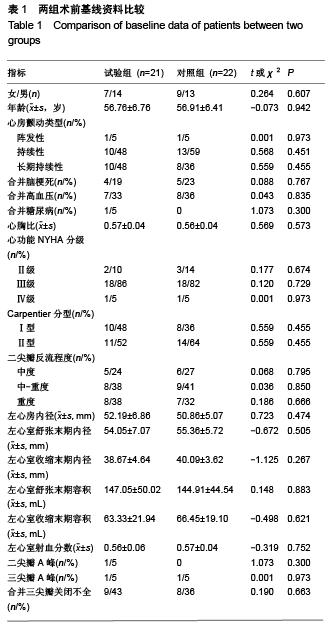
Chinese Journal of Tissue Engineering Research ›› 2015, Vol. 19 ›› Issue (52): 8522-8528.doi: 10.3969/j.issn.2095-4344.2015.52.028
Artificial valve ring implantation and Cox Maze III procedure in treatment of degenerative mitral annulus dilatation complicated by atrial fibrillation
Han Jin-song1, 2, Wang Hui-shan2, Wang Zeng-wei2, Yin Zong-tao2, Han Hong-guang2, Jin Yan2, Liu Yu2, Zhao Ke-yan2, Yu Yan2, Zhao Yang2, Chen Zhao-hui2
- 1Department of Cardiovascular Surgery, Xijing Hospital, the Fourth Military Medical University of Chinese PLA, Xi’ an 710032, Shaanxi Province, China; 2Department of Cardiovascular Surgery, General Hospital of Shenyang Military Area Command, Shenyang 110016, Liaoning Province, China
-
Received:2015-11-02Online:2015-12-17Published:2015-12-17 -
Contact:Wang Hui-shan, M.D., Chief physician, Professor, Doctoral supervisor, Department of Cardiovascular Surgery, General Hospital of Shenyang Military Area Command, Shenyang 110016, Liaoning Province, China -
About author:Han Jin-song, Studying for doctorate, Associate chief physician, Department of Cardiovascular Surgery, Xijing Hospital, the Fourth Military Medical University of Chinese PLA, Xi’an 710032, Shaanxi Province, China; Department of Cardiovascular Surgery, General Hospital of Shenyang Military Area Command, Shenyang 110016, Liaoning Province, China -
Supported by:the Natural Science Foundation of Liaoning Province of China, No. 2014020065; Earl Bakken Scholarship for Thoracic and Cardiovascular Surgery, Chinese Medical Association in 2014
CLC Number:
Cite this article
Han Jin-song, Wang Hui-shan, Wang Zeng-wei, Yin Zong-tao, Han Hong-guang, Jin Yan, Liu Yu, Zhao Ke-yan, Yu Yan, Zhao Yang, Chen Zhao-hui. Artificial valve ring implantation and Cox Maze III procedure in treatment of degenerative mitral annulus dilatation complicated by atrial fibrillation[J]. Chinese Journal of Tissue Engineering Research, 2015, 19(52): 8522-8528.
share this article
| [1] Gillinov AM,Saltman AE.Ablation of atrial fibrillation with concomitant cardiac surgery.Semin Thorac Cardiovasc Surg. 2007;19(1):25-32.
[2] Lee R,McCarthy PM,Wang EC,et al.Midterm survival in patients treated for atrial fibrillation: a propensity-matched comparison to patients without a history of atrial fibrillation.J Thorac Cardiovasc Surg.2012;143(6):1341-1351.
[3] Chugh SS,Havmoeller R,Narayanan K,et al.Worldwide epidemiology of atrial fi brillation: a Global Burden of Disease 2010 Study.Circulation.2014;129(8):837-847.
[4] Ad N,Henry L,Massimiano P,et al.The state of surgical ablation for atrial fibrillation in patients with mitral valve disease.Curr Opin Cardiol.2013; 28(2):170-180.
[5] Gammie JS,Haddad M,Milford-Beland S,et al.Atrial fibrillation correction surgery: lessons from the Society of Thoracic Surgeons National Cardiac Database.Ann Thorac Surg. 2008; 85(3):909-914.
[6] Ad N,Suri RM,Gammie JS,et al.Surgical ablation of atrial fibrillation trends and outcomes in North America.J Thorac Cardiovasc Surg.2012;144(5):1051-1060.
[7] Stulak JM,Dearani JA,Sundt TM 3rd,et al.Superiority of cut-and-sew technique for the Cox maze procedure: Comparison with radiofrequency ablation.J Thorac Cardiovasc Surg.2007;133(4):1023-1027.
[8] Gillinov AM,Gelijns AC,Parides MK,et al.Surgical ablation of atrial fibrillation during mitral-valve surgery.N Engl J Med. 2015;372(15):1399-1409.
[9] Saint LL,Damiano RJ Jr,Cuculich PS,et al.Incremental risk of the Cox-maze IV procedure for patients with atrial fibrillation undergoing mitral valve surgery. J Thorac Cardiovasc Surg. 2013;146(5):1072-1077.
[10] Ad N,Henry L,Hunt S,et al.Do we increase the operative risk by adding the Cox Maze III procedure to aortic valve replacement and coronary artery bypass surgery? J Thorac Cardiovasc Surg.2012;143(4):936-944.
[11] 韩劲松,王辉山,尹宗涛,等.人工腱索移植治疗二尖瓣脱垂78 例[J].中国胸心血管外科临床杂志,2013,20(4):425-429.
[12] 韩劲松,王辉山,尹宗涛,等.两种人工瓣环置入修复单纯二尖瓣环扩张引起的二尖瓣关闭不全的比较[J].中国组织工程研究, 2015, 19(16):2578-2582.
[13] 汪曾炜,张宝仁,朱家麟,等.慢性房颤合并二尖瓣病的迷宫手术[J].中华外科杂志,1997, 35(11):670-674.
[14] 王辉山,汪曾炜,李新民,等.141例心脏手术同期施行双极射频迷宫手术治疗房颤[J].中华胸心血管外科杂志,2009,25(6):371-374.
[15] Yin Z,Wang H,Wang Z,et al.The midterm results of radiofrequency ablation and vagal denervation in the surgical treatment of long-standing atrial fibrillation associated with rheumatic heart disease.Thorac Cardiovasc Surg.2015; 63(3):250-256.
[16] 韩劲松,王辉山,尹宗涛,等.二尖瓣人工腱索移植术的近中期疗效[J].中华医学杂志,2013, 93(34):2730-2732.
[17] Yap CH,Prior D,Kenny J,et al. Is there still a role for the classical Cox-Maze III? ANZ J Surg.2006;76(5):351-355.
[18] Ailawadi G,Gerdisch MW,Harvey RL,et al.Exclusion of the left atrial appendage with a novel device: early results of a multicenter trial. J Thorac Cardiovasc Surg. 2011;142(5): 1002-1009.
[19] Starcka CT,Steffelb J,Emmerta MY,et al.Epicardial left atrial appendage clip occlusion also provides the electrical isolation of the left atrial appendage. Inter CardioVasc Thorac Surg. 2012;15(3):416-418.
[20] Slater AD,Tatooles AJ,Coffey A,et al.Prospective Clinical Study of a Novel Left Atrial Appendage Occlusion Device.Ann Thorac Surg.2012;93(6):2035-2040.
[21] Ngaage DL,Schaff HV,Mullany CJ,et al.Influence of preoperative atrial fibrillation on late results of mitral repair: is concomitant ablation justified? Ann Thorac Surg. 2007;84(2): 434-42; discussion 442-443.
[22] Yoo JS,Kim JB,Jung SH,et al.Impact of the maze procedure and postoperative atrial fibrillation on progression of functional tricuspid regurgitation in patients undergoing degenerative mitral repair. Eur J Cardiothorac Surg. 2013; 43(3): 520-525.
[23] Garcia-Villarreal OA,Fernández-Ceseña E,Vega-Hernández R.Cox maze III procedure: the best alternative in surgery for atrial fibrillation.J Thorac Cardiovasc Surg. 2014;148(1): 368-369.
|
| [1] | Chen Ziyang, Pu Rui, Deng Shuang, Yuan Lingyan. Regulatory effect of exosomes on exercise-mediated insulin resistance diseases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 4089-4094. |
| [2] | Chen Yang, Huang Denggao, Gao Yuanhui, Wang Shunlan, Cao Hui, Zheng Linlin, He Haowei, Luo Siqin, Xiao Jingchuan, Zhang Yingai, Zhang Shufang. Low-intensity pulsed ultrasound promotes the proliferation and adhesion of human adipose-derived mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3949-3955. |
| [3] | Yang Junhui, Luo Jinli, Yuan Xiaoping. Effects of human growth hormone on proliferation and osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3956-3961. |
| [4] | Sun Jianwei, Yang Xinming, Zhang Ying. Effect of montelukast combined with bone marrow mesenchymal stem cell transplantation on spinal cord injury in rat models [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3962-3969. |
| [5] | Gao Shan, Huang Dongjing, Hong Haiman, Jia Jingqiao, Meng Fei. Comparison on the curative effect of human placenta-derived mesenchymal stem cells and induced islet-like cells in gestational diabetes mellitus rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3981-3987. |
| [6] | Hao Xiaona, Zhang Yingjie, Li Yuyun, Xu Tao. Bone marrow mesenchymal stem cells overexpressing prolyl oligopeptidase on the repair of liver fibrosis in rat models [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3988-3993. |
| [7] | Liu Jianyou, Jia Zhongwei, Niu Jiawei, Cao Xinjie, Zhang Dong, Wei Jie. A new method for measuring the anteversion angle of the femoral neck by constructing the three-dimensional digital model of the femur [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3779-3783. |
| [8] | Meng Lingjie, Qian Hui, Sheng Xiaolei, Lu Jianfeng, Huang Jianping, Qi Liangang, Liu Zongbao. Application of three-dimensional printing technology combined with bone cement in minimally invasive treatment of the collapsed Sanders III type of calcaneal fractures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3784-3789. |
| [9] | Qian Xuankun, Huang Hefei, Wu Chengcong, Liu Keting, Ou Hua, Zhang Jinpeng, Ren Jing, Wan Jianshan. Computer-assisted navigation combined with minimally invasive transforaminal lumbar interbody fusion for lumbar spondylolisthesis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3790-3795. |
| [10] | Hu Jing, Xiang Yang, Ye Chuan, Han Ziji. Three-dimensional printing assisted screw placement and freehand pedicle screw fixation in the treatment of thoracolumbar fractures: 1-year follow-up [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3804-3809. |
| [11] | Shu Qihang, Liao Yijia, Xue Jingbo, Yan Yiguo, Wang Cheng. Three-dimensional finite element analysis of a new three-dimensional printed porous fusion cage for cervical vertebra [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3810-3815. |
| [12] | Wang Yihan, Li Yang, Zhang Ling, Zhang Rui, Xu Ruida, Han Xiaofeng, Cheng Guangqi, Wang Weil. Application of three-dimensional visualization technology for digital orthopedics in the reduction and fixation of intertrochanteric fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3816-3820. |
| [13] | Sun Maji, Wang Qiuan, Zhang Xingchen, Guo Chong, Yuan Feng, Guo Kaijin. Development and biomechanical analysis of a new anterior cervical pedicle screw fixation system [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3821-3825. |
| [14] | Lin Wang, Wang Yingying, Guo Weizhong, Yuan Cuihua, Xu Shenggui, Zhang Shenshen, Lin Chengshou. Adopting expanded lateral approach to enhance the mechanical stability and knee function for treating posterolateral column fracture of tibial plateau [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3826-3827. |
| [15] | Zhu Yun, Chen Yu, Qiu Hao, Liu Dun, Jin Guorong, Chen Shimou, Weng Zheng. Finite element analysis for treatment of osteoporotic femoral fracture with far cortical locking screw [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3832-3837. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||