Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (6): 1580-1591.doi: 10.12307/2026.594
Previous Articles Next Articles
Bibliometric and visual analysis of the research status and trends of senescence in osteoporosis
Zhang Haiwen1, Zhang Xian2, Xu Taichuan1, Li Chao2
- 1Nanjing University of Chinese Medicine, Wuxi 210023, Jiangsu Province, China; 2Affiliated Wuxi Hospital of Nanjing University of Chinese Medicine, Wuxi 214000, Jiangsu Province, China
-
Received:2024-12-06Accepted:2025-02-20Online:2026-02-28Published:2025-07-19 -
Contact:Zhang Xian, Chief physician, Doctoral supervisor, Affiliated Wuxi Hospital of Nanjing University of Chinese Medicine, Wuxi 214000, Jiangsu Province, China -
About author:Zhang Haiwen, MS candidate, Nanjing University of Chinese Medicine, Wuxi 210023, Jiangsu Province, China -
Supported by:National TCM Advantageous Specialty Construction Project of National Administration of Traditional Chinese Medicine, No. [2024]90 (to ZX); Jiangsu Province Basic Research Program, No. BK20231147 (to ZX); Jiangsu Province TCM Science and Technology Development Program Project, No. MS2021044 (to ZX)
CLC Number:
Cite this article
Zhang Haiwen, Zhang Xian, Xu Taichuan, Li Chao. Bibliometric and visual analysis of the research status and trends of senescence in osteoporosis[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1580-1591.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
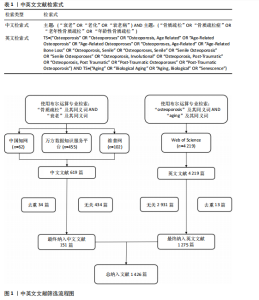
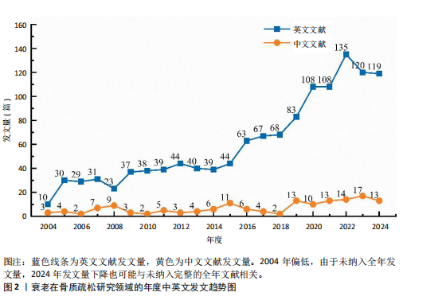
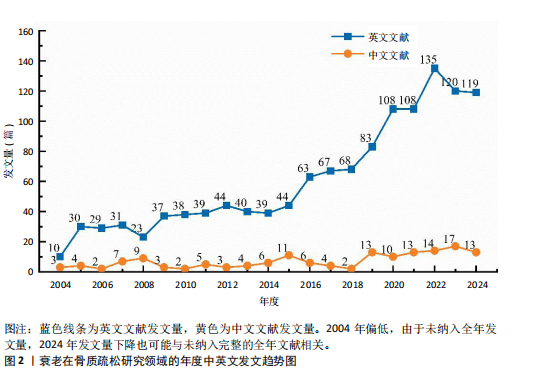
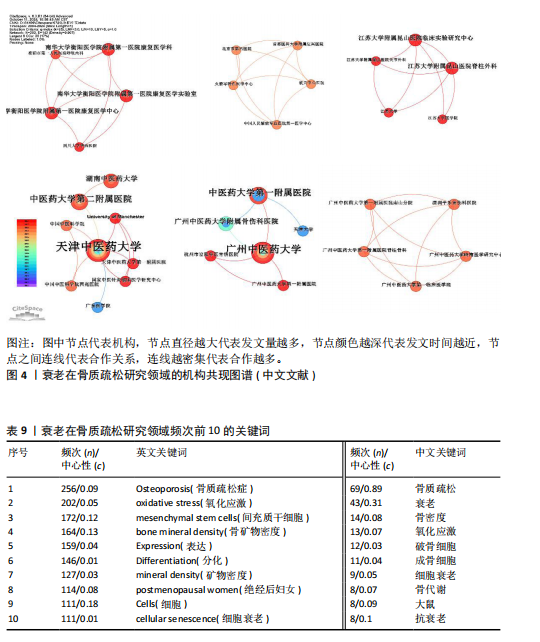
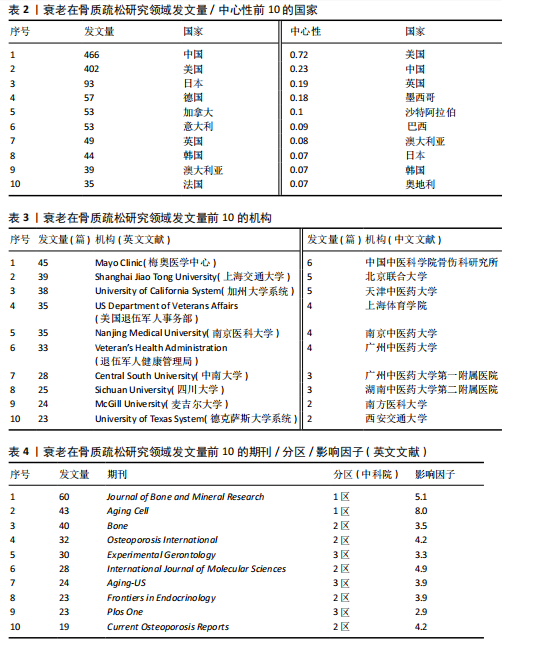
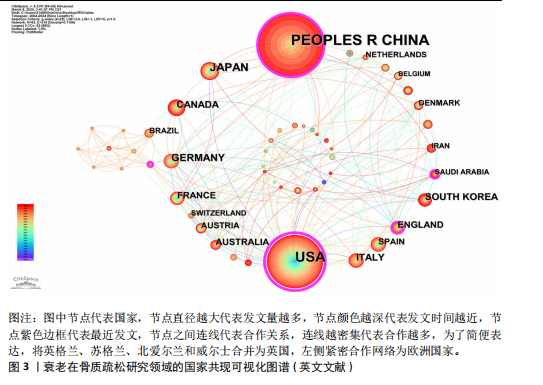
2.1 年度发文量分析 数据统计显示2004-2024年期间,文献量呈现持续增长趋势,2004-2015年发文量为相对停滞阶段,年发文量不足50篇,而2016-2018年为缓慢增长阶段,最后由2018年的68篇至今2024年的119篇为文献激增阶段,而中文文献呈缓慢增长趋势。从总发文趋势来看,近6年来衰老在骨质疏松领域的研究正受到热切关注,见图2。 2.2 发文国家和机构分析 通过将文献导入CiteSpace软件后,从英文文献中统计发文国家,通过分析得到节点N=64个,连线E=110条,表明发文国家为64个,合作次数为110次。中国(470篇)发文数量最多,其次是美国(402篇)、日本(93篇),中国和美国发文量远远领先于其他国家,占总发文量的63.4%;从发文国家的中心性上看,美国(0.72)以极高的中心性位列第一,其次是中国(0.22)、英国(0.19),见表2。衰老在骨质疏松领域中国发文量最多,但在中心性上美国领先于其他国家,说明美国在该领域的影响力十分突出。从国家合作上看,尽管欧洲国家单独发文量小,但形成了一个紧密的合作网络,见图3。 在机构分析中,英文文献得出节点N=443个,连线E=565条,前10个发文机构有6个为美国机构,其余4个为中国机构,Mayo Clinic(梅奥医学中心)发文量为45篇,位列发文机构第一,其次是Shanghai Jiao Tong University(上海交通大学)39篇,University of California System(加州大学系统)38篇。在中文文献中,中国中医科学院骨伤科研究所发文量为6篇,位列第一,其次是北京联合大学和天津中医大学,发文量均为5篇,见表3。国内的机构合作主要以广州中医药大学和天津中医药大学形成的两个合作网络为主,见图4。 2.3 期刊分析 英文文献共在455种期刊上发表,其中以期刊《Journal of"
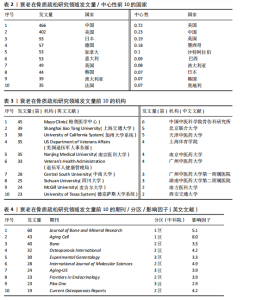
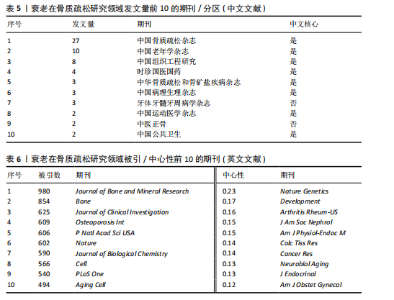
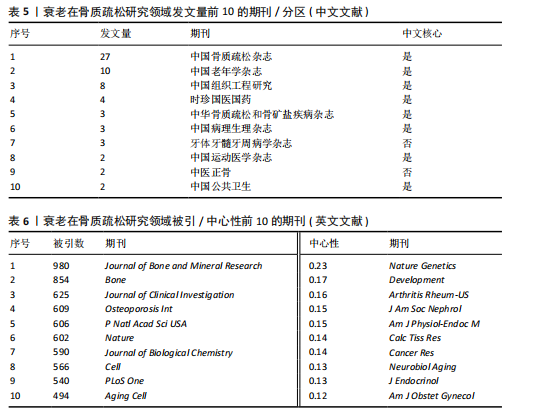
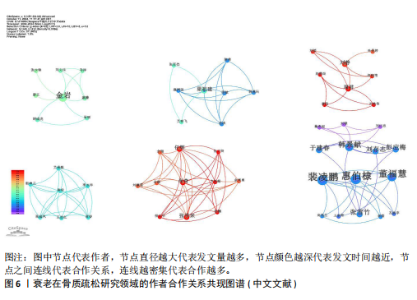
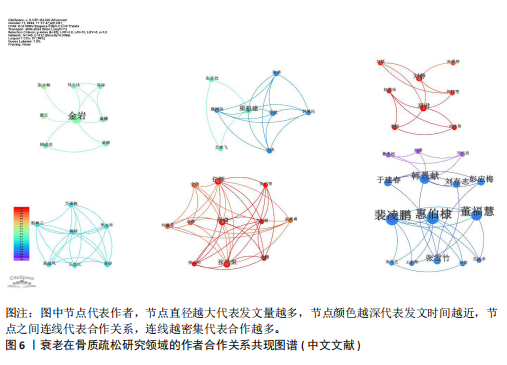
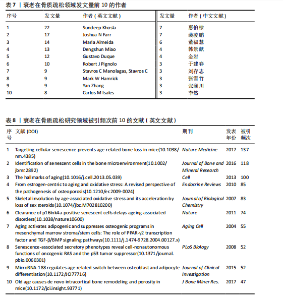
Bone and Mineral Research》发表60篇论文为最多,第二是《Aging Cell》43篇,而第三是《Bone》40篇。在发表量前10期刊中有7个是中科院升级版(2023)分区2区及以上的期刊,其中《Aging Cell》的影响因子8.0为最高,见表4。而中文文献共在84种期刊上发表,其中最值得关注的是发表了27篇的《中国骨质疏松杂志》和发表了10篇的《中国老年学杂志》,在发表量前10的期刊中只有2个不是中文核心期刊,见表5。 在英文文献共被引期刊分析中,共有746种期刊被引用,《Journal of Bone and Mineral Research》被引最多(n=980次),其次是《Bone》(n=854次)和《Journal of Bone and Mineral Research》(n=625次)。在进行中心性计算后得到《Nature Genetics》《Development》《Arthritis Rheum-US》等10本期刊的中心性大于0.1,说明这些期刊对衰老在骨质疏松研究领域发展具有支撑作用,是比较关键的核心期刊,见表6。 2.4 发文作者分析 通过CiteSpace分析,1 275篇英文文献由623位作者发表,有763次的合作关系,151篇中文文献由345位作者发表,有512次的合作关系。普赖斯特定律可用于评估科学文献中作者分布的均匀性,并确定有影响力的核心作者[15]。通过使用公式W≈0.749*(wmax)1/2 (其中wmax表示最多产作者的发表论文数),可以确定一位作者被归类为核心作者,其所需的最低发表论文数为W[16]。衰老在骨质疏松领域的英文文献wmax=22,见表7,得W≈3.51,表示发文数量为4篇及以上的作者才算核心作者。此外,这定律的另一公式K= kmax (其中kmax表示全部作者数)可以计算出核心发文的作者数K[17],英文作者kmax=623,得出K=24.95,表明该领域有25名核心作者。 对英文文献作者合作网络共现分析后,Q值为0.95,S值为0.99,表明共现的相似度较高,(Q,S)=0.97,表明聚类结果比较好,其中以Sundeep Khosla、Joshua Nicholas Joshua、苗登"
顺、Gustavo Duque等人为主的合作网络为主要核心,而苗登顺、Gustavo Duque和张扬为核心的合作网络范围最广,见图5。对中文文献的作者共现分析,得出前6个较大的合作网络,且各合作网络未见明显合作关系,可以看出国内的作者合作较少,对于多团队的合作度不足,见图6。 2.5 文献共被引分析 使用CiteSpace软件对英文文献进行共被引文献分析,得出914篇共被引用文章,发生2 626次互相引用关系。表8列出了被引量最高的前10篇文献,其中有3篇是不低于被引用100次的文章,“Targeting cellular senescence prevents age-related bone loss in mice”(n=137次)被引用量最高,另外是“Identification of senescent cells in the bone micro environment”(n=118次)和“The hallmarks of aging”(n=100次)。 共被引是指2篇文献共同被1篇文献引用,这篇文献为施引文献,阅读施引文献,可以进一步了解该领域的研究前沿和热点[18]。对共被引文献通过LLR算法进行聚类后发现,共被引文献共有23个集群,取前6个聚类,分别为#0-#5,排列依据聚类内文献数降序排列,分别标记为#0 cellular senescence(细胞衰老)、#1 bone microarchitecture(骨微结构)、#2 mesenchymal stem cell(间充质干细胞)、#3 mesenchymal stem cell fate(间充质干细胞命运)、#4 senscence- accelerate mouse(衰老加速小鼠)、#5 bone biology(骨生物学)。 各聚类轮廓值均高于0.8(范围0.813-0.902),表明内部结构具有良好同质性,具体特征如下: #0细胞衰老(122节点,S=0.883)表征的研究前沿聚焦于衰老细胞在年龄相关疾病中的致病机制。施引文献系统揭示了衰老相关分泌表型的分子通路,创新性提出通过senolytics(衰老细胞清除剂)和senomorphics(衰老表型调节剂)实现多病共治的干预策略[19]。#1骨微结构(90节点,S=0.876)突破传统雌激素中心理论范"
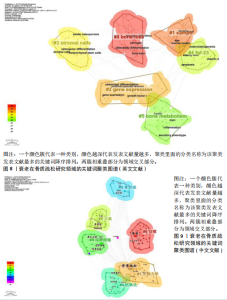
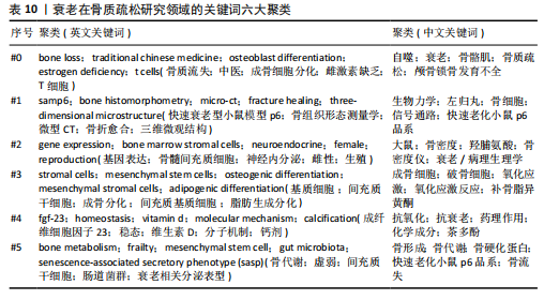
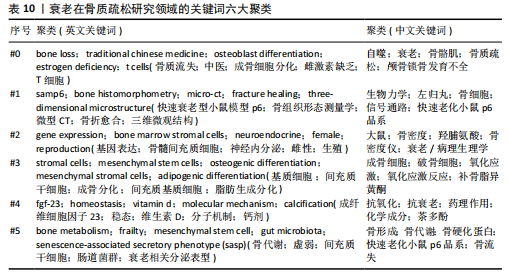
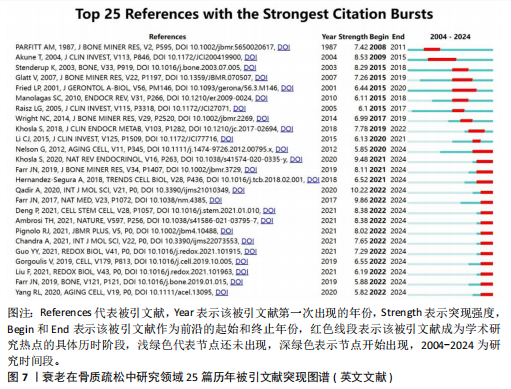
式,从衰老与氧化应激的整合视角重构骨质疏松病理机制,为开发非激素依赖的多维度治疗体系提供了理论依据[20]。#2间充质干细胞(82节点,S=0.863)的研究进展系统阐释了氧化应激与自噬调控在年龄相关性骨病中的双重作用机制,揭示了维持干细胞稳态对骨质疏松防治的关键意义[21]。#3间充质干细胞命运(78节点,S=0.880)从细胞衰老动态演变角度,阐明骨细胞SASP信号对骨质疏松的级联效应。研究突破传统“促形成-抑吸收”的治疗框架,提出衰老细胞靶向清除、SASP信号阻断、干细胞基因活化及中药多靶点干预等创新策略[22]。#4衰老加速小鼠(77节点,S=0.902)通过模式动物研究确证骨细胞衰老与骨质疏松的因果关联,重点解析抗氧化应激和自噬调节在延缓骨衰老中的分子机制[23]。 #5骨生物学(75节点,S=0.813)聚焦sirtuins蛋白家族,系统揭示其通过表观遗传调控影响骨代谢平衡的作用网络,为开发基于sirtuins激活剂的精准治疗策略提供科学依据[24]。 文献共被引突现图谱中,突现文献为该时间段被热点关注的文献,随着时间推移,突现文献的不断演进,也说明该领域研究方向的不断变化,了解突现文献也就意味着理解该领域演进的历史趋势[25]。对英文文献进行共被引文献的突现分析发现,最早文献爆发被引发生在1986年,题目为“Age-related changes in bone mass in the senescence-accelerated mouse(SAM): SAM-R/3 and SAM-P/6 as new murine models for senile osteoporosis”,这篇文献也是2004年来被引时间最长的爆发突现文献,此外文献“Link age of decreased bone mass with impaired osteoblastogenesis in a murine model of accelerated senescence”的强度(13.18)最高,见图7。 2.6 关键词分析 2.6.1 关键词共现分析 英文文献中关键词有518个,中文文献中关键词有194个,对出现频次前10的关键词进行了统计,其中n为频次,c为中心性,中心性大于0.1,则表明该关键词为核心关键词。在英文文献中有osteoporosis(n=256,c=0.09)、oxidative stress(n=202,c=0.05)、mesenchymal stem cells(n=172,c=0.12)等关键词;在中文文献中以骨质疏松、衰老、骨密度等关键词为主,见表9。 2.6.2 关键词聚类分析 对中英文文献关键词进行聚类分析,英文文献得出Q值=0.72 > 0.7,S值=0.87 > 0.7,Q/S=0.78,说明聚类合理,见图8。聚类后选取了前6个关键词,依据关键词数量降序排列,分别是“bone loss”“samp6”“gene expression”“stromal cells”“fgf-23”和“bone metabolism”,见表10。聚类#0反映了衰老在骨质疏松症中骨质流失机制方面的研究;聚类#1反映了衰老在骨质疏松症中骨组织模型的研究;聚类#2反映了衰老在骨质疏松症中基因表达的研究;聚类#3反映了衰老在骨质疏松症中骨间充质细胞分化的研究;聚类#4反映了衰老在骨质疏松症中细胞因子对骨质疏松的影响;聚类#5反映了衰老在骨质疏松症中代谢组的研究。在中文文献中有关键词194个,前6个依次为“自噬”“生物力学”“大鼠”“成骨细胞”“抗氧化”“骨形成”聚类,见图9。 2.6.3 关键词突现分析 关键词的突出显现,表明该关键词在某一年份受到大量关注,有大量关于该关键词的论文发表,随着时间推移和科学的发展,会产生每一个阶段的突现关键词,通过分析不同时间阶段的关键词,研"
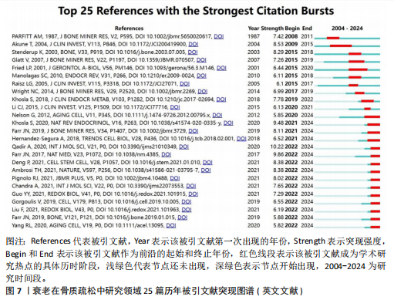
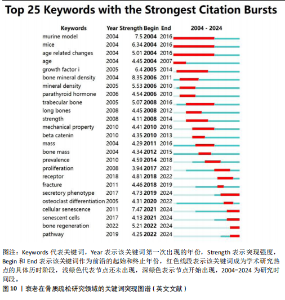
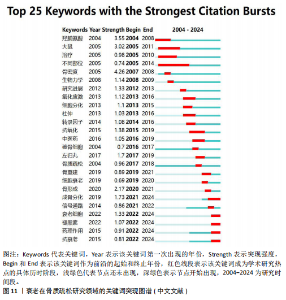
究人员可以很好地了解相关研究领域的历史研究热点轨迹,把握前沿热点[26]。使用CiteSpace软件分析中英文文献近20年的爆发热点关键词,其中英文文献中以“bone mineral density”(8.35)为强度最大,见图10。中文文献中 “骨密度”(4.26)强度最大,而“不同部位”的关注时间最长(9年),见图11。 在英文文献中关键词突现图谱,见图10,可以分为4个阶段:第一段为2004-2006年,标志性关键词是小鼠模型,这个时间段多关注于动物实验和内分泌调节方面;第二阶段为2008-2014年,这个时间段主要关注于肌肉机械功能强度对骨量的影响;第三阶段为2015-2018年,这个时间段多关注衰老在骨质疏松症研究中的受体;第四阶段为2019-2024年,主要关键词有衰老相关分泌型、细胞衰老、衰老细胞、骨再生、通路。自2019年以来衰老相关分泌表型有34篇文章涉及,细胞衰老有77篇文章涉及,衰老细胞有18篇文章涉及,骨再生13篇文章涉及。衰老相关分泌表型、细胞衰老、通路之间的相互作用最近在学术文献中引起极大关注。 在中文文献中关键词突现图谱,见图11,可以根据突现年份将近20年的关键词划分为4个阶段:第一阶段为2004-2008年,“不同部位”为最有代表性,这段时期多关注于骨密度;第二阶段为2012-2020年,抗氧化为代表词,这一阶段多为中医药通过抗氧化干预治疗老年性骨质疏松;第三阶段为2021-2024年,成骨分化最具有代表性,其在骨形成和对抗衰老细胞领域备受关注。中医药对抗衰老有独特的多靶点、多通路、多效应机制的优势,近年来中文文献领域也因为随着衰老治疗发展,开始关注抗衰老方向的研究。"
| [1] HUO R, WEI C, HUANG X, et al. Mortality associated with osteoporosis and pathological fractures in the United States (1999-2020): a multiple-cause-of-death study. J Orthop Surg Res. 2024;19(1):568. [2] XIAO PL, CUI AY, HSU CJ, et al. Global, regional prevalence, and risk factors of osteoporosis according to the World Health Organization diagnostic criteria: a systematic review and meta-analysis. Osteoporos Int. 2022;33(10):2137-2153. [3] LÓPEZ-OTÍN C, BLASCO MA, PARTRIDGE L, et al. The hallmarks of aging. Cell. 2013; 153(6):1194-1217. [4] KENNEDY BK, BERGER SL, BRUNET A, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709-713. [5] DOBSON PF, DENNIS EP, HIPPS D, et al. Mitochondrial dysfunction impairs osteogenesis, increases osteoclast activity, and accelerates age related bone loss. Sci Rep. 2020;10(1):11643. [6] MANSOUR A, MEZOUR MA, BADRAN Z, et al. Extracellular Matrices for Bone Regeneration: A Literature Review. Tissue Eng Part A. 2017;23(23-24):1436-1451. [7] SINGH L, BRENNAN TA, RUSSELL E, et al. Aging alters bone-fat reciprocity by shifting in vivo mesenchymal precursor cell fate towards an adipogenic lineage. Bone. 2016; 85:29-36. [8] MA Y, QI M, AN Y, et al. Autophagy controls mesenchymal stem cell properties and senescence during bone aging. Aging Cell. 2018;17(1):e12709. [9] KIRKLAND JL, TCHKONIA T, ZHU Y, et al. The Clinical Potential of Senolytic Drugs. J Am Geriatr Soc. 2017;65(10):2297-2301. [10] HAMBRIGHT WS, MU X, GAO X, et al. The Senolytic Drug Fisetin Attenuates Bone Degeneration in the Zmpste24-/- Progeria Mouse Model. J Osteoporos. 2023;2023:5572754. [11] KOKOL P, BLAŽUN VOŠNER H, ZAVRŠNIK J. Application of bibliometrics in medicine: a historical bibliometrics analysis. Health Info Libr J. 2021;38(2):125-138. [12] 牛奔,朱晓倩,杨辰,等.基于CiteSpace的国内外医疗大语言模型研究热点演进及趋势分析[J/OL].中国全科医学,1-9[2025-02-10].http://kns.cnki.net/kcms/detail/13.1222.R.20240912.1606.003.html. [13] 宋浩然,张玉强,谷娜,等.基于Citespace对人工智能在骨创伤研究的可视化分析[J].中国组织工程研究,2025, 29(3):493-502. [14] 杨泽雨,支亮,王佳,等.高频重复经颅磁刺激研究热点宏观角度的可视化分析[J].中国组织工程研究,2026,30(5):1320-1330. [15] DE SOLLA PRICE DJ. Little Science, Big Science and beyond. New York: Columbia University Press,1963. [16] JIA HF, LI HZ, RONG YF, et al. Knowledge Mapping of Macrophages in Osteoporosis: A Bibliometric Analysis (1999-2023). Orthop Surg. 2024;16(10):2326-2343. [17] LÓPEZ-MUÑOZ F, WEINREB RN, MOGHIMI S, et al. A Bibliometric and Mapping Analysis of Glaucoma Research between 1900 and 2019. Ophthalmol Glaucoma. 2022;5(1):16-25. [18] CHEN C, HU Z, LIU S, et al. Emerging trends in regenerative medicine: a scientometric analysis in CiteSpace. Expert Opin Biol Ther. 2012;12(5):593-608. [19] KAUR J, FARR JN. Cellular senescence in age-related disorders. Transl Res. 2020;226:96-104. [20] MANOLAGAS SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31(3):266-300. [21] ALMEIDA M, O’BRIEN CA. Basic biology of skeletal aging: role of stress response pathways. J Gerontol A Biol Sci Med Sci. 2013;68(10):1197-1208. [22] WANG T, HUANG S, HE C. Senescent cells: A therapeutic target for osteoporosis. Cell Prolif. 2022;55(12):e13323. [23] MANOLAGAS SC, PARFITT AM. What old means to bone. Trends Endocrinol Metab. 2010;21(6):369-374. [24] LI Q, CHENG JC, JIANG Q, et al. Role of sirtuins in bone biology: Potential implications for novel therapeutic strategies for osteoporosis. Aging Cell. 2021;20(2): e13301. [25] CHEN C. Science Mapping: A Systematic Review of the Literature. JDIS. 2017;2(2):1-40. [26] 彭皓,陈奇刚,申震.H型血管在不同骨骼疾病中研究热点的可视化分析[J].中国组织工程研究,2026,30(3):760-769. [27] ZHU Y, TCHKONIA T, PIRTSKHALAVA T, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14(4):644-658. [28] FARR JN, XU M, WEIVODA MM, et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med. 2017;23(9):1072-1079. [29] 裴凌鹏,董福慧,惠伯棣.D-半乳糖致衰老作用对大鼠骨质的影响[J].中医正骨, 2007,19(3):1-3. [30] 裴凌鹏,董福慧,惠伯棣.虾青素对D-半乳糖老龄大鼠骨质影响[J].中国公共卫生,2007,23(3):297-299. [31] 裴凌鹏,惠伯棣,董福慧.角黄素对D-半乳糖致衰老大鼠抗氧化功能影响[J].中国公共卫生,2007,23(4):453-454. [32] 裴凌鹏,惠伯棣,王东晖,等.反式白藜芦醇对D-半乳糖致老大鼠骨质影响研究[J].北京中医药大学学报,2007,30(5):317-322. [33] MATSUSHITA M, TSUBOYAMA T, KASAI R, et al. Age-related changes in bone mass in the senescence-accelerated mouse (SAM). SAM-R/3 and SAM-P/6 as new murine models for senile osteoporosis. Am J Pathol. 1986;125(2):276-283. [34] JILKA RL, WEINSTEIN RS, TAKAHASHI K, et al. Linkage of decreased bone mass with impaired osteoblastogenesis in a murine model of accelerated senescence. J Clin Invest. 1996;97(7):1732-1740. [35] STENDERUP K, JUSTESEN J, CLAUSEN C, et al. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33(6):919-926. [36] KHOSLA S, FARR JN, TCHKONIA T, et al. The role of cellular senescence in ageing and endocrine disease. Nat Rev Endocrinol. 2020;16(5):263-275. [37] KHOSLA S. Odanacatib: location and timing are everything. J Bone Miner Res. 2012;27(3):506-508. [38] KHOSLA S, FARR JN, KIRKLAND JL. Inhibiting Cellular Senescence: A New Therapeutic Paradigm for Age-Related Osteoporosis. J Clin Endocrinol Metab. 2018;103(4): 1282-1290. [39] QADIR A, LIANG S, WU Z, et al. Senile Osteoporosis: The Involvement of Differentiation and Senescence of Bone Marrow Stromal Cells. Int J Mol Sci. 2020; 21(1):349. [40] DI MICCO R, KRIZHANOVSKY V, BAKER D, et al. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol. 2021;22(2):75-95. [41] FARR JN, FRASER DG, WANG H, et al. Identification of Senescent Cells in the Bone Microenvironment. J Bone Miner Res. 2016;31(11):1920-1929. [42] COPPÉ JP, DESPREZ PY, KRTOLICA A, et al. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99-118. [43] DEMARIA M, OHTANI N, YOUSSEF SA, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31(6):722-733. [44] TAKASUGI M, YOSHIDA Y, HARA E, et al. The role of cellular senescence and SASP in tumour microenvironment. FEBS J. 2023; 290(5):1348-1361. [45] KIRKLAND JL, TCHKONIA T. Senolytic drugs: from discovery to translation. J Intern Med. 2020;288(5):518-536. [46] WANG Y, CHE L, CHEN X, et al. Repurpose dasatinib and quercetin: Targeting senescent cells ameliorates postmenopausal osteoporosis and rejuvenates bone regeneration. Bioact Mater. 2023;25:13-28. [47] CHAN WL, BUCHER CH, GOLDES J, et al. Targeting TGF-β signaling, oxidative stress, and cellular senescence rescues osteoporosis in gerodermia osteodysplastica. Aging Cell. 2024;23(12): e14322. [48] SONAR Y. Therapeutic potential of boosting NAD+ levels in aging and age-related bone loss. Queensland University of Technology, 2023. [49] 吴钰坤,韩杰,温帅波.骨折愈合过程中Runx2基因的作用机制[J].中国组织工程研究,2021,25(14):2274-2279. [50] UNGURIANU A, ZANFIRESCU A, MARGINĂ D. Sirtuins, resveratrol and the intertwining cellular pathways connecting them. Ageing Res Rev. 2023;88:101936. [51] FALVINO A, CARIATI I, BONANNI R, et al. Cellular senescence as a key factor in osteoporosis:the role of SIRT1. Int J Bone Frag. 2023; 3(3):100-104. [52] KANG X, CHEN L, YANG S, et al. Zuogui Wan slowed senescence of bone marrow mesenchymal stem cells by suppressing Wnt/β-catenin signaling. J Ethnopharmacol. 2022;294:115323. [53] ZENG J, XIAO Q, LI X, et al. Advanced oxidation protein products aggravate age‑related bone loss by increasing sclerostin expression in osteocytes via ROS‑dependent downregulation of Sirt1. Int J Mol Med. 2021;47(6):108. [54] WANG Y, XIE F, HE Z, et al. Senescence-Targeted and NAD+-Dependent SIRT1-Activated Nanoplatform to Counteract Stem Cell Senescence for Promoting Aged Bone Regeneration. Small. 2024;20(12): e2304433. [55] WU W, FU J, GU Y, et al. JAK2/STAT3 regulates estrogen-related senescence of bone marrow stem cells. J Endocrinol. 2020;245(1):141-153. [56] ZHANG Y, YU X, ZHOU C, et al. Targeting cellular senescence in senile osteoporosis: therapeutic potential of traditional Chinese medicine. Front Med (Lausanne). 2023;10:1288993. [57] 伍子贤,方志超,林锋,等. 龟板提取物抑制细胞衰老缓解SOP小鼠骨量丢失及海马退变[J].中国骨质疏松杂志, 2024,30(3):318-326. [58] 黄勇,董克芳,王凡,等.三种补肾方含药血清对衰老骨髓间充质干细胞中COLⅠ和ALP的影响[J].湖南中医药大学学报,2022,42(7):1082-1086. [59] 张天驰,李沐哲,牛园园,等.温肾通络止痛方抑制巨噬细胞衰老改善BMSC成骨分化和老年性骨质疏松模型小鼠骨丢失的研究[J].南京中医药大学学报,2024, 40(3):249-260. [60] 钟培瑞,何晓艳,廖瑛,等.P53/P21通路在电针抑制骨质疏松大鼠模型成骨细胞衰老中的机制[J].实用医学杂志,2023, 39(2):192-197. |
| [1] | Xu Canli, He Wenxing, Wang Yuping, Ba Yinying, Chi Li, Wang Wenjuan, Wang Jiajia. Research context and trend of TBK1 in autoimmunity, signaling pathways, gene expression, tumor prevention and treatment [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(在线): 1-11. |
| [2] | Lai Jiaming, , Song Yuling, Chen Zixi, Wei Jinghuan, Cai Hao, , Li Guoquan, . Screening of diagnostic markers for endothelial cell Senescence in mice with radiation-induced heart disease and analysis of immune infiltration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1450-1463. |
| [3] | Wang Yaping, Gao Tianyun, Wang Bin. Senescence of human bone marrow mesenchymal stromal cells with increasing age is not dependent on the mediation of endogenous retroviruses [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 10-20. |
| [4] | Wu Zhijing, Li Jiali, Zhang Jiaxin, Wang Tangrong, Zheng Yuzhou, Sun Zixuan. Alpha-ketoglutarate engineered small extracellular vesicles delay skin aging [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 120-129. |
| [5] | Wang Jianxu, Dong Zihao, Huang Zishuai, Li Siying, Yang Guang. Interaction between immune microenvironment and bone aging and treatment strategies [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6509-6519. |
| [6] | Li Xingfu, Zhou Guangqian, Lu Wei. Main pathways, roles, and regulatory mechanisms of intercellular mitochondrial transfer [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(25): 5443-5453. |
| [7] | Han Fang, Shu Qing, Jia Shaohui, Tian Jun. Electrotactic migration and mechanisms of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(23): 4984-4992. |
| [8] | Li Wenming, Li Yonghang, Yan Caiping, Wang Xingkuan, Xiang Chao, Zhang Yuan, Jiang Ke, Chen Lu. Injectable hydrogel microspheres that enhance autophagy can improve cartilage microenvironment and resist chondrocyte senescence [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(22): 4710-4719. |
| [9] | Gao Feng, Wang Jiliang, Wang Hongbo, Yang Yongsheng, Liu Yuan, Fu Su. Passage-associated senescence decreases osteogenic activity of MC3T3-E1 cells via primary cilia [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(18): 3741-3746. |
| [10] | Zhong Liying, Li Shundong, Wang Cong. miR-212-3p regulates senescence of bone marrow mesenchymal stem cells by targeting MAPK3 [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(13): 2690-2697. |
| [11] | Liu Xin, Hu Man, Zhao Wenjie, Zhang Yu, Meng Bo, Yang Sheng, Peng Qing, Zhang Liang, Wang Jingcheng. Cadmium promotes senescence of annulus fibrosus cells via activation of PI3K/Akt signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1217-1222. |
| [12] | Jia Ruiying, Mei Jie, He Qiang, Li Dan, Sun Xin, Qian Weiqing, Liu Zhen. Catalpol from Rehmannia glutinosa regulates senescence in ATDC5 chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(34): 5467-5472. |
| [13] | Zhao Wenjing, Liu Baikun, Li Qiulian, Chen Xi. Effects of long-term subculture on biological characteristics of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(31): 4926-4930. |
| [14] | Shi Weili, Liu Shanshan, Chang Hongbo, Gao Haixia, Wang Xinzhou, Qin Nan, Wu Hong. Vascular endothelial growth factor combined with basic fibroblast growth factor improves replicative senescence of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(31): 4958-4963. |
| [15] | Li Zhaojin, Zheng Pengcheng, Kong Jianda, Zhu Tengqi, Jiang Fugao. Review of PGC-1α role in exercise anti-aging in different tissues and organs [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(29): 4717-4725. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||