Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (14): 3652-3662.doi: 10.12307/2026.077
Previous Articles Next Articles
Conductive hydrogel with cell-free fat extract repairs spinal cord injuries in rats
Yang Biao1, 2, Wu Zhonghuan1, 2, Jiang Fugui1, 2, He Chenglong1, 2, Li Tingdong1, 2
- 1Department of Orthopedics, People’s Hospital of Qiandongnan Miao and Dong Autonomous Prefecture, Kaili 556000, Guizhou Province, China; 2Qiandongnan Clinical Medical Research Center, Kaili 556000, Guizhou Province, China
-
Received:2025-03-06Accepted:2025-05-17Online:2026-05-18Published:2025-09-12 -
Contact:Yang Biao, MD, Associate chief physician, Department of Orthopedics, People’s Hospital of Qiandongnan Miao and Dong Autonomous Prefecture, Kaili 556000, Guizhou Province, China; Qiandongnan Clinical Medical Research Center, Kaili 556000, Guizhou Province, China -
About author:Yang Biao, MD, Associate chief physician, Department of Orthopedics, People’s Hospital of Qiandongnan Miao and Dong Autonomous Prefecture, Kaili 556000, Guizhou Province, China; Qiandongnan Clinical Medical Research Center, Kaili 556000, Guizhou Province, China -
Supported by:Qiandongnan Prefecture Basic Research Program, Qiandongnan Kehe Foundation [2023] No. 21 (to YB)
CLC Number:
Cite this article
Yang Biao, , Wu Zhonghuan, , Jiang Fugui, , He Chenglong, , Li Tingdong, . Conductive hydrogel with cell-free fat extract repairs spinal cord injuries in rats[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3652-3662.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
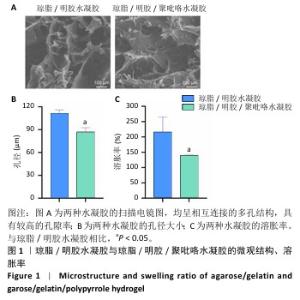
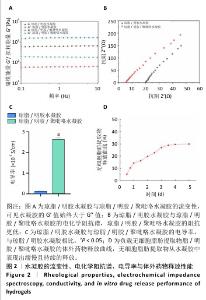
2.1 水凝胶的表征结果 扫描电镜下可见琼脂/明胶水凝胶与琼脂/明胶/聚吡咯水凝胶呈相互连接的多孔结构,具有较高的孔隙率(图1A)。琼脂/明胶水凝胶的孔径平均约为111 μm,琼脂/明胶/聚吡咯水凝胶的孔径平均约为87 μm,两组间孔径比较差异有显著性意义(P < 0.05),见图1B。琼脂/明胶/聚吡咯水凝胶的孔径小于琼脂/明胶水凝胶,可能是因为聚吡咯的疏水性导致含水量降低,但这两种水凝胶均能为细胞黏附和增殖提供充足的空间。如图1C所示,琼脂/明胶/聚吡咯水凝胶的溶胀率低于琼脂/明胶水凝胶(140%,215%,P < 0.05),这种差异表明聚吡咯的疏水性会影响水凝胶的溶胀比,而Fe3+进一步交联会降低溶胀率。 通过频率扫描方式研究了水凝胶结构的稳定性。如图2A所示,在0.1-10 Hz频率范围内,琼脂/明胶水凝胶与琼脂/明胶/聚吡咯水凝胶的G′值始终大于G′′值,表明它们具有典型的黏弹性行为。与琼脂/明胶水凝胶相比,琼脂/明胶/聚吡咯水凝胶具有更低的阻抗(图2B)。如图2C所示,琼脂/明胶/聚吡咯水凝胶的电导率显著高于琼脂/明胶水凝胶(2.60×10-3,0.13×10-3 S/m,P < 0.05),这有利于在组织之间传导电信号。体外药物释放实验显示,无细胞脂肪提取物从琼脂/明胶/聚吡咯水凝胶中表现出缓慢且持续的释放,5 d后累计释放率为31%左右,见图2D,表明无细胞脂肪提取物在微环境中的浓度在较长时间内保持恒定。"

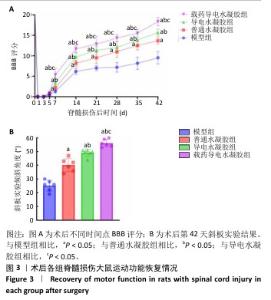
2.2 水凝胶修复大鼠脊髓损伤实验结果 2.2.1 实验动物数量分析 80只大鼠全部进入结果分析。 2.2.2 各组大鼠运动功能评估 随着脊髓损伤的进展,各组大鼠右侧后肢均出现弛缓性瘫痪,随后以时间依赖性的方式得到适当恢复,然而恢复程度在各组之间有所不同。由图3A可见,术后第7,14,21,28,35,42天,载药导电水凝胶组BBB评分高于模型组、普通水凝胶组、导电水凝胶组(P < 0.05),普通水凝胶组、导电水凝胶组BBB评分高于模型组(P < 0.05),导电水凝胶组BBB评分高于普通水凝胶组(P < 0.05)。斜板实验结果显示,载药导电水凝胶组斜板倾斜角度大于模型组、普通水凝胶组、导电水凝胶组(P <0.05),普通水凝胶组、导电水凝胶组斜板倾斜角度大于模型组(P < 0.05),导电水凝胶组斜板倾斜角度大于普通水凝胶组(P <0.05),见图3B。 "

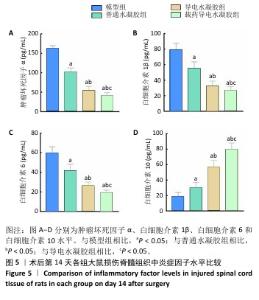
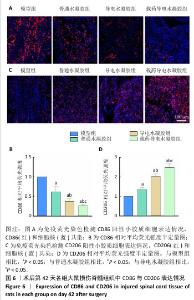
2.2.4 脊髓组织炎症因子水平检测 如图5所示,术后14 d,与模型组比较,普通水凝胶组、导电水凝胶组和载药导电水凝胶组肿瘤坏死因子α、白细胞介素1β、白细胞介素6水平均降低(P < 0.05),白细胞介素10水平升高(P < 0.05);与普通水凝胶组、导电水凝胶组比较,载药导电水凝胶组肿瘤坏死因子α、白细胞介素1β、白细胞介素6水平降低(P < 0.05),白细胞介素10水平升高(P < 0.05);与普通水凝胶组比较,导电水凝胶组肿瘤坏死因子α、白细胞介素1β、白细胞介素6水平降低(P < 0.05),白细胞介素10水平升高(P < 0.05)。 此次研究通过CD68(M1小胶质细胞/巨噬细胞标记物)与CD206(M2小胶质细胞/巨噬细胞标记物)免疫荧光染色检测小胶质细胞/巨噬细胞来评估脊髓半切损伤术后42 d的炎症反应。如图6A,B所示,普通水凝胶组、导电水凝胶组和载药导电水凝胶组CD68表达均低于模型组(P < 0.05),载药导电水凝胶组CD68表达低于普通水凝胶组、导电水凝胶组(P < 0.05),导电水凝胶组CD68表达低于普通水凝胶组(P < 0.05)。如图6C,D所示,普通水凝胶组、导电水凝胶组和载药导电水凝胶组CD206表达均高于模型组(P < 0.05),载药导电水凝胶组CD206表达高于普通水凝胶组、导电水凝胶组(P < 0.05),导电水凝胶组CD206表达高于普通水凝胶组(P < 0.05)。结果表明,载药导电水凝胶植入可能对脊髓损伤后的炎症反应具有积极作用。 "

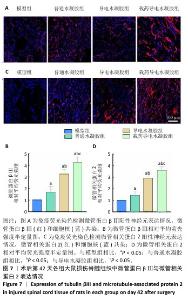
2.2.5 脊髓组织内源性神经干细胞分化情况 采用免疫荧光染色进一步验证水凝胶对体内内源性神经干细胞诱导分化的影响,未成熟神经元用微管蛋白βⅢ标记,成熟神经元用微管相关蛋白2标记。 由图7所示,各组大鼠脊髓损伤部位均表达微管蛋白βⅢ与微管相关蛋白2,与模型组相比,普通水凝胶组、导电水凝胶组和载药导电水凝胶组微管蛋白βⅢ与微管相关蛋白2表达均升高(P < 0.05);与普通水凝胶组、导电水凝胶组相比,载药导电水凝胶组微管蛋白βⅢ与微管相关蛋白2表达升高 (P < 0.05);导电水凝胶组微管蛋白βⅢ与微管相关蛋白2表达高于普通水凝胶组(P < 0.05)。结果表明,在炎症微环境下神经干细胞更容易分化为星形胶质细胞而非神经元,而载药导电水凝胶可以逆转这种抑制作用,促进神经干细胞向神经元方向分化。"

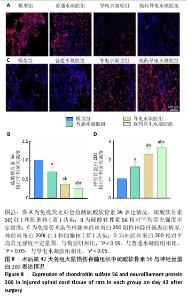
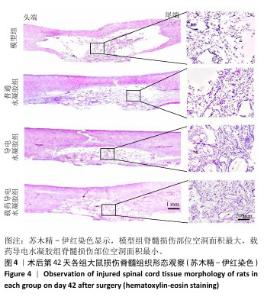
2.2.6 脊髓组织内胶质瘢痕形成及轴突、髓鞘再生情况 为了更全面地了解载药导电水凝胶在脊髓损伤后的治疗意义,进一步研究了神经胶质瘢痕、轴突和髓鞘的密度和状况。通过硫酸软骨素56免疫荧光染色反映神经形胶质瘢痕形成情况,由图8A,B所示,模型组硫酸软骨素56表达高于其他3组(P < 0.05),载药导电水凝胶组硫酸软骨素56表达低于普通水凝胶组、导电水凝胶组(P < 0.05),导电水凝胶组硫酸软骨素56表达低于普通水凝胶组(P < 0.05)。结果表明载药导电水凝胶组在预防瘢痕形成方面发挥了非常重要的作用。 由于神经丝蛋白200是神经丝再生的主要指标,因此通过神经丝蛋白200免疫荧光染色检测术后6周损伤脊髓组织轴突再生效果。如图8C,D所示,模型组神经丝蛋白200表达低于其他3组(P < 0.05),载药导电水凝胶组神经丝蛋白200表达高于普通水凝胶组、导电水凝胶组(P < 0.05),导电水凝胶组神经丝蛋白200表达高于普通水凝胶组(P < 0.05)。 通过髓鞘碱性蛋白免疫荧光染色评估轴突的髓鞘再生程度。如图9A所示,模型组髓鞘碱性蛋白表达低于其他3组,载药导电水凝胶组髓鞘碱性蛋白表达高于普通水凝胶组、导电水凝胶组,导电水凝胶组髓鞘碱性蛋白表达高于普通水凝胶组。牢固蓝染色结果显示,模型组髓鞘不完整、脱髓鞘增加,经过普通水凝胶、导电水凝胶及载药导电水凝胶治疗后髓鞘保存情况得到改善,载药导电水凝胶组髓鞘保存最好,鞘壁更厚、轴突更明显,见图9B。透射电子显微镜下可见模型组脊髓空洞形成较多、完整的有髓神经纤维较少,经普通水凝胶、导电水凝胶及载药导电水凝胶治疗后脱髓鞘现象均得到改善,髓鞘数量、直径和厚度均明显增加,并且以载药导电水凝胶组改善效果最好,其次是导电水凝胶组,见图9C。"

| [1] ZHA X, ZHENG G, SKUTELLA T, et al. Microglia: a promising therapeutic target in spinal cord injury. Neural Regen Res. 2025;20(2):454-463. [2] HU X, XU W, REN Y, et al. Spinal cord injury: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2023;8(1):245. [3] BADHIWALA JH, WILSON JR, WITIW CD, et al. The influence of timing of surgical decompression for acute spinal cord injury: a pooled analysis of individual patient data. Lancet Neurol. 2021;20(2):117-126. [4] ZHANG Y, XIAO S, DAN F, et al. Phillygenin inhibits neuroinflammation and promotes functional recovery after spinal cord injury via TLR4 inhibition of the NF-κB signaling pathway. J Orthop Translat. 2024;48:133-145. [5] ZHANG W, LIU M, REN J, et al. Magnetic Nanoparticles and Methylprednisolone Based Physico-Chemical Bifunctional Neural Stem Cells Delivery System for Spinal Cord Injury Repair. Adv Sci (Weinh). 2024;11(21):e2308993. [6] TAN Y, LAI T, LI Y, et al. An oil-in-gel type of organohydrogel loaded with methylprednisolone for the treatment of secondary injuries following spinal cord traumas. J Control Release. 2024;374:505-524. [7] HE LW, GUO XJ, ZHAO C, et al. Rehabilitation Training after Spinal Cord Injury Affects Brain Structure and Function: From Mechanisms to Methods. Biomedicines. 2023;12(1):41. [8] RONG Y, WANG J, HU T, et al. Ginsenoside Rg1 Regulates Immune Microenvironment and Neurological Recovery After Spinal Cord Injury Through MYCBP2 Delivery via Neuronal Cell-Derived Extracellular Vesicles. Adv Sci (Weinh). 2024;11(31):e2402114. [9] LIU Z, LAI J, KONG D, et al. Advances in electroactive bioscaffolds for repairing spinal cord injury. Biomed Mater. 2024;19(3):032005. [10] ZHANG B, WANG W, GAO P, et al. Injectable, Electroconductive, Free Radical Scavenging Silk Fibroin/Black Phosphorus/Glycyrrhizic Acid Nanocomposite Hydrogel for Enhancing Spinal Cord Repair. Adv Healthc Mater. 2024;13(18):e2304300. [11] YANG B, LIANG C, CHEN D, et al. A conductive supramolecular hydrogel creates ideal endogenous niches to promote spinal cord injury repair. Bioact Mater. 2022;15:103-119. [12] YANG Q, SU S, LIU S, et al. Exosomes-loaded electroconductive nerve dressing for nerve regeneration and pain relief against diabetic peripheral nerve injury. Bioact Mater. 2023;26:194-215. [13] LIU M, LI W, ZHOU X, et al. Cell-Free Fat Extract Improves Ovarian Function and Fertility in Mice With Advanced Age. Front Endocrinol (Lausanne). 2022;13:912648. [14] DING J, WEI C, XU Y, et al. 3D printing of Ceffe-infused scaffolds for tailored nipple-like cartilage development. BMC Biotechnol. 2024; 24(1):25. [15] WU D, LI X, WANG X, et al. Cell-free fat extract protects septic lethality via restraining NLRP3 inflammasome activation. Am J Transl Res. 2022;14(7):5201-5214. [16] JIA Z, KANG B, CAI Y, et al. Cell-free fat extract attenuates osteoarthritis via chondrocytes regeneration and macrophages immunomodulation. Stem Cell Res Ther. 2022;13(1):133. [17] QIU E, GONG Y, YAO J, et al. A dual aperture (mesoporous and macroporous) system loaded with cell-free fat extract to optimize bone regeneration microenvironment. J Mater Chem B. 2023;11(4):826-836. [18] SUN Y, CHEN D, DAI T, et al. Cell-free fat extract promotes axon regeneration and retinal ganglion cells survival in traumatic optic neuropathy. Front Cell Neurosci. 2024;18:1344853. [19] BASSO DM, BEATTIE MS, BRESNAHAN JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996; 139(2):244-256. [20] ZHENG B, TUSZYNSKI MH. Regulation of axonal regeneration after mammalian spinal cord injury. Nat Rev Mol Cell Biol. 2023; 24(6):396-413. [21] SERAFIN A, RUBIO MC, CARSI M, et al. Electroconductive PEDOT nanoparticle integrated scaffolds for spinal cord tissue repair. Biomater Res. 2022;26(1):63. [22] MICHEL-FLUTOT P, CHENG L, THOMAS SJ, et al. PTEN inhibition promotes robust growth of bulbospinal respiratory axons and partial recovery of diaphragm function in a chronic model of cervical contusion spinal cord injury. Exp Neurol. 2024;378:114816. [23] FAN L, LIU C, CHEN X, et al. Exosomes-Loaded Electroconductive Hydrogel Synergistically Promotes Tissue Repair after Spinal Cord Injury via Immunoregulation and Enhancement of Myelinated Axon Growth. Adv Sci (Weinh). 2022;9(13):e2105586. [24] WANG L, ZHAO H, HAN M, et al. Electromagnetic Cellularized Patch with Wirelessly Electrical Stimulation for Promoting Neuronal Differentiation and Spinal Cord Injury Repair. Adv Sci(Weinh). 2024;11(30):e2307527. [25] ZHANG Y, YAO A, WU J, et al. Conductive Hydrogel Restores Electrical Conduction to Promote Neurological Recovery in a Rat Model. Tissue Eng Part A. 2024;30(17-18):577-587. [26] YIN W, YANG C, LIU D, et al. Mussel shell-derived pro-regenerative scaffold with conductive porous multi-scale-patterned microenvironment for spinal cord injury repair. Biomed Mater. 2024; 19(3):30. [27] GUAN P, FAN L, ZHU Z, et al. M2 microglia-derived exosome-loaded electroconductive hydrogel for enhancing neurological recovery after spinal cord injury. J Nanobiotechnology. 2024;22(1):8. [28] ZHENG Y, NüTZL M, SCHACKEL T, et al. Biomaterial scaffold stiffness influences the foreign body reaction, tissue stiffness, angiogenesis and neuroregeneration in spinal cord injury. Bioact Mater. 2025;46: 134-149. [29] KAN T, RAN Z, SUN L, et al. Cell-free fat extract-loaded microneedles attenuate inflammation-induced apoptosis and mitochondrial damage in tendinopathy. Mater Today Bio. 2023;22:100738. [30] FU Z, GU Q, WANG L, et al. Cell-free fat extract regulates oxidative stress and alleviates Th2-mediated inflammation in atopic dermatitis. Front Bioeng Biotechnol. 2024;12:1373419. [31] LI D, LI Q, XU T, et al. Pro-vasculogenic Fibers by PDA-Mediated Surface Functionalization Using Cell-Free Fat Extract (CEFFE). Biomacromolecules. 2024;25(3):1550-1562. [32] KANG B, JIA Z, DONG Y, et al. Recombinant human annexin A5 accelerates diabetic wounds healing by regulating skin inflammation. Regen Ther. 2024;27:342-353. [33] XU GY, XU S, ZHANG YX, et al. Cell-Free Extracts from Human Fat Tissue with a Hyaluronan-Based Hydrogel Attenuate Inflammation in a Spinal Cord Injury Model through M2 Microglia/Microphage Polarization. Small. 2022;18(17):e2107838. [34] LIU M, ZHANG W, HAN S, et al. Multifunctional Conductive and Electrogenic Hydrogel Repaired Spinal Cord Injury via Immunoregulation and Enhancement of Neuronal Differentiation. Adv Mater. 2024;36(21):e2313672. [35] ZHAO H, XIONG T, CHU Y, et al. Biomimetic Dual-Network Collagen Fibers with Porous and Mechanical Cues Reconstruct Neural Stem Cell Niche via AKT/YAP Mechanotransduction after Spinal Cord Injury. Small. 2024;20(32):e2311456. [36] RIBEIRO BF, DA CRUZ BC, DE SOUSA BM, et al. Cell therapies for spinal cord injury: a review of the clinical trials and cell-type therapeutic potential. Brain. 2023;146(7): 2672-2693. [37] MORITZ C, FIELD-FOTE EC, TEFERTILLER C, et al. Non-invasive Spinal Cord Electrical Stimulation for Arm and Hand Function in Chronic Tetraplegia: A Safety and Efficacy Trial. Nat Med. 2024;30(5): 1276-1283. [38] WEI F, YANG W, WANG H, et al. Reactive oxygen species-scavenging biomaterials for neural regenerative medicine. Biomater Sci. 2025; 13(2):343-363. [39] LIU D, LU G, SHI B, et al. ROS-Scavenging Hydrogels Synergize with Neural Stem Cells to Enhance Spinal Cord Injury Repair via Regulating Microenvironment and Facilitating Nerve Regeneration. Adv Healthc Mater. 2023;12(18):e2300123. [40] MAGUROVA M, BACOVA M, PAPCUNOVA S, et al. Exploring synergistic effects: Atorvastatin and electrical stimulation in spinal cord injury therapy. IBRO Neurosci Rep. 2025;18:389-399. [41] XIAO L, XIE P, MA J, et al. A Bioinspired Injectable, Adhesive, and Self-Healing Hydrogel with Dual Hybrid Network for Neural Regeneration after Spinal Cord Injury. Adv Mater. 2023; 35(41):e2304896. [42] PETROSYAN HA, ALESSI V, LASEK K, et al. AAV Vector Mediated Delivery of NG2 Function Neutralizing Antibody and Neurotrophin NT-3 Improves Synaptic Transmission, Locomotion, and Urinary Tract Function after Spinal Cord Contusion Injury in Adult Rats. J Neurosci. 2023;43(9):1492-1508. [43] WU Z, HAN T, DONG Y, et al. Acid-sensing ion channel-1 contributes to the failure of myelin sheath regeneration following spinal cord injury by transcellular delivery of PGE2. Cell Mol Biol Lett. 2024;29(1):149. [44] NIE M, TIAN Y, XIAO Y, et al. Enhancing high-quality fat survival: a novel strategy using cell-free fat extract. FASEB J. 2024;38(14):e23733. [45] QIN C, QI Z, PAN S, et al. Advances in conductive hydrogel for spinal cord injury repair and regeneration. Int J Nanomedicine. 2023;18: 7305-7333. [46] TRIVISONNO A, ALEXANDER RW, BALDARI S, et al. Intraoperative strategies for minimal manipulation of autologous adipose tissue for cell- and tissue-based therapies: concise review. Stem Cells Transl Med. 2019;8(12):1265-1271. |
| [1] | Guo Yuchao, Ni Qianwei, Yin Chen, Jigeer·Saiyilihan, Gao Zhan . Quaternized chitosan hemostatic materials: synthesis, mechanism, and application [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2091-2100. |
| [2] | Liu Xiaohong, Zhao Tian, Mu Yunping, Feng Wenjin, Lyu Cunsheng, Zhang Zhiyong, Zhao Zijian, Li Fanghong. Acellular dermal matrix hydrogel promotes skin wound healing in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 395-403. |
| [3] | Kong Xiaojuan, Tan Zhenyu, Lei Lei. Repair of rat endometrial injury by using miR-424-5p modified exosome/Poloxam 407 hydrogel [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3626-3635. |
| [4] | Gong Yukang, Ye Gaoqi, Wang Chenhao, Chen Dejin, Gao Wenshan. Effects and mechanisms of natural polyphenol-based hydrogels in promoting bone repair [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3675-3686. |
| [5] | Li Qingshan, Li Runmeng, Gao Yuyang, Han Gang, Chen Jiying, Guo Quanyi. Magnetocaloric antitumor and osteogenic properties of magnetic bioactive glass scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3494-3503. |
| [6] | Wang Hao, He Qin, Wang Pingxi, Zhang Jun, Wu Zhilin. Deferoxamine-loaded strontium alginate hydrogel promotes the repair of skull injury in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3609-3617. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||