Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (6): 1220-1229.doi: 10.12307/2025.283
Previous Articles Next Articles
SR9009 combined with indolepropionic acid alleviates inflammation in C2C12 myoblasts through the nuclear factor-kappa B signaling pathway
Ji Huihui1, Jiang Xu1, Zhang Zhimin1, Xing Yunhong2, Wang Liangliang2, Li Na2, Song Yuting1, Luo Xuguang3, Cui Huilin1, Cao Ximei1, 4
- 1Department of Histology and Embryology, 2School of Forensic Medicine, 3Department of Microbiology and Immunology, 4The Transformation Pilot-Base of Shanxi Clinical Cell Therapy, Shanxi Medical University, Jinzhong 030604, Shanxi Province, China
-
Received:2024-01-30Accepted:2024-03-09Online:2025-02-28Published:2024-06-21 -
Contact:Cao Ximei, Associate professor, Master’s supervisor, Department of Histology and Embryology, Shanxi Medical University, Jinzhong 030604, Shanxi Province, China; The Transformation Pilot-Base of Shanxi Clinical Cell Therapy, Shanxi Medical University, Jinzhong 030604, Shanxi Province, China -
About author:Ji Huihui, Master candidate, Department of Histology and Embryology, Shanxi Medical University, Jinzhong 030604, Shanxi Province, China -
Supported by:Research Project Supported by Shanxi Scholarship Council of China, No. 2023-095 (to CXM); Natural Science Foundation of Shanxi Province (General Program), No. 202303021211113 (to CXM)
CLC Number:
Cite this article
Ji Huihui, Jiang Xu, Zhang Zhimin, Xing Yunhong, Wang Liangliang, Li Na, Song Yuting, Luo Xuguang, Cui Huilin, Cao Ximei. SR9009 combined with indolepropionic acid alleviates inflammation in C2C12 myoblasts through the nuclear factor-kappa B signaling pathway[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1220-1229.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

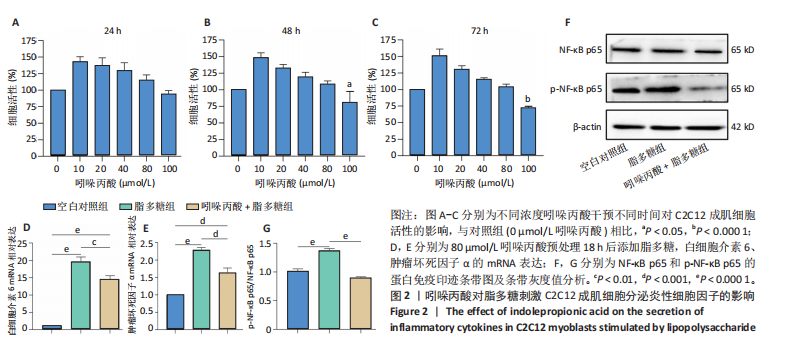
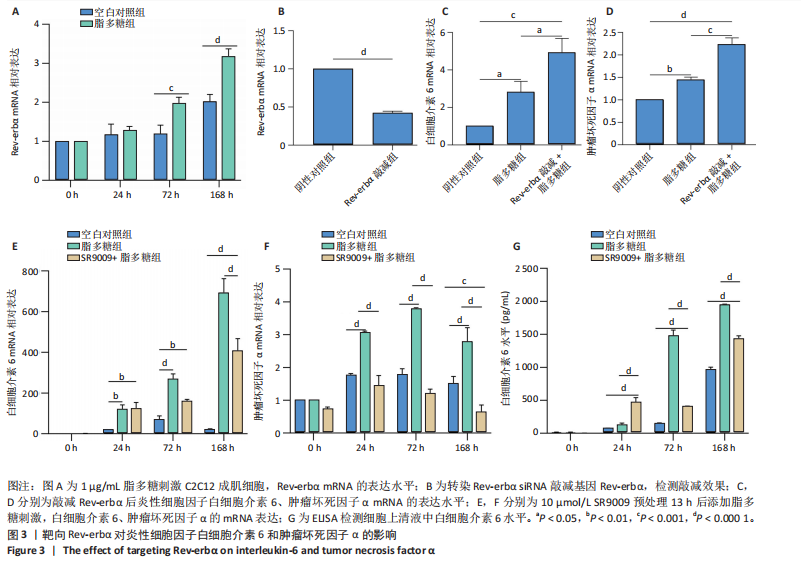
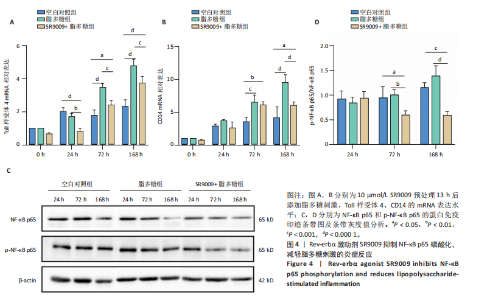
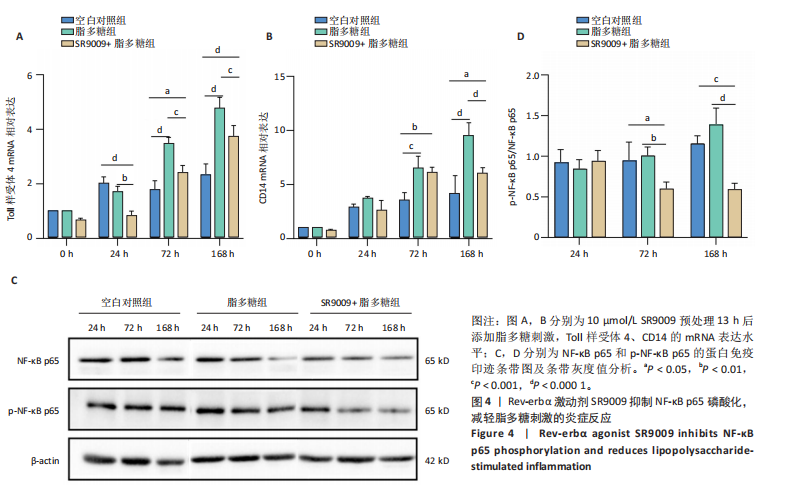
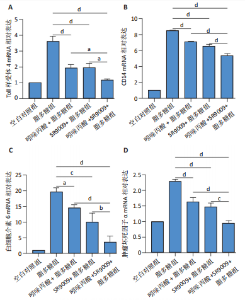
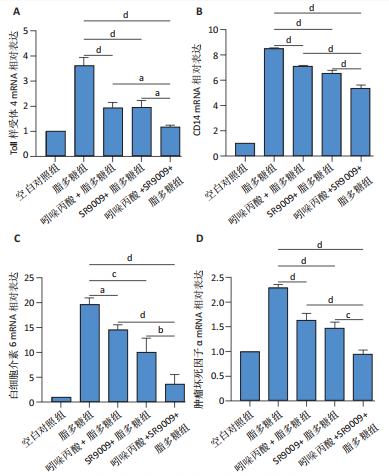
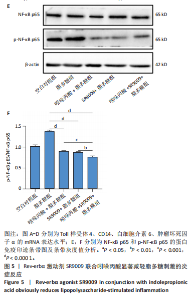
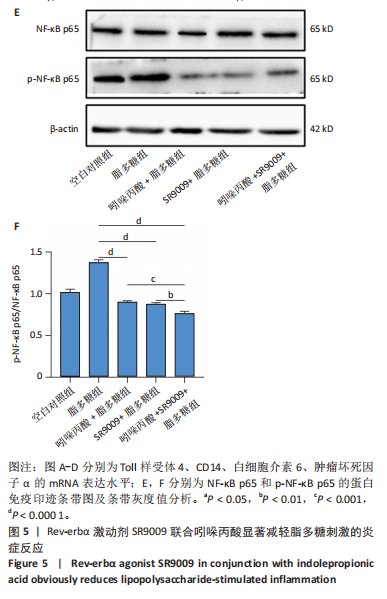
2.1 脂多糖刺激C2C12成肌细胞分泌白细胞介素6和肿瘤坏死因子α,并激活NF-κB信号通路 C2C12成肌细胞分化过程中添加1 μg/mL脂多糖诱导炎症反应,与对照组相比,C2C12成肌细胞生长减慢、细胞融合和肌管的形成被抑制。实验观察期内,脂多糖时间依赖性地抑制肌管形成(图1A)。RNA转录组测序结合KEGG通路分析结果显示,脂多糖刺激C2C12成肌细胞分化过程中的炎症反应与肿瘤坏死因子α、NF-κB及其相关信号通路的激活相关(图1B)。 验证实验结果显示,与对照组相比,C2C12成肌细胞分化过程中添加脂多糖上调NF-κB p65和p-NF-κB p65蛋白表达,且p-NF-κB p65/NF-κB p65的比值随脂多糖刺激时间延长显著升高(图1C,D),提示脂多糖刺激C2C12成肌细胞条件下,NF-κB信号通路被激活。RT-qPCR结果显示,脂多糖刺激组细胞中白细胞介素6 mRNA表达较对照组显著升高,且随时间依赖性升高(图1E),肿瘤坏死因子α mRNA表达随脂多糖刺激时间依赖性升高,72 h达到小高峰后略有下降(图1F)。ELISA检测细胞上清液中白细胞介素6水平,其与mRNA的表达呈现相同升高趋势(图1G)。以上结果支持脂多糖刺激C2C12成肌细胞条件下,NF-κB信号通路被激活,诱发炎症反应。 2.2 吲哚丙酸抑制脂多糖刺激的C2C12成肌细胞炎症反应 已知吲哚丙酸能通过NF-κB信号通路发挥抗炎作用[14-16]。 为进一步验证C2C12成肌细胞分化过程中脂多糖刺激的炎症反应与NF-κB信号通路有关,在生长培养基中添加吲哚丙酸培养18 h后,转入含1 μg/mL脂多糖的分化培养基,并维持吲哚丙酸干预剂量培养72 h。CCK-8检测吲哚丙酸的细胞毒性,筛选最佳给药浓度。结果显示,随着吲哚丙酸作用时间延长,吲哚丙酸浓度> 80 μmol/L对C2C12成肌细胞造成明显毒性损伤;吲哚丙酸浓度< 80 μmol/L时未对C2C12成肌细胞活性造成显著性影响,且低浓度吲哚丙酸表现出促增殖作用,以10 μmol/L最显著(图2A-C)。结合利用吲哚丙酸抑炎的实验目的,选择80 μmol/L作为吲哚丙酸的使用浓度进行后续实验。与脂多糖组相比,吲哚丙酸+脂多糖组干预72 h后白细胞介素6、肿瘤坏死因子α mRNA表达水平显著降低(图2D,E);NF-κB p65和p-NF-κB p65蛋白表达水平下调,尤以p-NF-κB p65下调显著,p-NF-κB p65/NF-κB p65比值较脂多糖组显著降低(图2F,G)。 2.3 靶向抑制或激活Rev-erbα对炎性细胞因子白细胞介素6和肿瘤坏死因子α的影响 KEGG通路分析结果显示昼夜节律钟基因参与调节炎症反应(图1B)。研究表明,Rev-erbα参与调节巨噬细胞的炎症反应[17]。靶向Rev-erbα是否参与调节脂多糖刺激C2C12成肌细胞的炎症反应值得进一步研究。脂多糖组Rev-erbα mRNA的表达水平呈时间依赖性升高(图3A)。通过Rev-erbα siRNA有效敲减Rev-erbα,并用RT-qPCR确定其抑制效率达58%以上(图3B)。Rev-erbα敲减组白细胞介素6和肿瘤坏死因子α mRNA表达水平较脂多糖组显著上调(图3C,D)。提示,敲减Rev-erbα加重脂多糖刺激C2C12成肌细胞的炎症反应。 吡咯衍生物SR9009是Rev-erbα的激动剂,可以增强Rev-erbα依赖性阻遏因子活性[18]。10 μmol/L SR9009预处理C2C12成肌细胞后给予脂多糖刺激,白细胞介素6和肿瘤坏死因子α mRNA表达水平较脂多糖组呈现下降趋势(图3E,F);细胞上清液中白细胞介素6水平的表达趋势与其mRNA水平基本一致(图3G)。提示,药理性激活Rev-erbα能够发挥抗炎效应。 2.4 Rev-erbα激动剂SR9009抑制NF-κB p65磷酸化,减轻脂多糖刺激的炎症反应 TLR4是与脂多糖结合的主要受体,TLR4/CD14信号通路是介导脂多糖刺激引发炎症反应的重要通路[19-20]。随脂多糖刺激时间延长,TLR4、CD14 mRNA的表达水平依赖性增加。当SR9009药理性激活Rev-erbα后,SR9009+脂多糖组较脂多糖组TLR4、CD14 mRNA的表达水平下调,尤以72 h以后显著(图4A,B)。NF-κB是TLR4介导的信号通路中的关键转录因子。蛋白免疫印迹实验进一步检测脂多糖刺激C2C12成肌细胞分化过程中NF-κB信号通路的变化,结果显示p-NF-κB p65/NF-κB p65蛋白比值较对照组时间依赖性升高。激动剂SR9009预处理后,SR9009+脂多糖组p-NF-κB p65/NF-κB p65蛋白比值逐渐下调且显著低于脂多糖组(图4C,D)。同一时间点NF-κB p65的表达水平在脂多糖组和SR9009+脂多糖组之间无显著差异。提示,SR9009预处理通过下调TLR4、CD14 mRNA的表达,抑制NF-κB p65激活,以72 h后显著,进而减轻脂多糖刺激的炎症反应。 2.5 Rev-erbα激动剂SR9009联合吲哚丙酸具有更强的抗炎作用 为评价脂多糖刺激C2C12成肌细胞分化过程中Rev-erbα激动剂SR9009联合吲哚丙酸共培养条件下NF-κB信号通路的变化,检测脂多糖刺激72 h时TLR4、CD14、炎性细胞因子白细胞介素6、肿瘤坏死因子α mRNA的表达,结果显示SR9009+脂多糖组和吲哚丙酸+脂多糖组TLR4、CD14、白细胞介素6、肿瘤坏死因子α mRNA的表达均显著低于脂多糖组;SR9009和吲哚丙酸联合后,上述因子的表达水平进一步下降,尤以白细胞介素6的下降更为显著(图5A-D)。SR9009和吲哚丙酸联合后,p-NF-κB p65/NF-κB p65蛋白比值较SR9009+脂多糖组或吲哚丙酸+脂多糖组均显著下调(图5E,F)。提示,Rev-erbα激动剂SR9009联合吲哚丙酸具有更强的抗炎作用。"

| [1] ZHANG-SUN ZY, XU XZ, ESCAMES G, et al. Targeting NR1D1 in organ injury: challenges and prospects. Mil Med Res. 2023;10(1):62. [2] MAYEUF-LOUCHART A, THOREL Q, DELHAYE S, et al. Rev-erb-α regulates atrophy-related genes to control skeletal muscle mass. Sci Rep. 2017;7(1):14383. [3] RAZA GS, SODUM N, KAYA Y, et al. Role of Circadian Transcription Factor Rev-Erb in Metabolism and Tissue Fibrosis. Int J Mol Sci. 2022; 23(21):12954. [4] WANG J, LIU Z, XU Y, et al. Enterobacterial LPS-inducible LINC00152 is regulated by histone lactylation and promotes cancer cells invasion and migration. Front Cell Infect Microbiol. 2022;12:913815. [5] AFROZ R, TANVIR EM, TANIA M, et al. LPS/TLR4 Pathways in Breast Cancer: Insights into Cell Signalling. Curr Med Chem. 2022;29(13): 2274-2289. [6] SINGER-ENGLAR T, BARLOW G, MATHUR R. Obesity, diabetes, and the gut microbiome: an updated review. Expert Rev Gastroenterol Hepatol. 2019;13(1):3-15. [7] ONO Y, SAKAMOTO K. Lipopolysaccharide inhibits myogenic differentiation of C2C12 myoblasts through the Toll-like receptor 4-nuclear factor-κB signaling pathway and myoblast-derived tumor necrosis factor-α. PLoS One. 2017;12(7):e0182040. [8] YU D, FANG X, XU Y, et al. Rev-erbα can regulate the NF-κB/NALP3 pathway to modulate lipopolysaccharide-induced acute lung injury and inflammation. Int Immunopharmacol. 2019;73:312-320. [9] ZHAO G, ZHANG T, WU H, et al. MicroRNA let-7c Improves LPS-Induced Outcomes of Endometritis by Suppressing NF-κB Signaling. Inflammation. 2019;42(2):650-657. [10] WANG S, LIN Y, YUAN X, et al. REV-ERBα integrates colon clock with experimental colitis through regulation of NF-κB/NLRP3 axis. Nat Commun. 2018;9(1):4246. [11] WOLFF SEC, WANG XL, JIAO H, et al. The Effect of Rev-erbα Agonist SR9011 on the Immune Response and Cell Metabolism of Microglia. Front Immunol. 2020;11:550145. [12] PARIOLLAUD M, GIBBS JE, HOPWOOD TW, et al. Circadian clock component REV-ERBα controls homeostatic regulation of pulmonary inflammation. J Clin Invest. 2018;128(6):2281-2296. [13] SHI J, TONG R, ZHOU M, et al. Circadian nuclear receptor Rev-erbα is expressed by platelets and potentiates platelet activation and thrombus formation. Eur Heart J. 2022;43(24):2317-2334. [14] ZHUANG H, REN X, JIANG F, et al. Indole-3-propionic acid alleviates chondrocytes inflammation and osteoarthritis via the AhR/NF-κB axis. Mol Med. 2023;29(1):17. [15] DU L, QI R, WANG J, et al. Indole-3-Propionic Acid, a Functional Metabolite of Clostridium sporogenes, Promotes Muscle Tissue Development and Reduces Muscle Cell Inflammation. Int J Mol Sci. 2021;22(22):12435. [16] ZHUANG H, LI B, XIE T, et al. Indole-3-aldehyde alleviates chondrocytes inflammation through the AhR-NF-κB signalling pathway. Int Immunopharmacol. 2022;113(Pt A):109314. [17] SATO S, SAKURAI T, OGASAWARA J, et al. A circadian clock gene, Rev-erbα, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. J Immunol. 2014; 192(1):407-417. [18] ZHAO W, CUI L, HUANG X, et al. Activation of Rev-erbα attenuates lipopolysaccharide-induced inflammatory reactions in human endometrial stroma cells via suppressing TLR4-regulated NF-κB activation. Acta Biochim Biophys Sin (Shanghai). 2019;51(9):908-914. [19] FU YJ, XU B, HUANG SW, et al. Baicalin prevents LPS-induced activation of TLR4/NF-κB p65 pathway and inflammation in mice via inhibiting the expression of CD14. Acta Pharmacol Sin. 2021;42(1):88-96. [20] ZANONI I, OSTUNI R, MAREK LR, et al. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell. 2011;147(4):868-880. [21] LUO X, YANG X, YANG Y, et al. The interrelationship between inflammatory cytokines and skeletal muscle decay from the viewpoint of circadian rhythms. Arch Physiol Biochem. 2022;128(6):1559-1565. [22] MOHAMMAD S, THIEMERMANN C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front Immunol. 2021;11:594150. [23] ZHU G, CHENG Z, LIN C, et al. MyD88 Regulates LPS-induced NF-ĸB/MAPK Cytokines and Promotes Inflammation and Malignancy in Colorectal Cancer Cells. Cancer Genomics Proteomics. 2019;16(6): 409-419. [24] CANI PD, AMAR J, IGLESIAS MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761-1772. [25] 赖明慧,肖卫华.骨骼肌是内分泌器官[J].自然杂志,2020,42(6): 463-470. [26] LAVINE KJ, SIERRA OL. Skeletal muscle inflammation and atrophy in heart failure. Heart Fail Rev. 2017;22(2):179-189. [27] CHOI MC, JO J, PARK J, et al. NF-κB Signaling Pathways in Osteoarthritic Cartilage Destruction. Cells. 2019;8(7):734. [28] 滕益霖,席德双,冯彦斌,等.吲哚丙酸抑制小胶质细胞M1极化治疗脊髓损伤[J].中国组织工程研究,2024,28(31):5010-5016. [29] SUNDAR IK, RASHID K, SELLIX MT, et al. The nuclear receptor and clock gene REV-ERBα regulates cigarette smoke-induced lung inflammation. Biochem Biophys Res Commun. 2017;493(4):1390-1395. [30] URIZ-HUARTE A, DATE A, ANG H, et al. The transcriptional repressor REV-ERB as a novel target for disease. Bioorg Med Chem Lett. 2020; 30(17):127395. [31] CIESIELSKA A, MATYJEK M, KWIATKOWSKA K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol Life Sci. 2021;78(4):1233-1261. [32] ZHANG Y, LIANG X, BAO X, et al. Toll-like receptor 4 (TLR4) inhibitors: Current research and prospective. Eur J Med Chem. 2022;235:114291. [33] LI Y, SHI J, LIU Z, et al. Regulation of the migration of colorectal cancer stem cells via the TLR4/MyD88 signaling pathway by the novel surface marker CD14 following LPS stimulation. Oncol Lett. 2023;27(2):60. [34] KHANMOHAMMADI S, REZAEI N. Role of Toll-like receptors in the pathogenesis of COVID-19. J Med Virol. 2021;93(5):2735-2739. [35] SHIRJANG S, MANSOORI B, SOLALI S, et al. Toll-like receptors as a key regulator of mesenchymal stem cell function: An up-to-date review. Cell Immunol. 2017;315:1-10. [36] YANG X, HAGHIAC M, GLAZEBROOK P, et al. Saturated fatty acids enhance TLR4 immune pathways in human trophoblasts. Hum Reprod. 2015;30(9):2152-2159. [37] ZHOU M, YI Y, HONG L. Oridonin Ameliorates Lipopolysaccharide-Induced Endometritis in Mice via Inhibition of the TLR-4/NF-κBpathway. Inflammation. 2019;42(1):81-90. [38] TANG J, XU L, ZENG Y, et al. Effect of gut microbiota on LPS-induced acute lung injury by regulating the TLR4/NF-kB signaling pathway. Int Immunopharmacol. 2021;91:107272. [39] LAM MT, CHO H, LESCH HP, et al. Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription. Nature. 2013;498(7455):511-515. |
| [1] | Chen Yixian, Chen Chen, Lu Liheng, Tang Jinpeng, Yu Xiaowei. Triptolide in the treatment of osteoarthritis: network pharmacology analysis and animal model validation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 805-815. |
| [2] | Chi Wenxin, Zhang Cunxin, Gao Kai, Lyu Chaoliang, Zhang Kefeng. Mechanism by which nobiletin inhibits inflammatory response of BV2 microglia [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1321-1327. |
| [3] | Yu Ting, Lyu Dongmei, Deng Hao, Sun Tao, Cheng Qian. Icariin pretreatment enhances effect of human periodontal stem cells on M1-type macrophages [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1328-1335. |
| [4] | Bai Jing, Zhang Xue, Ren Yan, Li Yuehui, Tian Xiaoyu. Effect of lncRNA-TNFRSF13C on hypoxia-inducible factor 1alpha in periodontal cells by modulation of #br# miR-1246 #br# [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 928-935. |
| [5] | Deng Ran, Wei Yi, Ji Xiaowei. Induction of M1/M2 polarization of macrophages by lipopolysaccharides and titanium particles in peri-implant tissues [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7415-7422. |
| [6] | Zhao Xuemei, Wang Rui, Ao · Wuliji, Bao Shuyin, Jiang Xiaohua. Effects of Agiophyllum Oligo Saccharides on inflammation and apoptosis of mouse synovial cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6939-6946. |
| [7] | Fang Jun, Wei Wei, Xue Yating, Cui Chenlong, Wei Jiasheng, Shi Xiao, Yang Lijuan, Yang Baozhong. M2 macrophage-derived exosomes promote microglia M2-type polarization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(25): 5320-5327. |
| [8] | Zhang Xingzhou, Wei Ming, Dong Guoqiang, Du Wei, Luo Yiwen, Zhang Nan . Mechanism of postoperative abdominal adhesion formation and therapeutic prospect of mesenchymal stem cell exosomes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(1): 147-155. |
| [9] | Yang Peng, Tang Yubin, Yang Jing, Liu Jian, Yao Runjie, Chen Lin, Su Nan. Protective effect of ulinastatin on acute bone loss in sepsis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(35): 5649-5655. |
| [10] | Teng Yilin, Xi Deshuang, Feng Yanbin, Liang Yu, Deng Hao, Zeng Gaofeng, Zong Shaohui. Indolepropionic acid inhibition of microglial cell M1 polarization for treatment of spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(31): 5010-5016. |
| [11] | Yu Jingwen, Guo Minfang, Zhang Bingxin, Mu Bingtao, Meng Tao, Zhang Huiyu, Ma Cungen, Yin Jinzhu, Song Lijuan, Yu Jiezhong. Astragaloside inhibits astrocyte activation and inflammatory response induced by inflammation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(31): 5022-5028. |
| [12] | Zhang Tiandong, Peng Qingping, Liu Huan, Feng Jianguo, Yi Qian, Huang Wenhua. Semen cuscutae in the treatment of osteoarthritis: network pharmacology analysis and experimental validation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(28): 4516-4521. |
| [13] | Huang Jie, Jiang Qiang, Han Jiaheng, Liu Jiang, Zhang Yan, Lu Zhencao, Ding Yu. Mechanism by which interleukin-1beta regulates the expression of Semaphorin 3A to induce intervertebral disc degeneration [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(23): 3680-3685. |
| [14] | Han Yue, Wang Yufei, Liu Wanqing, Dong Ming, Niu Weidong. Effects of icariin on proliferation and differentiation of MC3T3-E1 cells in an inflammatory environment [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(23): 3709-3714. |
| [15] | Yang Qihang, Pu Rui, Chen Ziyang, Leng Siyi, Song Yongjing, Liu Hui, Du Guangyou. Role and mechanism of intestinal flora metabolites in obesity regulation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 308-314. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||