Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (5): 968-977.doi: 10.12307/2025.293
Previous Articles Next Articles
Effect of fibroblast growth factor receptor 1 inhibitor on bone destruction in rats with collagen-induced arthritis
Han Haihui1, 2, 3, Meng Xiaohui1, 2, 3, Xu Bo1, 2, 3, Ran Lei1, 2, 3, Shi Qi1, 2, 3, Xiao Lianbo1, 2, 3
- 1Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China; 2Guanghua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai 200052, China; 3Institute of Arthritis Research of Integrated Traditional Chinese and Western Medicine, Shanghai Academy of Traditional Chinese Medicine, Shanghai 200052, China
-
Received:2024-01-15Accepted:2024-02-29Online:2025-02-18Published:2024-06-03 -
Contact:Xiao Lianbo, MD, Chief physician, Doctoral supervisor, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China; Guanghua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai 200052, China; Institute of Arthritis Research of Integrated Traditional Chinese and Western Medicine, Shanghai Academy of Traditional Chinese Medicine, Shanghai 200052, China -
About author:Han Haihui, MD candidate, Physician, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China; Guanghua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai 200052, China; Institute of Arthritis Research of Integrated Traditional Chinese and Western Medicine, Shanghai Academy of Traditional Chinese Medicine, Shanghai 200052, China -
Supported by:Shanghai Natural Science Foundation (General Program), No. 22ZR1453100 (to XLB); Changning District Health Commission Key Specialties, No. 20231003 (to XLB)
CLC Number:
Cite this article
Han Haihui, Meng Xiaohu, Xu Bo, Ran Le, Shi Qi, Xiao Lianbo. Effect of fibroblast growth factor receptor 1 inhibitor on bone destruction in rats with collagen-induced arthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 968-977.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
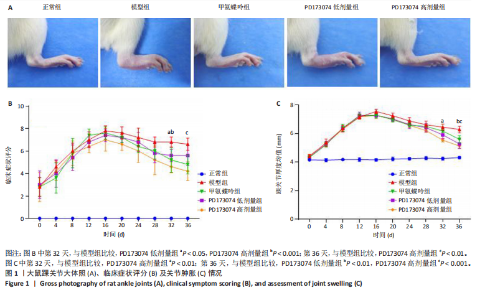
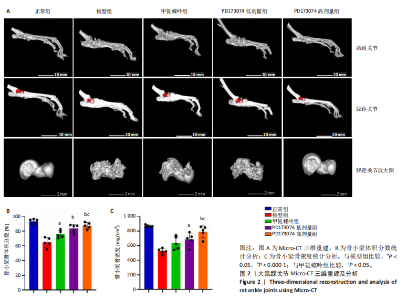
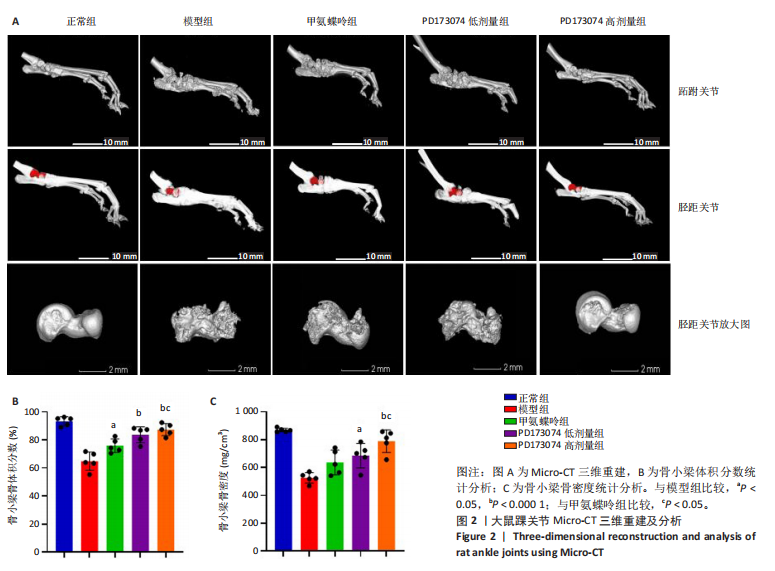
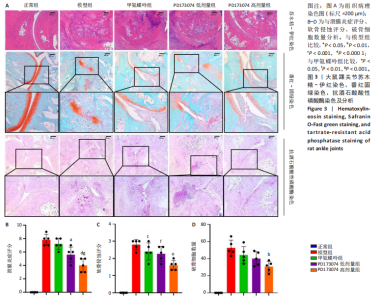
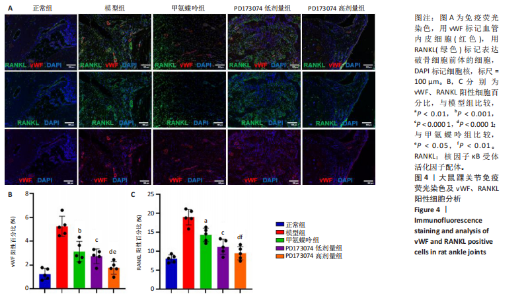
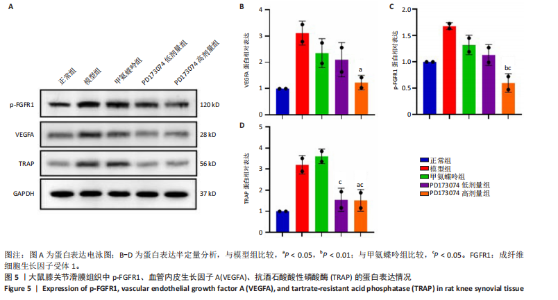
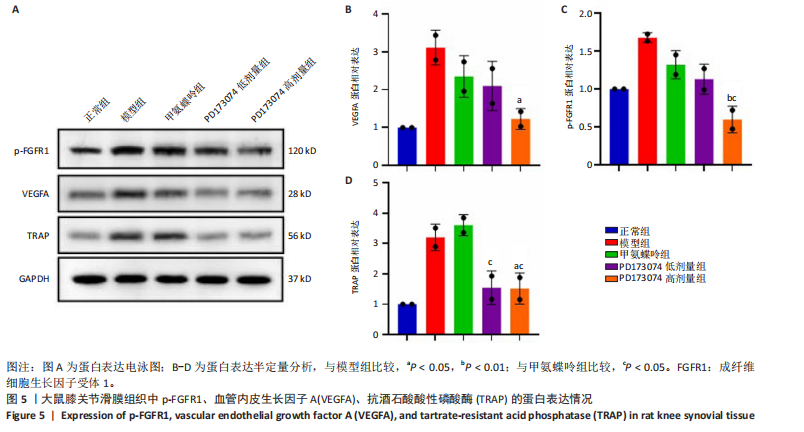
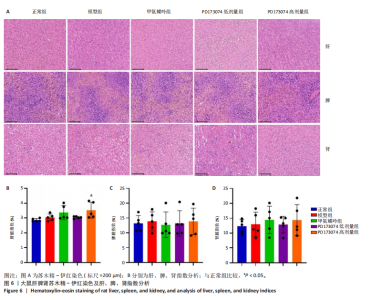
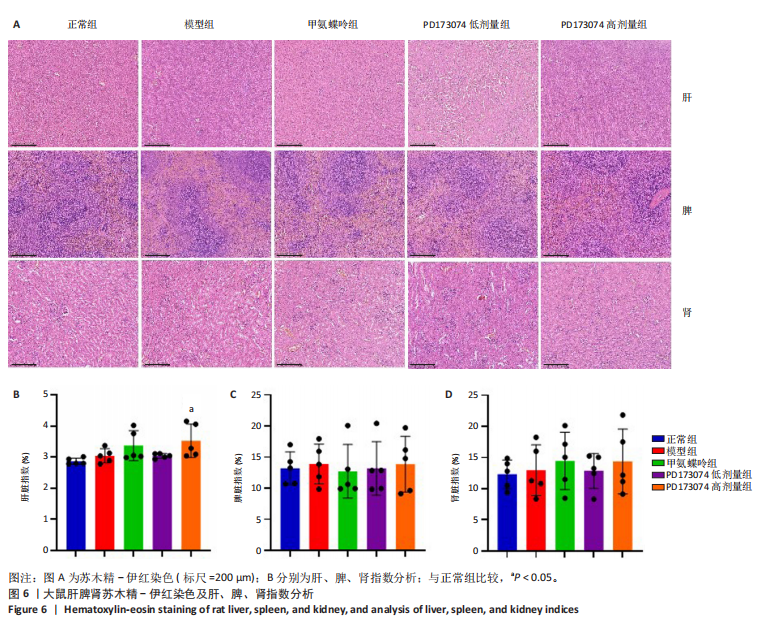
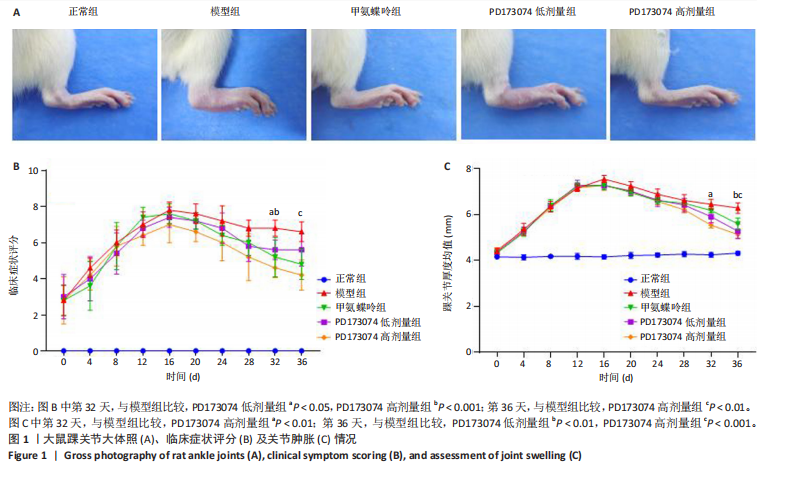
2.1 实验动物数量分析 此次实验选用大鼠25只,每组5只,实验过程中气体吸入麻醉稳定可控,Ⅱ型胶原诱导关节炎模型相对稳定,腹腔注射给药方式安全、操作规范,实验期间无动物死亡,最终分析纳入分析25只。 2.2 PD173074减轻了胶原诱导性关节炎大鼠临床症状以及关节肿胀 为了研究PD173074对胶原诱导性关节炎大鼠的治疗效果,设计了如图所示的实验流程(图1)。从二次免疫后开始评估,发现经过胶原诱导性关节炎造模的大鼠临床评分和踝关节厚度随着时间变化显著升高。治疗前3周,各组之间的临床症状评分、踝关节厚度无明显差异。 2.2.1 临床症状评分 在治疗3周后,即第32天时,统计分析表明,与模型组症状评分(6.80±0.45)相比,PD173074低剂量组(5.60±0.55)和PD173074高剂量组(4.60±0.55)评分显著降低(P < 0.05,P < 0.001),而两个PD173074剂量组之间差异无显著性意义。在第36天时,模型组评分(6.60±0.55)显著高于甲氨蝶呤组(4.80±0.84)(P < 0.05)。 2.2.2 踝关节厚度 在第32天时,PD173074高剂量组踝关节厚度(5.54±0.12) mm显著小于模型组(6.45±0.22) mm (P < 0.01),而其他治疗组与模型组均未见明显差异。在第36天时,踝关节厚度甲氨蝶呤组为(5.60±0.25) mm、PD173074低剂量组为(5.28±0.32) mm、PD173074高剂量组为(5.13±0.16) mm,均显著小于模型组(6.29±0.23) mm(P < 0.05,P < 0.01,P < 0.001),PD173074高剂量组小于甲氨蝶呤组(P < 0.05)。 2.3 PD173074延缓了Ⅱ型胶原诱导关节炎模型大鼠踝关节骨破坏,减少了骨质丢失,具有较好的骨保护作用 实验采用micro-CT三维重建了大鼠踝关节并进一步重建了距骨的三维图像(图2)。与对照的模型组相比差异显著,这说明大鼠的骨破坏模型已经成功建立。在模型组中,可以清楚地看到胫距关节、跖跗关节、近端和远端趾间关节均发生了显著的关节破坏,这种骨破坏表现为关节间隙的消失、明显的骨刺形成以及远端趾间关节的畸形,尤其是在胫距关节区域。图2显示了大鼠踝关节距骨骨小梁的数据分析(n=5)。骨显微结构分析显示PD173074对骨破坏有保护作用。 2.3.1 骨小梁骨体积分数 是指骨小梁体积与组织体积的比值,较低的数值表明骨小梁减少,这是骨质疏松症的特征之一。未经过治疗的模型组(64.88±6.45)%显示骨小梁骨体积分数百分比显著低于正常对照组(93.38±3.15)%(P < 0.000 1);而经过甲氨蝶呤、PD173074治疗后,骨小梁骨体积分数相对模型组显著升高,并且PD173074高剂量组(87.41±4.33)%要优于甲氨蝶呤组(75.94±4.88)%(P < 0.05)。 2.3.2 骨小梁骨密度 代表松质骨骨密度,是指在松质骨,即充满骨小梁的骨组织区域内,骨矿物质的密度。模型组骨密度中羟基磷灰石(524.63±37.71) mg/cm3较正常对照组(868.43±13.96) mg/cm3显著降低,PD173074低剂量组(685.04±88.2) mg/cm3和PD173074高剂量组(789.45±80.94) mg/cm3显著高于模型组(P < 0.05,P < 0.000 1),并且PD173074高剂量组优于甲氨蝶呤组(636.5±88.77) mg/cm3(P < 0.05)。 2.4 PD173074减轻了Ⅱ型胶原诱导关节炎模型大鼠踝关节病理损伤,减少破骨细胞形成 见图3。 2.4.1 苏木精-伊红染色 分析显示,正常对照组的大鼠踝关节滑膜细胞保持了正常形态,细胞核清楚可辨,细胞质染色均匀,没有显著的炎症细胞积聚或渗透,血管增生不明显,软骨边缘平滑,结构正常,骨结构未见损坏,骨小梁紧凑有序。而在模型组中,大鼠踝关节滑膜显示出侵蚀现象,伴随大量炎症细胞的积聚和渗透,滑膜细胞染色呈深蓝色,关节结构遭到严重破坏。在药物干预各组的关节都表现出了不同程度的结构变化。踝关节滑膜炎症评分发现,PD173074低剂量组(5.6±1.1)和PD173074高剂量组(4.0±1.0)明显低于模型组(7.8±0.8)(P < 0.05,P < 0.000 1)。 2.4.2 Safranin O/Fast Green染色 结果发现,模型组软骨大量丢失,提示明显的软骨损伤;骨小梁排列稀疏,可见明显的骨量流失。经过治疗后,甲氨蝶呤组及PD173074低剂量组与模型组相比,软骨侵蚀评分无明显差异,而PD173074高剂量组(1.6±0.3)与模型组(2.8±0.3)相比显著下降(P < 0.001)。此外,PD173074高剂量组软骨侵蚀评分显著低于甲氨蝶呤组(2.4±0.5)及PD173074低剂量组(2.27±0.43)(P < 0.01,P < 0.05)。 2.4.3 抗酒石酸酸性磷酸酶染色 用抗酒石酸酸性磷酸酶染色标记破骨细胞,对各组大鼠踝关节的阳性染色数量进行统计分析。模型组阳性染色数量(52.6±9.0)明显多于正常对照组,而经过治疗后PD173074高剂量组(30.6±6.2)阳性染色细胞数量有明显下降(P < 0.01)。 2.5 PD173074抑制了关节周围血管生成,并降低了RANKL的表达 通过免疫荧光染色,用vWF标记血管内皮细胞,用RANKL标记表达破骨细胞前体的细胞,DAPI标记细胞核,见图4。对vWF和RANKL进行双色免疫荧光共染,分析vWF阳性染色和RANKL阳性染色在滑膜感兴趣区域ROI(Region of Interest)中的比例。发现模型组vWF和RANKL表达要明显高于正常对照组,而经过治疗,各组均能降低vWF的表达。并且PD173074高剂量组vWF和RANKL阳性比例要低于甲氨蝶呤组(P < 0.05,P < 0.01),但与PD173074低剂量组无明显统计学差异。 2.6 PD173074治疗后大鼠滑膜中p-FGFR1、血管内皮生长因子A、抗酒石酸酸性磷酸酶表达情况 Western blot检测结果发现,PD173074能够抑制FGFR1磷酸化蛋白表达(P < 0.05),并且抑制了滑膜组织中血管内皮生长因子A和抗酒石酸酸性磷酸酶蛋白的表达(P < 0.05),见图5。 2.7 PD173074治疗后大鼠肝、脾、肾无明显变化 对大鼠肝、脾、肾内脏进行苏木精-伊红染色,发现各组内脏均无明显的病理改变,未发现有明显的炎性细胞聚集,组织浸润,空泡形成,大量纤维连接等表现。计算每只大鼠的肝脏、脾脏、肾脏的质量与各自体质量的比值,分别得到肝脏指数、脾脏指数和肾脏指数。研究中发现肝脏指数中PD173074高剂量组高于正常组(P < 0.05),而甲氨蝶呤与PD173074低剂量组没有明显差异,见图6。"
| [1] [No authors listed].Rheumatoid arthritis. Nat Rev Dis Primers. 2018; 4(1):18001. [2] WINTHROP KL, WEINBLATT ME, CROW MK, et al. Unmet need in rheumatology: reports from the Targeted Therapies meeting 2018. Ann Rheum Dis. 2019;78(7):872-878. [3] MENG X, CHEN Z, LI T, et al. Role and Therapeutic Potential for Targeting Fibroblast Growth Factor 10/FGFR1 in Relapsed Rheumatoid Arthritis. Arthritis Rheumatol. 2024;76(1):32-47. [4] COLLIN-OSDOBY P, ROTHE L, BEKKER S, et al. Basic fibroblast growth factor stimulates osteoclast recruitment, development, and bone pit resorption in association with angiogenesis in vivo on the chick chorioallantoic membrane and activates isolated avian osteoclast resorption in vitro. J Bone Miner Res. 2002;17(10):1859-1871. [5] TAN Q, CHEN B, WANG Q, et al. A novel FGFR1-binding peptide attenuates the degeneration of articular cartilage in adult mice. Osteoarthritis Cartilage. 2018;26(12):1733-1743. [6] MCKENZIE J, SMITH C, KARUPPAIAH K, et al. Osteocyte Death and Bone Overgrowth in Mice Lacking Fibroblast Growth Factor Receptors 1 and 2 in Mature Osteoblasts and Osteocytes. J Bone Miner Res. 2019;34(9): 1660-1675. [7] LEE C, CHEN R, SUN G, et al. VEGF-B prevents excessive angiogenesis by inhibiting FGF2/FGFR1 pathway. Signal Transduct Target Ther. 2023; 8(1):305. [8] OBERKERSCH RE, PONTARIN G, ASTONE M, et al.Aspartate metabolism in endothelial cells activates the mTORC1 pathway to initiate translation during angiogenesis. Dev Cell. 2022;57(10):1241-1256.e8. [9] ZHU X, QIU C, WANG Y, et al. FGFR1 SUMOylation coordinates endothelial angiogenic signaling in angiogenesis. Proc Natl Acad Sci U S A. 2022;119(26):e2202631119. [10] TAN Q, WANG Z, WANG Q, et al. A novel FGFR1-binding peptide exhibits anti-tumor effect on lung cancer by inhibiting proliferation and angiogenesis. Int J Biol Sci. 2018;14(10):1389-1398. [11] MOHAMMADI M, FROUM S, HAMBY JM, et al. Crystal structure of an angiogenesis inhibitor bound to the FGF receptor tyrosine kinase domain. EMBO J. 1998;17(20):5896-5904. [12] ANREDDY N, PATEL A, SODANI K, et al. PD173074, a selective FGFR inhibitor, reverses MRP7 (ABCC10)-mediated MDR. Acta Pharm Sin B. 2014;4(3):202-207. [13] XU W, XIE Y, WANG Q, et al. A novel fibroblast growth factor receptor 1 inhibitor protects against cartilage degradation in a murine model of osteoarthritis. Sci Rep. 2016;6:24042. [14] KRENN V, PERINO G, RÜTHER W, et al. 15 years of the histopathological synovitis score, further development and review:A diagnostic score for rheumatology and orthopaedics. Pathol Res Pract. 2017;213(8): 874-881. [15] DOUNI E, SFIKAKIS PP, HARALAMBOUS S, et al. Attenuation of inflammatory polyarthritis in TNF transgenic mice by diacerein: comparative analysis with dexamethasone, methotrexate and anti-TNF protocols. Arthritis Res Ther. 2004;6(1):R65-R72. [16] ABBASI M, MOUSAVI MJ, JAMALZEHI S, et al. Strategies toward rheumatoid arthritis therapy; the old and the new. J Cell Physiol. 2019; 234(7):10018-10031. [17] ORNITZ DM. FGF signaling in the developing endochondral skeleton. Cytokine Growth Factor Rev. 2005;16(2):205-213. [18] XIE Y, SU N, YANG J, et al. FGF/FGFR signaling in health and disease. Signal Transduct Target Ther. 2020;5(1):181. [19] KRONENBERG HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332-336. [20] ORNITZ DM, ITOH N. New developments in the biology of fibroblast growth factors. WIREs Mech Dis. 2022;14(4):e1549. [21] ORNITZ DM, ITOH N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4(3):215-266. [22] TURNER N, GROSE R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10(2):116-129. [23] SU N, JIN M, CHEN L. Role of FGF/FGFR signaling in skeletal development and homeostasis: learning from mouse models. Bone Res. 2014;2:14003. [24] GOYAL L, KONGPETCH S, CROLLEY VE, et al. Targeting FGFR inhibition in cholangiocarcinoma. Cancer Treat Rev. 2021;95:102170. [25] ROSKOSKI R JR. The role of fibroblast growth factor receptor (FGFR) protein-tyrosine kinase inhibitors in the treatment of cancers including those of the urinary bladder. Pharmacol Res. 2020;151:104567. [26] WANG Y, CAI Y, JI J, et al. Discovery and identification of new non-ATP competitive FGFR1 inhibitors with therapeutic potential on non-small-cell lung cancer. Cancer Lett. 2014;344(1):82-89. [27] GARCÍA-GONZÁLEZ CM, BAKER J.Treatment of early rheumatoid arthritis: Methotrexate and beyond. Curr Opin Pharmacol. 2022;64: 102227. [28] WANG Y, WU H, DENG R. Angiogenesis as a potential treatment strategy for rheumatoid arthritis. Eur J Pharmacol. 2021;910:174500. [29] LIU J, ZHAO N, SU SH, et al. Anti-Arthritic Effect of Edaravone Against Complete Freund Adjuvant Induced Arthritis via Osteoclast Differentiation and HIF-1alpha-VEGF-ANG-1 Axis. Drug Des Devel Ther. 2023;17:519-534. [30] AO L, GAO H, JIA L, et al. Matrine inhibits synovial angiogenesis in collagen-induced arthritis rats by regulating HIF-VEGF-Ang and inhibiting the PI3K/Akt signaling pathway. Mol Immunol. 2022;141:13-20. [31] LI Y, LIU Y, WANG C, et al. Succinate induces synovial angiogenesis in rheumatoid arthritis through metabolic remodeling and HIF-1alpha/VEGF axis. Free Radic Biol Med. 2018;126:1-14. |
| [1] | Lai Pengyu, Liang Ran, Shen Shan. Tissue engineering technology for repairing temporomandibular joint: problems and challenges [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [2] | Han Haihui, Ran Lei, Meng Xiaohui, Xin Pengfei, Xiang Zheng, Bian Yanqin, Shi Qi, Xiao Lianbo. Targeting fibroblast growth factor receptor 1 signaling to improve bone destruction in rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1905-1912. |
| [3] | Chen Lijuan, Gao Xinxue, Wu Jin, Du Ying, Lyu Meijun, Sui Guoyuan, Jia Lianqun, Pan Guowei. Construction and evaluation of spleen-deficiency hyperlipidemia mouse models [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(29): 6237-6242. |
| [4] | Li Shuyuan, Yang Dawen, Zeng Zhanpeng, Cai Qunbin, Zhang Jingtao, Zhou Qishi. Application of induced membrane technique for repairing critical-sized bone defects: advantages and future development [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(28): 6083-6093. |
| [5] | Zhang Yibo, Lu Jianqi, Mao Meiling, Pang Yan, Dong Li, Yang Shangbing, Xiao Xiang. Rheumatoid arthritis and coronary atherosclerosis: data analysis of serum metabolite and inflammatory factor in the European population [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(24): 5263-5271. |
| [6] | Lai Pengyu, Liang Ran, Shen Shan. Tissue engineering technology for repairing temporomandibular joint: problems and challenges [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(22): 4804-4812. |
| [7] | Wu Xiuli, Yan Xiaoxia, Ren Zhiqiang, Sun Nan, Li Jinju. Modeling methods and evaluation criteria in animal models of steroid-induced osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(21): 4560-4567. |
| [8] | Xu Liang, Gulimila·Muhetaer, Ju Bowei, Li Ruoning. Molecular mechanism of allicin-targeted regulation of epidermal growth factor receptor and kynureninase in the treatment of rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(20): 4205-4214. |
| [9] | Tao Zihan, Saijilafu. Cycloastragenol promotes motor function recovery and cortical neuron regeneration in mice with spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(20): 4258-4265. |
| [10] | Liang Roujun, Zhan Lifen, Zeng Xuejiu, Ding Qiangsheng, Luo Xiaojing, Zhuo Yue, Ai Kun, Deng Shifeng, Xu Ming, Zhang Hong. Effect of the number of times to urinate on the modeling rate of neurogenic bladder model in rats after complete spinal cord transection [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(18): 3840-3847. |
| [11] | He Li, , Ren Lu, , Jiang Xiaoxi, , Liu Xuqian, , Li Chunhui, . Effects of 1,8-cineole on inflammatory response in a rat model of experimental periodontitis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(17): 3605-3613. |
| [12] | Qian Jiaming, Wang Xiaole, Fang Ting, Zhou Maosheng, Liu Fushui, . Animal model of cervical spondylosis and its internal molecular mechanism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(17): 3624-3631. |
| [13] | Qian Zuping, Chen Yong, Ran Yan, Da Jingjing, Zha Yan. Diabetic nephropathy model: animal model, two-dimensional cell simulation and three-dimensional organoid model [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(17): 3632-3640. |
| [14] | Yin Hao, Ji Meiqi, Hu Zhixiang, Wu Han, Lyu Heng, Li Shengyun, Li Lei, Zhai Chuntao, Lyu Yue. Comparison and evaluation of three different methods for preparing rat models of lumbar disc herniation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(14): 2930-2936. |
| [15] | Tian Dongzi, Shen Weiwei, Li Wenshuai, Shi Jie, Deng Xiaowen, Zhao Zhengrong, Liu Dengke, Liu Taotao, Cai Maolin, Gao Qiuming. Construction and evaluation of a model of chronic osteomyelitis in sheep tibia [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(14): 2937-2942. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||