Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (36): 7804-7815.doi: 10.12307/2025.753
Previous Articles Next Articles
Human amniotic mesenchymal stem cell exosomes repair radiation-induced submandibular gland damage in rats
Zhang Min1, Zhang Nini1, Huang Guilin2, Li Zhuangzhuang1, Wang Xue1, Wang Huike1
- 1Department of Maxillofacial Surgery, Stomatological Hospital Affiliated to Zunyi Medical University, Zunyi 563003, Guizhou Province, China; 2Fifth Affiliated (Zhuhai) Hospital of Zunyi Medical University, Zhuhai 519180, Guangdong Province, China
-
Received:2024-06-17Accepted:2024-08-17Online:2025-12-28Published:2025-03-12 -
Contact:Zhang Nini, MS, Chief physician, Department of Maxillofacial Surgery, Stomatological Hospital Affiliated to Zunyi Medical University, Zunyi 563003, Guizhou Province, China -
About author:Zhang Min, MS, Physician, Department of Maxillofacial Surgery, Stomatological Hospital Affiliated to Zunyi Medical University, Zunyi 563003, Guizhou Province, China -
Supported by:Guizhou Provincial Science and Technology Plan Project, No. ZK[2024]339 (to ZNN); "Future Clinical Famous Doctor" Project of Zunyi Medical University, No. 20211017 (to ZNN); Guizhou Provincial Clinical Key Construction Project, No. [2017]24 (to HGL)
CLC Number:
Cite this article
Zhang Min, Zhang Nini, Huang Guilin, Li Zhuangzhuang, Wang Xue, Wang Huike. Human amniotic mesenchymal stem cell exosomes repair radiation-induced submandibular gland damage in rats[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7804-7815.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
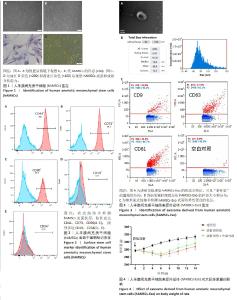
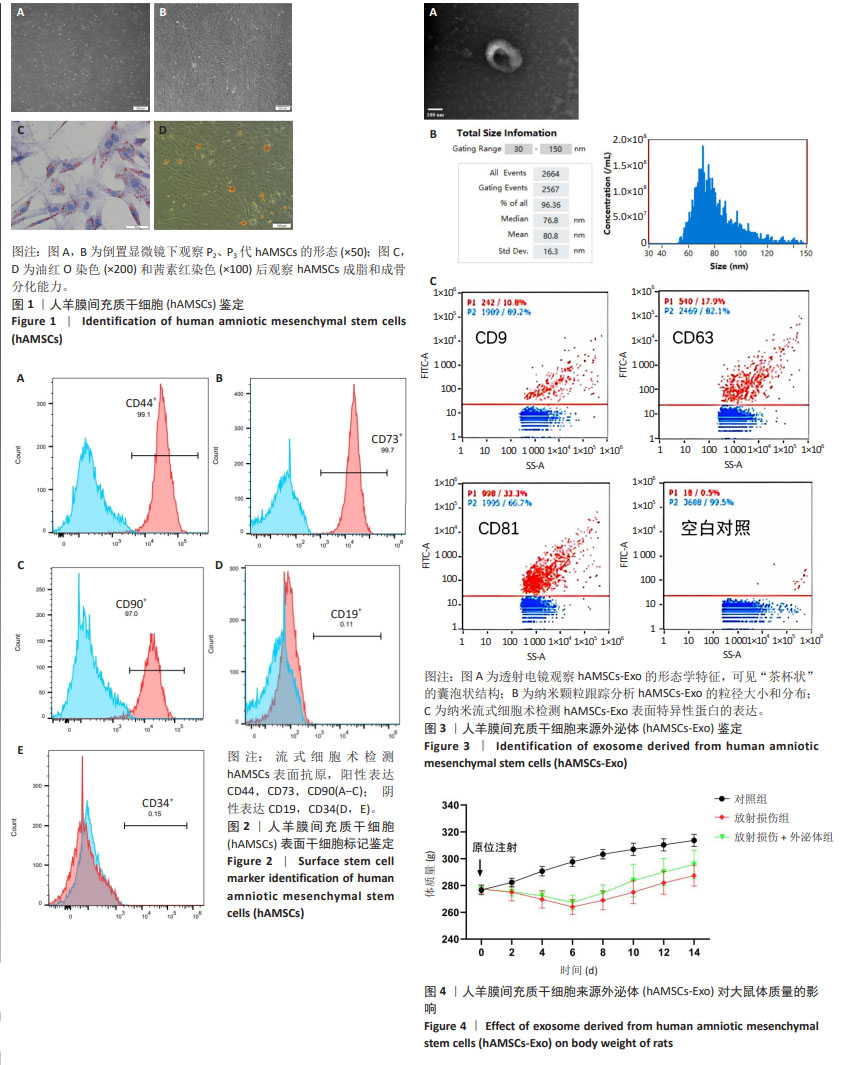
2.1 hAMSC、hAMSC-Exo的分离鉴定 此次实验采用酶消化法获得 hAMSCs,P2、P3代 hAMSCs 细胞排列紧密,相互黏附,形态均一,呈漩涡状贴壁生长(图1A,B)。hAMSCs在成脂分化诱导培养基、成骨分化诱导培养基试剂诱导条件下,观察到被染成橘红色的脂滴颗粒形成;经成骨诱导3周后,茜素红染色后镜下观察可见诱导细胞内出现散落分布的橘红球形小团块,证明钙结节已经形成(图1C,D)。流式细胞分析仪检测发现 hAMSCs 阳性表达CD44、CD73、CD90;阴性表达CD19、CD34(图2)。电镜观察到hAMSCs-Exo为类圆形的双层膜结构,中间凹陷,呈“茶杯状”的囊泡状结构(图3A)。纳米跟踪分析技术进行粒径分析结果表明颗粒粒径在30-150 nm之间,平均粒径为80.8 nm (图3B)。利用纳米流式细胞术检测与对照组(hAMSCs蛋白裂解液)相比,hAMSCs-Exo 膜上参与 CD9、CD63、CD81跨膜蛋白运输(图3C)。 2.2 动物实验结果 2.2.1 各组大鼠体质量变化 实验过程中各组大鼠无脱失,连续14 d监测体质量结果显示,相比于对照组,经放射处理过的大鼠在6 d内体质量均呈减轻趋势;7-14 d,相比于放射损伤组,放射损伤+外泌体组大鼠体质量增加,于第14 天时恢复至与对照组体质量接近的程度。见图4。"
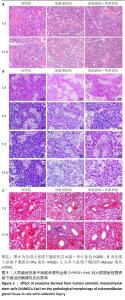
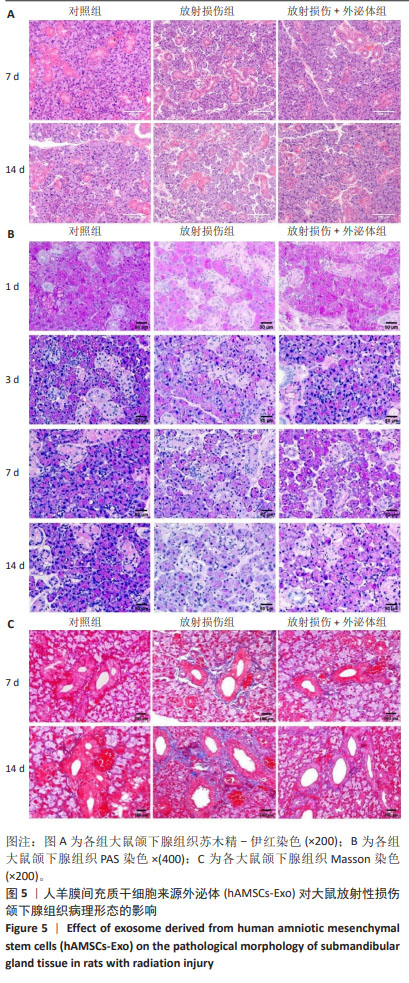
2.2.2 hAMSCs-Exo对大鼠放射性损伤颌下腺病理形态影响 苏木精-伊红染色结果如图5A所示,第7,14天,放射损伤组腺泡萎缩,核仁减少,结构疏松、紊乱,部分胞浆内出现空泡化改变,导管萎缩变形;而与放射损伤组比较,14 d时,放射损伤+外泌体组的组织结构及形态恢复,主要表现为核仁增多,胞浆内空泡化减少,萎缩的腺泡细胞形态较饱满,间质疏松结构得到明显改善。 PAS染色结果如图5B所示,第3天时,放射损伤组PAS阳性酶原颗粒含量相对减少;第7天时,放射损伤组腺泡胞质内可见酶原颗粒分布不均、数量、密度变少;而第14天时,放射损伤组腺泡开始出现萎缩,结构混乱,部分呈空泡样变性,阳性酶原颗粒明显减少、甚至部分颗粒已消失;第3,7,14天时,与放射损伤组比较,放射损伤+外泌体组阳性酶原颗粒数量、密度分布逐渐增多。 Masson染色如图5C所示,第7,14天时,放射损伤组间质及导管阳性胶原纤维数量、密度增多,纤维化组织面积明显增多;而与放射损伤组相比,放射损伤+外泌体组间质及导管阳性胶原纤维数量、密度减少,纤维化组织面积降低。 "
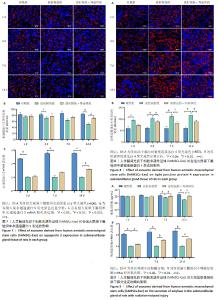
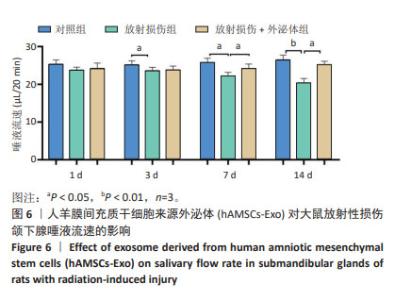
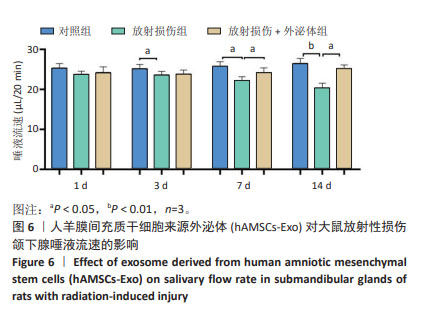
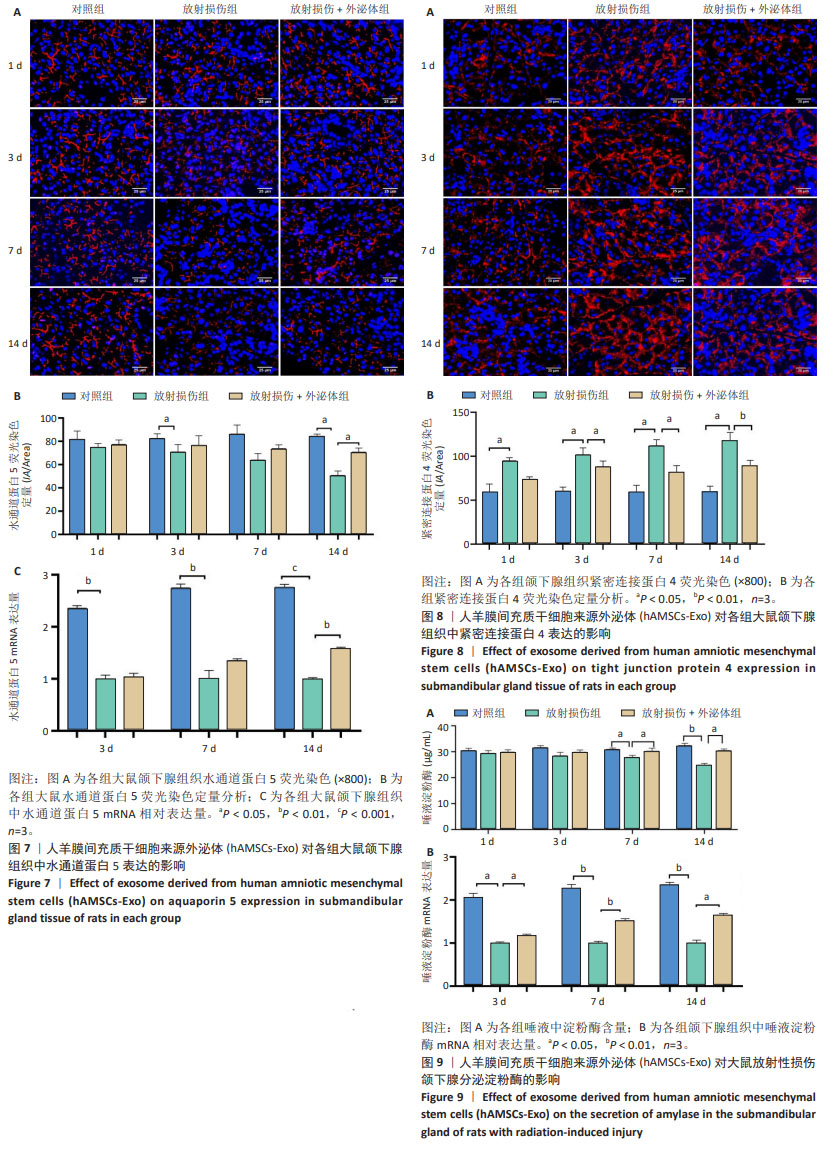
各组颌下腺组织腺泡顶侧膜的水通道蛋白5荧光分布变化见图7A,各组水通道蛋白5蛋白荧光定量分析见图7B。第3,14天,放射损伤组水通道蛋白5分布、荧光定量强度呈现依赖性减弱(P < 0.05);而第14天时,与放射损伤组相比,放射损伤+外泌体组荧光定量强度显著增强(P < 0.05)。 各组水通道蛋白5 mRNA表达分布变化见图7C,其中3,7,14 d,放射损伤组水通道蛋白5 mRNA表达量逐渐降低(P < 0.01,P < 0.001);第14天时,与放射损伤组相比,放射损伤+外泌体组水通道蛋白5 mRNA表达量显著升高(P < 0.01)。 各组颌下腺组织中紧密连接蛋白4荧光分布见图8A,各组蛋白荧光定量分析见图8B。其中第1,3,7,14天,各组放射损伤组中紧密连接蛋白4分布、荧光定量强度均呈现明显增强(P < 0.05);而第3,7,14天,较放射损伤组相比,放射损伤+外泌体组荧光分布、定量强度出现依赖性减弱(P < 0.05);且第14天时,紧密连接蛋白4荧光分布、定量强度显著降低(P < 0.01)。 2.2.4 各组大鼠唾液淀粉酶水平比较 ELISA结果见图9A, 第1,3天,各组唾液淀粉酶水平未见明显变化;第7天时,放射损伤组唾液淀粉酶水平开始降低(P < 0.05);14 d时,放射损伤组唾液淀粉酶水平随时间推移持续降低(P < 0.01);7,14 d,与放射损伤组比较,放射损伤+外泌体组唾液淀粉酶水平升高(P < 0.05)。 各组颌下腺组织唾液淀粉酶 mRNA表达变化见图9B,放射后第3天时,放射损伤组唾液淀粉酶mRNA表达量开始降低(P < 0.05);7,14 d,放射损伤组唾液淀粉酶mRNA 表达量持续降低(P < 0.01),而第7天时较放射损伤组相比,放射损伤+外泌体组唾液淀粉酶mRNA表达量显著升高(P < 0.01);第14 天时,唾液淀粉酶mRNA表达量升高(P < 0.05)。 "
| [1] SUNG H, FERLAY J, SIEGEL RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [2] SROUSSI HY, EPSTEIN JB, BENSADOUN RJ, et al. Common oral complications of head and neck cancer radiation therapy: mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med. 2017;6: 2918-2931. [3] LI SS, WU CZ, QIAO XH, et al. Advances on mechanism and treatment of salivary gland in radiation injury. Hua Xi Kou Qiang Yi Xue Za Zhi. 2021;39:99-104. [4] ZHANG XM, HUANG Y, CONG X, et al. Parasympathectomy increases resting secretion of the submandibular gland in minipigs in the long term. Cell Physiol. 2018;234:9515-9524. [5] ALMANSOORI AA, HARIHARAN A, CAO UMN, et al. Drug Therapeutics Delivery to the Salivary Glands: Intraglandular and Intraductal Injections. Adv Exp Med Biol. 2023;1436:119-130. [6] PIRAINO LR, BENOIT DSW, DELOUISE LA, et al. Salivary Gland Tissue Engineering Approaches: State of the Art and Future Directions. Cells. 2021;10(7):1723. [7] SONG W, LIU H, SU Y, et al. Current developments and opportunities of pluripotent stem cells-based therapies for salivary gland hypofunction. Front Cell Dev Biol. 2024;12:1346996. [8] ChANSAENROJ A, YODDMUANG S, FERREIRA J, et al. Trends in Salivary Gland Tissue Engineering: From Stem Cells to Secretome and Organoid Bioprinting. Tissue Eng Part B Rev. 2021;27:155-165. [9] MARINKOVIC M, TRAN ON, WANG HZ, et al. Autologous mesenchymal stem cells offer a new paradigm for salivary gland regeneration. Int J Oral Sci. 2023;15(1):18. [10] KALLURI R, LEBLEU VS. The biology function and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. [11] DAI S, WEN Y, LUO P, et al. Therapeutic implications of exosomes in the treatment of radiation injury. Burns Trauma. 2022;10:tkab043. [12] KIMIZ-GEBOLOGLU I, ONCEL SS. Exosomes: Large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J Control Release. 2022;347:533-543. [13] CHANSAENROJ A, ADINE C, CHAROENLAPPANIT S, et al. Magnetic bioassembly platforms towards the generation of extracellular vesicles from human salivary gland functional organoids for epithelial repair. Bioact Mater. 2022;18:151-163. [14] XIAO XY, ZHANG NN, LONG YZ, et al. Repair mechanism of radiation-induced salivary gland injury by hypoxia-pretreated human urine-derived stem cell exosomes. Oral Dis. 2024;30(3):1234-1241. [15] LIU QW, HUANG QM, WU HY, et al. Characteristics and Therapeutic Potential of Human Amnion-Derived Stem Cells. Int J Mol Sci. 2021; 22(2):970. [16] HU ZM, LUO Y, NI RH, et al. Biological importance of human amniotic membrane in tissue engineering and regenerative medicine. Mater Today Bio. 2023;22:780-790. [17] 崔田宁,张霓霓,龙远铸,等.低氧预处理人羊膜间充质干细胞外泌体修复放射性损伤唾液腺的实验研究[J].口腔医学研究,2022, 38(12):1145-1150. [18] PORCHERI C, MITSIADIS TA. Physiology, Pathology and Regeneration of Salivary Glands. Cells. 2019;8(9):976. [19] LI J, SUDIWALA S, BERTHOIN L, et al. Long-term functional regeneration of radiation-damaged salivary glands through delivery of a neurogenic hydrogel. 2022;8(51):eadc8753. [20] KHOURY ZH, SULTAN AS. Prosthodontic implications of saliva and salivary gland dysfunction. J Prosthodont. 2023;32(9):766-775. [21] 卢浩,张赐童,柳康,等.大鼠腮腺主导管结扎损伤诱导腺体萎缩的组织学变化[J].中华老年口腔医学杂志,2015,13(2):85-87. [22] KIMBERLY J, JASMER, KRISTY E, et al. Radiation-Induced Salivary Gland Dysfunction: Mechanisms, Therapeutics and Future Directions. J Clin Med. 2020;9:100-105 [23] HYE J, HONG, JAE M, et al. Thermoresponsive fiber-based microwells capable of formation and retrieval of salivary gland stem cell spheroids for the regeneration of irradiation-damaged salivary glands. J Tissue Eng. 2022;13:575-581. [24] NAM K, DOS SANTOS HT, MASLOW FM, et al. Copper chelation reduces early collagen deposition and preserves saliva secretion in irradiated salivary glands. Heliyon. 2024;10(2):e24368. [25] 王英鑫.低氧预处理人羊膜间充质干细胞修复放射性涎腺损伤功能的研究[D]. 遵义:遵义医学院,2018. [26] TIAN Q, CHEN XJ, WEI HY, et al. Telocyte-Derived Exosomes Provide an Important Source of Wnts That Inhibits Fibrosis and Supports Regeneration and Repair of Endometrium. Cell Transplant. 2023:32: 9636897231212746. [27] CARLANDER AF, GUNDESTRUP AK, JANSSON PM, et al. Mesenchymal Stromal/Stem Cell Therapy Improves Salivary Flow Rate in Radiation-Induced Salivary Gland Hypofunction in Preclinical in vivo Models: A Systematic Review and Meta-Analysis. Stem Cell Rev Rep. 2024; 20(4):1078-1092. [28] ZAYED HM, KHEIR E, ABU AM, et al. Gingival-derived mesenchymal stem cell therapy regenerated the radiated salivary glands: functional and histological evidence in murine model. Stem Cell Res Ther. 2024; 15(1):46. [29] XU XY, XIONG G, ZhANG M, et al. Sox9 cells are required for salivary gland regeneration after radiation damage via the Wnt/β-catenin pathway. J Genet Genomics. 2022;49:230-239. [30] KAZUO, H, CHEN JY, TAKAHIRO, et al. Dynamics of Salivary Gland AQP5 under Normal and Pathologic Conditions. Int J Mol Sci. 2020;21: 303-310. [31] LAI Z, YIN H, CABRERA PJ, et al. Aquaporin gene therapy corrects Sjögren’s syndrome phenotype in mice. Proc Natl Acad Sci. 2016; 113(20):5694-5699. [32] 崔荣兴,战丽彬,孙晓霞,等.阴虚津亏模型小鼠颌下腺AQP5及cAMP/PKA-CREB信号通路的表达与意义[J].中华中医药学刊, 2022,40(6):149-153. [33] AN HY, SHIN HS, CHOI JS, et al. Adipose Mesenchymal Stem Cell Secretome Modulated in Hypoxia for Remodeling of Radiation-Induced Salivary Gland Damage. PLoS One. 2015;10(11):e0141862. [34] KIM JH, JEONG BK, JANG SJ, et al. Alpha-Lipoic Acid Ameliorates Radiation-Induced Salivary Gland Injury by Preserving Parasympathetic Innervation in Rats. Int J Mol Sci. 2020;21(7):2260. [35] LI J, SUDIWALA S, BERTHOIN L, et al. Long-term functional regeneration of radiation-damaged salivary glands through delivery of a neurogenic hydrogel. Sci Adv. 2022;8(51):eade8753. [36] WU YH, XU H, YAO QT, et al. Effect of ionizing radiation on the secretion of the paracellular pathway in rat submandibular glands. Hua Xi Kou Qiang Yi Xue Za Zhi. 2021;39:267-273. [37] YAMAMURA Y, AOTA K, YAMANOI T, et al. DNA demethylating agent decitabine increases AQP5 expression and restores salivary function. J Dent Res. 2012;91:612-617. [38] CONG X, MAO XD, WU LL, et al. The role and mechanism of tight junctions in the regulation of salivary gland secretion. Oral Dis. 2024; 30:673-680. [39] 姚清婷,吴言辉,刘少华,等.电离辐射对大鼠腮腺旁细胞分泌途径损伤及紧密连接蛋白claudin-4的影响[J].上海口腔医学, 2022,31(4):359-366. [40] CONG X, ZHANG Y, LI J, et al. Claudin-4 is required for modulation of paracellular permeability by muscarinic acetylcholine receptor in epithelial cells. J Cell Sci. 2015;128:2271-2286. [41] XIANG RL, MEI M, CONG X, et al. Claudin-4 is required for AMPK-modulated paracellular permeability in submandibular gland cells. J Mol Cell Biol. 2014;6:486-497. [42] YAMAGISHI R, WAKAYAMA T, NAKATA H, et al. Expression and Localization of α-amylase in the Submandibular and Sublingual Glands of Mice. Acta Histochem Cytochem. 2014;47:95-102. [43] DE FF, TOMBOLINI M, MUSELLA A, et al. Radiation therapy and serum salivary amylase in head and neck cancer. Oncotarget. 2017;8: 90496-90500. [44] SHIN HS, LEE S, HONG HJ, et al. Stem cell properties of human clonal salivary gland stem cells are enhanced by three-dimensional priming culture in nanofibrous microwells. Stem Cell Res Ther. 2018;9(1):74. [45] KIM JM, CHOI ME, JEON EJ, et al. Cell-derived vesicles from adipose-derived mesenchymal stem cells ameliorate irradiation-induced salivary gland cell damage. Regen Ther. 2022;21:453-459. [46] DE MG, ÁGATHA N, NAGAI MA, et al. Apoptosis and proliferation during human salivary gland development. J Anat. 2019;234:830-838. [47] HU S, CHEN B, ZHOU J, et al. Dental pulp stem cell-derived exosomes revitalize salivary gland epithelial cell function in NOD mice via the GPER-mediated cAMP/PKA/CREB signaling pathway. J Transl Med. 2023;21(1):361. [48] LOMBAERT I, MOVAHEDEIA MM, ADINE C, et al. Concise Review: Salivary Gland Regeneration: Therapeutic Approaches from Stem Cells to Tissue Organoids. Stem Cells. 2017;35:97-105. [49] KANG S, YASUHARA R, TOKUMASU R, et al. Adipose-derived mesenchymal stem cells promote salivary duct regeneration via a paracrine effect. J Oral Biosci. 2023;65:104-110. |
| [1] | Miao Jiahang, Ma Sheng, Li Qupeng, Yu Huilin, Hu Tianyu, Gao Xiao, Feng Hu. Cervical lordosis ratio can be used as a decision-making indicator for selection of posterior surgical approach for multi-level cervical spondylotic myelopathy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1796-1802. |
| [2] | Yu Shuai, Liu Jiawei, Zhu Bin, Pan Tan, Li Xinglong, Sun Guangfeng, Yu Haiyang, Ding Ya, Wang Hongliang. Hot issues and application prospects of small molecule drugs in treatment of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1913-1922. |
| [3] | Cai Yaohao, Lang Lyu, Li Hong. Assessing the bone mass of the residual alveolar ridge in the first molar for implant placement by cone-beam computed tomography [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1572-1577. |
| [4] | Liu Lin, Liu Shixuan, Lu Xinyue, Wang Kan. Metabolomic analysis of urine in a rat model of chronic myofascial trigger points [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1585-1592. |
| [5] | Su Xiaoyang, Chen Wenting, Fu Yidan, Zhao Yan, Lan Danfeng, Yang Qiuping. Correlation between Mer receptor tyrosine kinase and diabetic peripheral neuropathy in Sprague-Dawley rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1593-1599. |
| [6] | Li Jun, Gong Jingjing, Sun Guobin, Guo Rui, Ding Yang, Qiang Lijuan, Zhang Xiaoli, Fang Zhanhai . miR-27a-3p promotes the proliferation of human hypertrophic scar fibroblasts by regulating mitogen-activated protein kinase signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1609-1617. |
| [7] | Jing Ruyi, Chen Yingxin, Cao Lei . Prognosis of deep lamellar keratoplasty versus penetrating keratoplasty in the treatment of stromal corneal dystrophy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1626-1633. |
| [8] | Wang Yida, Liu Jun, Wang Xiaoling, Wang Liyan, Yang Chengru, Zhang Xuexiao. Effects of wearable electronic device-based interventions on physical activity and sedentary behavior in healthy adolescents: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1693-1704. |
| [9] | Zheng Huakun, Yin Mingyue, Liu Qian. Effects of interval and continuous training on the quality of life in physically inactive adults: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1727-1740. |
| [10] | Hu Taotao, Liu Bing, Chen Cheng, Yin Zongyin, Kan Daohong, Ni Jie, Ye Lingxiao, Zheng Xiangbing, Yan Min, Zou Yong. Human amniotic mesenchymal stem cells overexpressing neuregulin-1 promote skin wound healing in mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1343-1349. |
| [11] | Jin Kai, Tang Ting, Li Meile, Xie Yuan. Effects of conditioned medium and exosomes of human umbilical cord mesenchymal stem cells on proliferation, migration, invasion, and apoptosis of hepatocellular carcinoma cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1350-1355. |
| [12] | Liu Qi, Li Linzhen, Li Yusheng, Jiao Hongzhuo, Yang Cheng, Zhang Juntao. Icariin-containing serum promotes chondrocyte proliferation and chondrogenic differentiation of stem cells in the co-culture system of three kinds of cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1371-1379. |
| [13] | Huang Ting, Zheng Xiaohan, Zhong Yuanji, Wei Yanzhao, Wei Xufang, Cao Xudong, Feng Xiaoli, Zhao Zhenqiang. Effects of macrophage migration inhibitory factor on survival, proliferation, and differentiation of human embryonic stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1380-1387. |
| [14] | He Longcai, Song Wenxue, Ming Jiang, Chen Guangtang, Wang Junhao, Liao Yidong, Cui Junshuan, Xu Kaya. An experimental method for simultaneous extraction and culture of primary cortical neurons and microglial cells from SD rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1395-1400. |
| [15] | Peng Hongcheng, Peng Guoxuan, Lei Anyi, Lin Yuan, Sun Hong, Ning Xu, Shang Xianwen, Deng Jin, Huang Mingzhi . Role and mechanism of platelet-derived growth factor BB in repair of growth plate injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1497-1503. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||