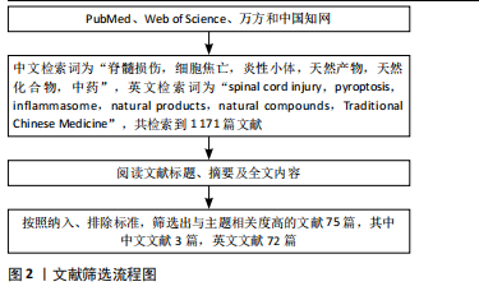
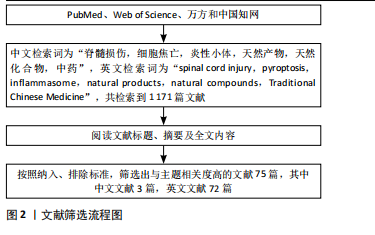
Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (30): 6520-6528.doi: 10.12307/2025.946
Previous Articles Next Articles
Natural products modulate pyroptosis for treatment of spinal cord injury
Zhang Xuesan, Zhang Zheng, Shen Le, Geng Qingqing, Tan Shusen, Lou Chunbiao, Han Kang
- The 960 Hospital of Joint Logistics Support Force of Chinese People’s Liberation Army, Jinan 250000, Shandong Province, China
-
Received:2024-10-21Accepted:2024-12-10Online:2025-10-28Published:2025-03-29 -
Contact:Han Kang, Associate chief physician, Master’s supervisor, The 960 Hospital of Joint Logistics Support Force of Chinese People’s Liberation Army, Jinan 250000, Shandong Province, China -
About author:Zhang Xuesan, Attending physician, The 960 Hospital of Joint Logistics Support Force of Chinese People’s Liberation Army, Jinan 250000, Shandong Province, China -
Supported by:Natural Science Foundation of Shandong Province, No. ZR2023MH206 (to HK); Shandong Province Medical and Health Science and Technology Development Program Project, No. 202204071065 (to HK)
CLC Number:
Cite this article
Zhang Xuesan, Zhang Zheng, Shen Le, Geng Qingqing, Tan Shusen, Lou Chunbiao, Han Kang. Natural products modulate pyroptosis for treatment of spinal cord injury[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6520-6528.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
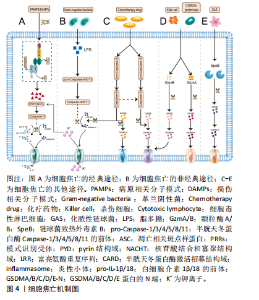
2.1.2 细胞焦亡的通路机制 焦亡具有细胞膜成孔特性,直径10-16 nm的裂孔可使胞内的炎症因子流出、胞外的水流入,最终引发细胞破裂,使内容物完全释放[19]。焦亡的途径主要有3条,包括与Caspase-1相关的经典途径,与Caspase-4/5/11相关的非经典途径以及由Caspase-3/8、颗粒酶A/B、链球菌致热外毒素B(streptococcal pyrogenic exotoxin B,SpeB)诱发的其他途径[12]。细胞焦亡机制见图4。 在经典途径中,炎性小体由模式识别受体(pattern recognition receptors,PRRs)、凋亡相关斑点样蛋白(apoptosis-related speck-like protein,ASC)和pro-Caspase-1组成[20]。PRRs是炎性小体的受体,作为传感器用来识别病原相关分子模式和损伤相关分子模式[21]。PRRs包括NOD样受体(the NOD-like receptors,NLR)家族(如NLRP1、NLRP3、NLRC4)、AIM2和PYRIN几种[20,22]。PRRs的类型决定了炎性小体的命名,如NLRP3炎性小体。在结构上,PRRs由pyrin结构域(PYD)、核苷酸结合和寡聚结构域(NACHT)以及富亮氨酸重复序列(LRR)组成,其中LRR在识别刺激信号中发挥核心作用[22]。此外,接头蛋白ASC包含一个PYD和一个半胱天冬蛋白酶激活招募结构域(CARD),通过PYD-PYD和CARD-CARD来招募PRRs和 pro-Caspase-1。需要强调的是,NLR家族中的NLRP1、NLRC4可以不经ASC的间接作用,直接使用其自身的CARD与pro-Caspase-1发生耦合[23-24]。炎性小体组装完成后会激活下游的效应蛋白Caspase-1,Caspase-1一方面可以切割GSDMD释放出C端和N端,其中N端可以募集到细胞膜内形成跨膜孔隙,另一方面Caspase-1能把白细胞介素1β/18前体切割成具有活性的白细胞介素1β/18,并经膜孔分泌到胞外。"
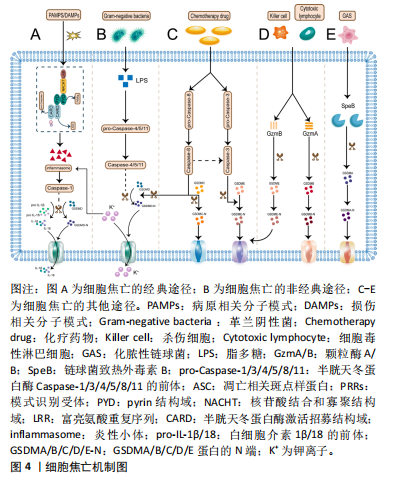
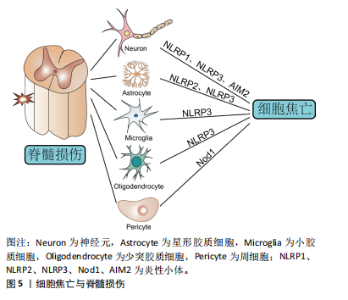
在非经典途径中,革兰阴性菌外膜中的脂多糖成分会刺激并激活来自人的Caspase-4/5和来自鼠的Caspase-11[25]。具体来说,pro-Caspase-4/5/11经其CARD结构域识别到胞质内的脂多糖后活化为成熟的Caspase-4/5/11并切割GSDMD分离出N端,进而导致细胞膜完整性丧失[26]。应注意的是,Caspase-4/5/11本身不能直接处理白细胞介素1β/18前体,白细胞介素1β/18的成熟分泌依赖于NLRP3/Caspase-1轴来完成[10],这一过程可能与K+外排有关[27]。 在其他途径中,化疗药物刺激下Caspase-3/8可分别促进GSDME/GSDMC分解出各自的N端,这些N端具有与GSDMD-N端相似的细胞膜成孔活性,从而引发焦亡[28-29]。 值得一提的是,Caspase-8还能直接切割GSDMD或经Caspase-3间接激活GSDME导致焦亡[30-31]。而颗粒酶A/B则是依赖于对GSMDB和GSDME的激活来诱发焦亡[32-33]。此外,SpeB可激活GSDMA,释放的GSDMA-N端同样可以插入细胞膜引发细胞焦亡[34]。 2.2 细胞焦亡与脊髓损伤 脊髓二次损伤会引起活性氧、K+和组织蛋白酶B等多种物质的释放,这些物质往往具有很强的促炎活性,导致脊髓炎症扩大,向更高的节段扩展,这也是病情加重的主要原因。近年来,随着对程序性细胞死亡研究的深入,越来越多的科学家关注到细胞焦亡这一促炎性裂解性程序性细胞死亡形式。最近的研究不仅证实了脊髓损伤后细胞焦亡通路的激活[35],还发现了细胞焦亡是调控脊髓损伤后炎症反应的有效手段[36-37]。 脊髓中的细胞主要有小胶质细胞、神经元、星形胶质细胞、少突胶质细胞和周细胞,几乎每种细胞都被证实了焦亡的发生[10]。其中,小胶质细胞的焦亡激活以NLRP3炎性小体的表达途径为主[38]。XU等[35]和WANG等[39]分别在脊髓损伤小鼠模型中发现NLRP3所引起的细胞焦亡主要发生在小胶质细胞中,还证实了胞外-5’-核苷酸酶(CD73)和Toll 样受体4(Toll-like receptor 4,TLR4)能通过不同的信号通路实现对细胞焦亡的控制。LIU等[36]在实验中验证了脊髓损伤后小胶质细胞的活化,并阐明了高级氧化蛋白产物(advanced oxidation protein products,AOPPs)诱导BV2小胶质细胞焦亡的相关途径。而在另一项相关研究中,核因子红细胞2相关因子2(nuclear factor E2-related factor 2,Nrf2)能与microRNA-146a启动子结合并将其激活,利用microRNA-146a对GSDMD的靶向抑制作用来抑制小胶质细胞焦亡[40]。由此可见,脊髓损伤后的小胶质细胞存在广泛的细胞焦亡激活,通过对相关通路的研究为脊髓损伤的治疗提供了新的靶点选择。此外,细胞焦亡也是脊髓损伤后神经元丢失的主要原因。神经元中的细胞焦亡模式依赖NLRP1、NLRP3和AIM2炎性小体途径[10]。一项大鼠颈椎脊髓损伤的实验证实了在损伤6 h后神经元中的NLRP1和ASC表达显著增加[17]。LIN等[41]发现了脊髓损伤后神经元中NLRP1和血红素加氧酶1(heme oxygenase-1,HO-1)的表达增强,并在体外实验中观察到HO-1可通过抑制NLRP1表达来保护神经元免于死亡。NLRP3炎性小体在细胞焦亡中极为关键,通过抑制NLRP3蛋白组装同样能减少神经元焦亡[42]。星形胶质细胞的焦亡以NLRP2炎性小体途径为主,其中P2X7受体和泛连接蛋白1在NLRP2的激活中起到重要作用[43]。最近的生信学研究还发现NLRP3 炎性小体介导的焦亡相关基因在星形胶质细胞中高度表达,同时观察到促红细胞生成素可以在体内外减少星形胶质细胞焦亡[44]。另外,少突胶质细胞与小胶质细胞类似,其焦亡激活同样以NLRP3炎性小体途径为主[45]。令人惊讶的是,这两类细胞的NLRP3相关焦亡过程都可被雌二醇缓解[46]。周细胞是血脊髓屏障的重要结构。研究发现周细胞焦亡与核苷酸寡聚化结构域1非典型途径激活有关,这一过程可被骨髓间充质干细胞衍生的外泌体所逆转[8]。目前来看,关于周细胞的焦亡机制仍缺乏经典途径的报道,需要对该方向进一步拓展探索。综上可见,脊髓损伤后脊髓中的多种细胞均表现出细胞焦亡通路的激活,这说明细胞焦亡可调控脊髓损伤的炎性程度,无疑是治疗脊髓损伤的新途径。细胞焦亡与脊髓损伤的关系见图5。"


2.3 天然产物调控细胞焦亡治疗脊髓损伤的研究 2.3.1 天然产物抑制NLRP3/Caspase-1经典焦亡途径治疗脊髓损伤 NLRP3炎性小体所介导的细胞焦亡是目前研究最广泛、最深入的焦亡途径。脊髓损伤后的多种损伤相关分子模式和病原相关分子模式会被NLRP3蛋白感知,激活炎性小体组装,形成NLRP3/ASC/Pro-Caspase-1蛋白复合体,活化的Caspase-1会发挥切割作用,形成GSDMD-N端并募集到细胞膜中形成裂孔,诱发细胞焦亡。天然产物是一种新兴的优势药用化合物,越来越多的研究为其调控细胞焦亡治疗脊髓损伤提供了可靠的证据。提取自蜘蛛香的环烯醚萜类成分具有明确的神经保护作用,能调控NLRP3/Caspase-1通路来抑制焦亡,减少脊髓损伤大鼠受损组织中的白细胞介素1β和白细胞介素18分泌,减轻神经炎症,从而修复受损的功能[47]。甘草甜素是从中草药甘草根中提取的主要成分,它同样可以抑制脊髓损伤大鼠NLRP3、ASC、Caspase-1以及白细胞介素1β和白细胞介素18的表达,从而减少脊髓中的细胞焦亡[48]。此外,该团队还观察到甘草甜素可以减少M1型小胶质细胞极化而增加M2型小胶质细胞极化,这能更好地发挥抗炎作用,加强神经保护。青藤碱是从青风藤中提取出的生物碱,具有抗炎和免疫抑制功能[49]。在体外细胞实验中,氧糖剥夺/复糖复氧模型诱导的BV2小胶质细胞的乳酸脱氢酶释放量及碘化丙啶染色阳性细胞数显著提升,即细胞焦亡被激活,而给予青藤碱后降低了NLRP3/Caspase-1的磷酸化水平,也就是抑制了BV2细胞的焦亡,从而减少炎症刺激[50]。简而言之,天然产物在直接抑制NLRP3/Caspase-1经典焦亡途径治疗脊髓损伤中表现出显著的优势,无疑是今后研究的热门方向。但众所周知,细胞死亡是一个极为复杂的过程,其他对细胞焦亡有影响的通路也不容忽视,所以作者在下文中对影响脊髓损伤中细胞焦亡的有关通路进行了提取、筛选和总结。 2.3.2 天然产物调控核因子κB(nuclear factor κB,NF-κB)相关通路抑制细胞焦亡治疗脊髓损伤 NF-κB是典型的促炎信号通路,在细胞炎症反应中起到关键性作用。在另一方面,NF-κB也是NLRP3和白细胞介素1β等物质激活的关键上游要素,后者的转录受到NF-κB通路的调控[51]。更深入的研究还发现细胞焦亡的关键执行者GSDMD蛋白也被NF-κB所影响[52]。总的来说,NF-κB除了能影响炎症反应的发生,还能作用于细胞焦亡途径的多种相关蛋白,是调控细胞焦亡的关键因子。 越来越多的研究证实NF-κB相关通路在天然产物调控脊髓损伤后的细胞焦亡水平方面展现出巨大的潜力。羽扇豆酮是天然三萜类化学成分,具有良好的抗炎活性,广泛分布在多种植物中[53]。脊髓损伤体内外实验显示,NF-κB通路中的关键蛋白p65过表达能促进NLRP3的表达,增加碘化丙啶阳性细胞数量,提高乳酸脱氢酶、白细胞介素1β、白细胞介素18水平,而羽扇豆酮的参与可以逆转p65的上述影响,说明羽扇豆酮可经NF-κB通路控制细胞焦亡和神经炎症[54]。此外,羽扇豆酮还能促进小胶质细胞从M1促炎表型向M2抗炎表型转变,更好地改善神经功能。与其相似的是丹皮酚,中药牡丹根提取物中的主要活性成分。最近的研究结果表明丹皮酚不仅能有效降低脊髓损伤后大鼠的脊髓水分含量,减轻对脊髓结构的损害,还可通过TLR4/髓样分化因子88(myeloid differentiation primary response protein 88,MyD88)/NF-κB相关通路促进小胶质细胞M2型极化,缓解NLRP3炎性小体的激活和细胞焦亡,减少白细胞介素1β、白细胞介素18的分泌[55]。再就是鹰嘴豆芽素A,一种具有雌激素作用的天然化合物,富含在豆科植物中。LI等[56]研究证实了鹰嘴豆芽素A可缓解 TLR4/NF-κB/NLRP3信号通路引起的细胞焦亡和炎症反应,并且能激活 Nrf2/HO-1 信号通路抑制脊髓损伤大鼠的氧化应激,同时还肯定了自噬激活在脊髓损伤后抑制细胞焦亡中的重要性。山奈酚也是一种黄酮类化合物,广泛存在于茶、羽衣甘蓝和西蓝花等多种植物中,具有抗炎和抗氧化特性。LIU等[57]发现山奈酚能够抑制脊髓损伤后小胶质细胞中NLRP3、ASC、Caspase-1和GSDMD蛋白水平。换句话说,山奈酚可以抑制NLRP3经典焦亡通路。同时,该团队还观察到山奈酚减少了人核因子κB抑制蛋白α的降解和磷酸化从而抑制了p65亚基的核易位,最终缓解了NF-κB通路对神经炎症的激活。但是该研究存在设计缺陷,实验中缺少了对NF-κB和NLRP3相关性的验证。除了以上天然产物外,白藜芦醇苷[58]、枸杞糖肽[59]、雷公藤红素均可通过抑制NF-κB/NLRP3相关通路改善脊髓损伤的炎症微环境并促进脊髓修复[60],在此不多赘述。 综上可见,虽然天然产物在抑制脊髓损伤后细胞焦亡和炎症反应方面具有巨大的潜在价值,通过对NF-κB炎性通路的控制也可以有效减少细胞焦亡的激活,但实验设计方面仍存在很多共性的问题,比如实验细胞多为小胶质细胞和神经元,对其他细胞类型的研究欠缺明显;再就是天然产物对细胞焦亡的影响局限于NLRP3经典途径,缺乏对非经典途径和NLRP1等其他经典途径的研究,这都是目前存在的问题,需要更多的研究补充完善。 2.3.3 天然产物经其他信号通路抑制细胞焦亡治疗脊髓损伤 Nrf2是抗氧化反应的关键转录因子。正常情况下,Nrf2与其抑制蛋白结合而稳定存在于细胞质中,当出现氧化应激和炎症刺激时Nrf2会与其抑制蛋白分离而转移到细胞核中,促进下游的抗氧化相关基因表达,其转录产物主要有HO-1、过氧化氢酶等[61]。研究表明Nrf2/HO-1通路在脊髓损伤中的潜在治疗作用[56]。橙皮素是来源于柑橘类水果的一种黄酮类天然化合物。在大鼠C5颈髓损伤的体内模型和脂多糖+三磷酸腺苷(ATP)诱导的体外小胶质细胞模型中,橙皮素显著增加了HO-1蛋白水平,同时显著降低了ASC、NLRP3、Caspase-P10、GSDID-N和白细胞介素1β的蛋白表达,然而这一系列趋势在Nrf2沉默后出现了逆转,这表明橙皮素是通过Nrf2/HO-1通路抑制了NLRP3焦亡途径的活化,减轻了神经炎症[62]。乙酰基-11-酮基-β-乳香酸是从乳香的药用树脂中提取得到的。在脂多糖+ATP建立的神经元损伤模型中,乙酰基-11-酮基-β-乳香酸能减少细胞乳酸脱氢酶的释放,减轻线粒体损伤和活性氧水平,而Nrf2抑制剂的加入同样逆转了上述趋势,也就是说乙酰基-11-酮基-β-乳香酸通过激活Nrf2通路缓解了线粒体损伤和活性氧的不利效应,进而抑制了活性氧引起的焦亡启动[63]。 除了Nrf2的抗氧化通路之外,叉头框蛋白O3a(forkhead box protein O3,FoxO3a)/硫氧还原蛋白结合蛋白(thioredoxin interacting protein,TXNIP)通路和磷脂酰肌醇3-激酶(phosphatidylinositol 3-kinase,PI3K)/蛋白激酶B(protein kinase B,AKT)通路也在抗细胞焦亡治疗脊髓损伤中发挥了不可或缺的作用。左洁仪等[64]研究证实了槲皮素可以通过抑制FoxO3a的降解来激活FoxO3a/TXNIP通路,以此增加神经细胞的活力,抑制细胞焦亡。而HU等[65]则在一系列实验中验证了天然化合物紫杉醇对脊髓损伤后神经炎症和小胶质细胞焦亡的抑制是通过PI3K/AKT通路来完成的。此外,特别值得一提的是提取自中国喜树的喜树碱。在神经退行性疾病的研究中,喜树碱能减少神经元的丢失,改善小鼠的运动表现,通过激活AKT/Nrf2/HO-1通路和抑制NF-κB通路促进小胶质细胞向M2抗炎表型的转化,抑制神经炎症[66]。而在脊髓损伤模型的研究中,喜树碱作为拓扑异构酶1的抑制剂,通过对拓扑异构酶1的抑制作用产生与其他天然产物类似的减轻焦亡的效应[67]。喜树碱是否能经其他通路抑制脊髓损伤后的细胞焦亡是亟需实验验证的新方向。 综上,在脊髓损伤的研究中,天然产物调节细胞焦亡水平的多条上游通路引起了学者的重视,并在相应的动物、细胞实验中得到了阐明。但目前所报道的通路仍然较少,对其他相关通路的验证将是后续研究的重点方向。另外,尚未见有关非经典焦亡途径的上游通路的研究,在此方面的探索仍属空白。 2.3.4 天然产物调控自噬抑制细胞焦亡治疗脊髓损伤 自噬作为一种细胞内降解系统,通过将受损的细胞器和蛋白质等细胞成分输送到溶酶体中进行降解和循环来实现细胞内环境的稳态[68]。细胞自噬和焦亡在损伤脊髓组织中有着广泛而复杂的交互串联。例如,脊髓损伤的继发损伤与线粒体受损和大量活性氧的生成密切相关[69]。而上调自噬通量可以降解受损的线粒体,减少活性氧的产生,间接通过抑制NF-κB通路来减少NLRP3炎性小体的激活,最终减少白细胞介素1β等炎症物质的释放。自噬还可以直接降解NLRP3和ASC等成分以及磷酸化炎性小体来影响焦亡[70]。总之,自噬会影响到受损脊髓细胞中的焦亡有关成分,继而影响焦亡的发生。 近年来,天然产物通过控制自噬途径来改善脊髓损伤后的细胞焦亡水平得到了广泛关注。桦木酸是一种天然的五环三萜类化合物,主要从白桦树的树皮中提取而来。来源于MITF-TFE家族的转录因子 EB(transcription factor EB,TFEB)是自噬和溶酶体生物发生的中心调节因子。桦木酸能增加5’磷酸腺苷激活蛋白酶(adenosine 5’-monophosphate (AMP)-activated protein kinase,AMPK)的磷酸化并抑制哺乳动物雷帕霉素靶点(mammalian target of rapamycin,mTOR)磷酸化,引起TFEB核易位而增加其表达,而在给予AMPK阻滞剂后不仅改变了三者的表达水平,还抑制了多个线粒体自噬相关蛋白的表达,增加了Caspase-1、NLRP3和GSDMD等焦亡有关蛋白的产生,这说明桦木酸能经AMPK/mTOR/TFEB通路增强线粒体自噬,从而减少活性氧积累,抑制细胞焦亡的发生,显著促进了脊髓损伤的恢复[71]。人参皂苷Rh2是红参中的一种化合物,具有神经元保护作用[72]。LIU等[73]研究发现人参皂苷Rh2在脊髓损伤后同样可经TFEB核易位来增加自噬通量,从而达到抑制焦亡、实现神经保护的作用。在自噬抑制剂3-甲基腺嘌呤的作用下,焦亡的抑制和运动功能的恢复也会被部分逆转。此外,他们利用网络药理学和分子对接技术推测出表皮生长因子受体(epidermal growth factor receptor,EGFR)/MAPK为TFEB的上游通路,并在后续实验中利用EGFR激活剂部分逆转了TFEB的表达,证实了人参皂苷Rh2可通过EGFR/MAPK/TFEB通路来调节自噬和细胞焦亡的发生,进而促进脊髓损伤后的功能恢复,但人参皂苷Rh2是否通过其他途径控制TFEB核易位并未做进一步研究。黄芩素来源于中草药黄芩的根部,是一种天然生物类黄酮。WU等[74]在脊髓缺血再灌注损伤的相关研究中发现黄芩素能增强神经元自噬,抑制细胞焦亡和内质网应激引起的细胞凋亡,表现出优秀的神经保护作用,而在给予3-甲基腺嘌呤抑制自噬后这些变化被显著抵消,这进一步提示了黄芩素抑制神经元焦亡和凋亡的作用是通过上调自噬水平来实现的,但该实验并未释清确切的调控通路,这应是后续研究补充完善的重点。在该团队的另一项研究中,他们还观察到源自黑胡椒等香料中的天然化合物胡椒碱在脊髓损伤中表现出与黄芩素极为相似的作用,即胡椒碱能增强脊髓损伤后神经元的自噬,抑制细胞焦亡和氧化应激,减轻炎症,促进脊髓功能恢复,但这些效应在给予自噬抑制剂3-甲基腺嘌呤后同样会发生逆转[75]。类似的是,该研究也没有说明具体的通路机制,而且研究设计缺乏阳性对照。 经过以上讨论,可以肯定的是多种天然产物能在脊髓损伤后通过上调自噬通量实现对细胞内焦亡激活相关因子的降解,从而减弱细胞焦亡,改善脊髓功能。但目前天然产物通过激活自噬抑制脊髓损伤后细胞焦亡水平的研究还处于初级阶段,未来应着重筛选具有更强自噬激活作用的天然产物,并探究其对脊髓损伤后细胞焦亡的作用机制。 调控脊髓损伤后细胞焦亡的天然产物及其相关机制汇总见表1。"

| [1] SHI Z, YUAN S, SHI L, et al. Programmed cell death in spinal cord injury pathogenesis and therapy. Cell Prolif. 2021;54(3):e12992. [2] GBD 2016 TRAUMATIC BRAIN INJURY AND SPINAL CORD INJURY COLLABORATORS. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019; 18(1):56-87. [3] GBD SPINAL CORD INJURIES COLLABORATORS. Global, regional, and national burden of spinal cord injury, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2023;22(11):1026-1047. [4] JIANG B, SUN D, SUN H, et al. Prevalence, Incidence, and External Causes of Traumatic Spinal Cord Injury in China: A Nationally Representative Cross-Sectional Survey. Front Neurol. 2022;12:784647. [5] LI X, YU Z, ZONG W, et al. Deficiency of the microglial Hv1 proton channel attenuates neuronal pyroptosis and inhibits inflammatory reaction after spinal cord injury. J Neuroinflammation. 2020;17(1):263. [6] MA Y, LI P, JU C, et al. Photobiomodulation Attenuates Neurotoxic Polarization of Macrophages by Inhibiting the Notch1-HIF-1α/NF-κB Signalling Pathway in Mice With Spinal Cord Injury. Front Immunol. 2022;13:816952. [7] MORTEZAEE K, KHANLARKHANI N, BEYER C, et al. Inflammasome: Its role in traumatic brain and spinal cord injury. J Cell Physiol. 2018; 233(7):5160-5169. [8] ZHOU Y, WEN LL, LI YF, et al. Exosomes derived from bone marrow mesenchymal stem cells protect the injured spinal cord by inhibiting pericyte pyroptosis. Neural Regen Res. 2022;17(1):194-202. [9] ANWAR MA, AL SHEHABI TS, EID AH. Inflammogenesis of Secondary Spinal Cord Injury. Front Cell Neurosci. 2016;10:98. [10] YIN J, GONG G, WAN W, et al. Pyroptosis in spinal cord injury. Front Cell Neurosci. 2022;16:949939. [11] SIMON DW, MCGEACHY MJ, BAYIR H, et al. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol. 2017;13(3):171-191. [12] LI Z, CHENG W, GAO K, et al. Pyroptosis: A spoiler of peaceful coexistence between cells in degenerative bone and joint diseases. J Adv Res. 2024. doi: 10.1016/j.jare.2024.06.010. [13] TOMASIK J, BASAK GW. Inflammasomes-New Contributors to Blood Diseases. Int J Mol Sci. 2022;23(15):8129. [14] ZYCHLINSKY A, PREVOST MC, SANSONETTI PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358(6382):167-169. [15] HILBI H, CHEN Y, THIRUMALAI K, et al. The interleukin 1beta-converting enzyme, caspase 1, is activated during Shigella flexneri-induced apoptosis in human monocyte-derived macrophages. Infect Immun. 1997;65(12):5165-5170. [16] COOKSON BT, BRENNAN MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9(3):113-114. [17] DE RIVERO VACCARI JP, LOTOCKI G, MARCILLO AE, et al. A molecular platform in neurons regulates inflammation after spinal cord injury. J Neurosci. 2008;28(13):3404-3414. [18] SHI J, ZHAO Y, WANG K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575): 660-665. [19] FENG S, FOX D, MAN SM. Mechanisms of Gasdermin Family Members in Inflammasome Signaling and Cell Death. J Mol Biol. 2018;430(18 Pt B):3068-3080. [20] ZHENG D, LIWINSKI T, ELINAV E. Inflammasome activation and regulation: toward a better understanding of complex mechanisms. Cell Discov. 2020;6:36. [21] XUE W, CUI D, QIU Y. Research Progress of Pyroptosis in Alzheimer’s Disease. Front Mol Neurosci. 2022;15:872471. [22] WU Q, DU J, BAE EJ, et al. Pyroptosis in Skeleton Diseases: A Potential Therapeutic Target Based on Inflammatory Cell Death. Int J Mol Sci. 2024;25(16):9068. [23] FAUSTIN B, LARTIGUE L, BRUEY JM, et al. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell. 2007;25(5):713-724. [24] LI Y, FU TM, LU A, et al. Cryo-EM structures of ASC and NLRC4 CARD filaments reveal a unified mechanism of nucleation and activation of caspase-1. Proc Natl Acad Sci U S A. 2018;115(43):10845-10852. [25] SHI J, ZHAO Y, WANG Y, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514(7521):187-192. [26] MAN SM, KARKI R, KANNEGANTI TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev. 2017;277(1):61-75. [27] RÜHL S, BROZ P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. Eur J Immunol. 2015;45(10):2927-2936. [28] WANG Y, GAO W, SHI X, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547(7661): 99-103. [29] FRITSCH M, GÜNTHER SD, SCHWARZER R, et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature. 2019;575(7784):683-687. [30] DEMARCO B, GRAYCZYK JP, BJANES E, et al. Caspase-8-dependent gasdermin D cleavage promotes antimicrobial defense but confers susceptibility to TNF-induced lethality. Sci Adv. 2020;6(47):eabc3465. [31] SARHAN J, LIU BC, MUENDLEIN HI, et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci U S A. 2018;115(46):E10888-E10897. [32] ZHOU Z, HE H, WANG K, et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science. 2020; 368(6494):eaaz7548. [33] ZHANG Z, ZHANG Y, XIA S, et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature. 2020;579(7799):415-420. [34] DENG W, BAI Y, DENG F, et al. Streptococcal pyrogenic exotoxin B cleaves GSDMA and triggers pyroptosis. Nature. 2022;602(7897):496-502. [35] XU S, WANG J, ZHONG J, et al. CD73 alleviates GSDMD-mediated microglia pyroptosis in spinal cord injury through PI3K/AKT/Foxo1 signaling. Clin Transl Med. 2021;11(1):e269. [36] LIU Z, YAO X, JIANG W, et al. Advanced oxidation protein products induce microglia-mediated neuroinflammation via MAPKs-NF-κB signaling pathway and pyroptosis after secondary spinal cord injury. J Neuroinflammation. 2020;17(1):90. [37] ZHU J, FU Y, TU G. Role of Smad3 inhibitor and the pyroptosis pathway in spinal cord injury. Exp Ther Med. 2020;20(2):1675-1681. [38] XU X, YIN D, REN H, et al. Selective NLRP3 inflammasome inhibitor reduces neuroinflammation and improves long-term neurological outcomes in a murine model of traumatic brain injury. Neurobiol Dis. 2018;117:15-27. [39] WANG J, ZHANG F, XU H, et al. TLR4 aggravates microglial pyroptosis by promoting DDX3X-mediated NLRP3 inflammasome activation via JAK2/STAT1 pathway after spinal cord injury. Clin Transl Med. 2022; 12(6):e894. [40] ZHANG D, MAO F, WANG S, et al. Role of Transcription Factor Nrf2 in Pyroptosis in Spinal Cord Injury by Regulating GSDMD. Neurochem Res. 2023;48(1):172-187. [41] LIN WP, XIONG GP, LIN Q, et al. Heme oxygenase-1 promotes neuron survival through down-regulation of neuronal NLRP1 expression after spinal cord injury. J Neuroinflammation. 2016;13(1):52. [42] JIANG W, HE F, DING G, et al. Elamipretide reduces pyroptosis and improves functional recovery after spinal cord injury. CNS Neurosci Ther. 2023;29(10):2843-2856. [43] MINKIEWICZ J, DE RIVERO VACCARI JP, Keane RW. Human astrocytes express a novel NLRP2 inflammasome. Glia. 2013;61(7):1113-1121. [44] SHAN W, WANG J, CHENG R, et al. Erythropoietin alleviates astrocyte pyroptosis by targeting the miR-325-3p/Gsdmd axis in rat spinal cord injury. Inflammopharmacology. 2024;32(1):523-536. [45] YANAGISAWA S, KATOH H, IMAI T, et al. The relationship between inflammasomes and the endoplasmic reticulum stress response in the injured spinal cord. Neurosci Lett. 2019;705:54-59. [46] ZENDEDEL A, MÖNNINK F, HASSANZADEH G, et al. Estrogen Attenuates Local Inflammasome Expression and Activation after Spinal Cord Injury. Mol Neurobiol. 2018;55(2):1364-1375. [47] 王静怡,尹杰,刘建成,等.蜘蛛香环烯醚萜类成分对急性脊髓损伤大鼠神经细胞焦亡的影响[J].中国康复理论与实践,2021,27(6): 653-660. [48] SU XQ, WANG XY, GONG FT, et al. Oral treatment with glycyrrhizin inhibits NLRP3 inflammasome activation and promotes microglial M2 polarization after traumatic spinal cord injury. Brain Res Bull. 2020; 158:1-8. [49] KIASALARI Z, AFSHIN-MAJD S, BALUCHNEJADMOJARAD T, et al. Sinomenine Alleviates Murine Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis through Inhibiting NLRP3 Inflammasome. J Mol Neurosci. 2021;71(2):215-224. [50] 陈应丛,王国涛,徐道剑.青藤碱调控NLRP3/caspase-1通路抑制BV-2小胶质细胞焦亡及炎症的机制研究[J].浙江中西医结合杂志, 2021,31(12):1094-1099. [51] CORNUT M, BOURDONNAY E, HENRY T. Transcriptional Regulation of Inflammasomes. Int J Mol Sci. 2020;21(21):8087. [52] LIU Z, GAN L, XU Y, et al. Melatonin alleviates inflammasome-induced pyroptosis through inhibiting NF-κB/GSDMD signal in mice adipose tissue. J Pineal Res. 2017;63(1):e12414. [53] LEE HS, KIM EN, JEONG GS. Lupenone Protects Neuroblastoma SH-SY5y Cells Against Methamphetamine-Induced Apoptotic Cell Death via PI3K/Akt/mTOR Signaling Pathway. Int J Mol Sci. 2020;21(5):1617. [54] LI F, SUN X, SUN K, et al. Lupenone improves motor dysfunction in spinal cord injury mice through inhibiting the inflammasome activation and pyroptosis in microglia via the nuclear factor kappa B pathway. Neural Regen Res. 2024;19(8):1802-1811. [55] ZHAO H, WANG X, LIU S, et al. Paeonol regulates NLRP3 inflammasomes and pyroptosis to alleviate spinal cord injury of rat. BMC Neurosci. 2022;23(1):16. [56] LI X, FU J, GUAN M, et al. Biochanin A attenuates spinal cord injury in rats during early stages by inhibiting oxidative stress and inflammasome activation. Neural Regen Res. 2024;19(9):2050-2056. [57] LIU Z, YAO X, SUN B, et al. Pretreatment with kaempferol attenuates microglia-mediate neuroinflammation by inhibiting MAPKs-NF-κB signaling pathway and pyroptosis after secondary spinal cord injury. Free Radic Biol Med. 2021;168:142-154. [58] LV R, DU L, LIU X, et al. Polydatin alleviates traumatic spinal cord injury by reducing microglial inflammation via regulation of iNOS and NLRP3 inflammasome pathway. Int Immunopharmacol. 2019;70:28-36. [59] JIANG Z, ZENG Z, HE H, et al. Lycium barbarum glycopeptide alleviates neuroinflammation in spinal cord injury via modulating docosahexaenoic acid to inhibiting MAPKs/NF-kB and pyroptosis pathways. J Transl Med. 2023;21(1):770. [60] DAI W, WANG X, TENG H, et al. Celastrol inhibits microglial pyroptosis and attenuates inflammatory reaction in acute spinal cord injury rats. Int Immunopharmacol. 2019;66:215-223. [61] CHENG Y, CHEN B, XIE W, et al. Ghrelin attenuates secondary brain injury following intracerebral hemorrhage by inhibiting NLRP3 inflammasome activation and promoting Nrf2/ARE signaling pathway in mice. Int Immunopharmacol. 2020;79:106180. [62] LIU Z, TU K, ZOU P, et al. Hesperetin ameliorates spinal cord injury by inhibiting NLRP3 inflammasome activation and pyroptosis through enhancing Nrf2 signaling. Int Immunopharmacol. 2023;118:110103. [63] WANG Y, XIONG Z, ZHANG Q, et al. Acetyl-11-Keto-β-Boswellic Acid Accelerates the Repair of Spinal Cord Injury in Rats by Resisting Neuronal Pyroptosis with Nrf2. Int J Mol Sci. 2023;25(1):358. [64] 左洁仪,徐汪洋,陈洪栋,等.槲皮素通过调控FoxO3a/TXNIP通路对神经细胞焦亡的影响[J].中药材,2024,47(5):1271-1276. [65] HU Z, XUAN L, WU T, et al. Taxifolin attenuates neuroinflammation and microglial pyroptosis via the PI3K/Akt signaling pathway after spinal cord injury. Int Immunopharmacol. 2023;114:109616. [66] HE D, FU S, ZHOU A, et al. Camptothecin Regulates Microglia Polarization and Exerts Neuroprotective Effects via Activating AKT/Nrf2/HO-1 and Inhibiting NF-κB Pathways In Vivo and In Vitro. Front Immunol. 2021;12:619761. [67] JIANG W, HE F, DING G, et al. Topoisomerase 1 inhibition modulates pyroptosis to improve recovery after spinal cord injury. FASEB J. 2022; 36(6):e22294. [68] LI Z, LI D, CHEN R, et al. Cell death regulation: A new way for natural products to treat osteoporosis. Pharmacol Res. 2023;187:106635. [69] CHEN X, CUI J, ZHAI X, et al. Inhalation of Hydrogen of Different Concentrations Ameliorates Spinal Cord Injury in Mice by Protecting Spinal Cord Neurons from Apoptosis, Oxidative Injury and Mitochondrial Structure Damages. Cell Physiol Biochem. 2018;47(1):176-190. [70] LV S, WANG H, LI X. The Role of the Interplay Between Autophagy and NLRP3 Inflammasome in Metabolic Disorders. Front Cell Dev Biol. 2021;9:634118. [71] WU C, CHEN H, ZHUANG R, et al. Betulinic acid inhibits pyroptosis in spinal cord injury by augmenting autophagy via the AMPK-mTOR-TFEB signaling pathway. Int J Biol Sci. 2021;17(4):1138-1152. [72] JIN GN, LU JM, LAN HW, et al. Protective effect of ginsenoside Rh2 against Toxoplasma gondii infection-induced neuronal injury through binding TgCDPK1 and NLRP3 to inhibit microglial NLRP3 inflammasome signaling pathway. Int Immunopharmacol. 2022;112:109176. [73] LIU R, JIANG L, CHEN Y, et al. Ginsenoside-Rh2 Promotes Functional Recovery after Spinal Cord Injury by Enhancing TFEB-Mediated Autophagy. J Agric Food Chem. 2024;72(26):14727-14746. [74] WU C, XU H, LI J, et al. Baicalein Attenuates Pyroptosis and Endoplasmic Reticulum Stress Following Spinal Cord Ischemia-Reperfusion Injury via Autophagy Enhancement. Front Pharmacol. 2020;11:1076. [75] ZHANG H, WU C, YU DD, et al. Piperine attenuates the inflammation, oxidative stress, and pyroptosis to facilitate recovery from spinal cord injury via autophagy enhancement. Phytother Res. 2023;37(2):438-451. |
| [1] | Yu Shuai, Liu Jiawei, Zhu Bin, Pan Tan, Li Xinglong, Sun Guangfeng, Yu Haiyang, Ding Ya, Wang Hongliang. Hot issues and application prospects of small molecule drugs in treatment of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1913-1922. |
| [2] | Li Kaiying, Wei Xiaoge, Song Fei, Yang Nan, Zhao Zhenning, Wang Yan, Mu Jing, Ma Huisheng. Mechanism of Lijin manipulation regulating scar formation in skeletal muscle injury repair in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1600-1608. |
| [3] | Wang Qiuyue, Jin Pan, Pu Rui . Exercise intervention and the role of pyroptosis in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1667-1675. |
| [4] | Wan Lingling, Wu Mengying, Zhang Yujiao, Luo Qingqing. Inflammatory factor interferon-gamma affects migration and apoptosis of human vascular smooth muscle cells through pyroptosis pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1422-1428. |
| [5] | Chi Wenxin, Zhang Cunxin, Gao Kai, Lyu Chaoliang, Zhang Kefeng. Mechanism by which nobiletin inhibits inflammatory response of BV2 microglia [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1321-1327. |
| [6] | Zhao Ruihua, Chen Sixian, Guo Yang, Shi Lei, Wu Chengjie, Wu Mao, Yang Guanglu, Zhang Haoheng, Ma Yong. Wen-Shen-Tong-Du Decoction promoting spinal cord injury repair in mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1118-1126. |
| [7] | Liu Lingyun, He Guixin, Qin Weibin, Song Hui, Zhang Liwen, Tang Weizhi, Yang Feifei, Zhu Ziyi, Ou Yangbin . Improvement of myocardial injury by traditional Chinese medicine: mitochondrial calcium homeostasis mediates macrophage autophagy and pyroptosis pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1276-1284. |
| [8] | Bai Jing, Zhang Xue, Ren Yan, Li Yuehui, Tian Xiaoyu. Effect of lncRNA-TNFRSF13C on hypoxia-inducible factor 1alpha in periodontal cells by modulation of #br# miR-1246 #br# [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 928-935. |
| [9] | Zhi Fang, Zhu Manhua, Xiong Wei, Lin Xingzhen. Analgesic effect of acupuncture in a rat model of lumbar disc herniation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 936-941. |
| [10] | Wang Rongrong, Huang Yushan, Li Xiangmiao, Bai Jinzhu. Prostaglandin E1 regulates vascular-related factors and protects microcirculatory function during the acute phase of traumatic spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 958-967. |
| [11] | Wang Yuru, Li Siyuan, Xu Ye, Zhang Yumeng, Liu Yang, Hao Huiqin. Effects of wogonin on joint inflammation in collagen-induced arthritis rats via the endoplasmic reticulum stress pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1026-1035. |
| [12] | Yang Bin, Tao Guangyi, Yang Shun, Xu Junjie, Huang Junqing . Visualization analysis of research hotspots of artificial intelligence in field of spinal cord nerve injury and repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 761-770. |
| [13] | Yu Hui, Yang Yang, Wei Ting, Li Wenli, Luo Wenqian, Liu Bin. Gadd45b alleviates white matter damage in chronic ischemic rats by modulating astrocyte phenotype [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7797-7803. |
| [14] | Guo Jia, Ren Yafeng, Li Bing, Huang Jing, Shang Wenya, Yang Yike, Liu Huiyao. Action mechanism of mesenchymal stem cell-derived exosomes carrying miRNAs in improving spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7827-7838. |
| [15] | Zheng Yitong, Wang Yongxin, Liu Wen, Amujite, Qin Hu. Action mechanism of intrathecal transplantation of human umbilical cord mesenchymal stem cell-derived exosomes for repair of spinal cord injury under neuroendoscopy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7743-7751. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||