Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (29): 6219-6227.doi: 10.12307/2025.774
Previous Articles Next Articles
Ginsenoside Rb1 alleviates cerebral ischemic injury in mice by regulating microglial polarization
Liu Ruojing1, 2, Zhao Xue3, Zhu Yizhen4, Fu Lingling1, 2, Zhu Junde1, 2
- 1Human Anatomy Teaching and Research Laboratory, School of Basic Medical Science, Guizhou Medical University, Gui’an 561113, Guizhou Province, China; 2Key Laboratory of Brain Function and Diseases Tissue Bank of Higher Education Institutions in Guizhou Province, Gui’an 561113, Guizhou Province, China; 3Guiqian International General Hospital, Guiyang 550024, Guizhou Province, China; 4Class 5, School of Clinical Medicine, Guizhou Medical University, Gui’an 561113, Guizhou Province, China
-
Received:2024-09-24Accepted:2024-11-12Online:2025-10-18Published:2025-03-07 -
Contact:Zhu Junde, MS, Professor, Doctoral supervisor, Human Anatomy Teaching and Research Laboratory, School of Basic Medical Science, Guizhou Medical University, Gui’an 561113, Guizhou Province, China; Key Laboratory of Brain Function and Diseases Tissue Bank of Higher Education Institutions in Guizhou Province, Gui’an 561113, Guizhou Province, China -
About author:Liu Ruojing, Master candidate, Human Anatomy Teaching and Research Laboratory, School of Basic Medical Science, Guizhou Medical University, Gui’an 561113, Guizhou Province, China; Key Laboratory of Brain Function and Diseases Tissue Bank of Higher Education Institutions in Guizhou Province, Gui’an 561113, Guizhou Province, China -
Supported by:National Natural Science Foundation of China, No. 81660243 (to ZJD); Natural Science Foundation of Guizhou Province, No. ZK[2023]323 (to ZJD); National Natural Science Foundation Cultivation Project of Guizhou Medical University, No. 20NSP006 (to ZJD)
CLC Number:
Cite this article
Liu Ruojing, Zhao Xue, Zhu Yizhen, Fu Lingling, Zhu Junde. Ginsenoside Rb1 alleviates cerebral ischemic injury in mice by regulating microglial polarization[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(29): 6219-6227.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
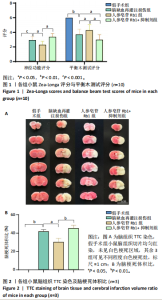
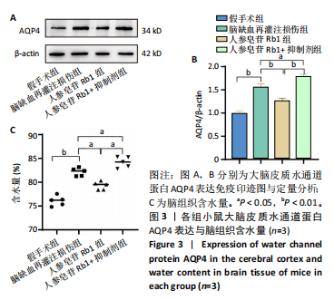
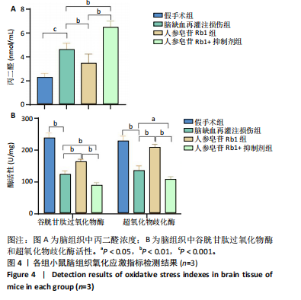
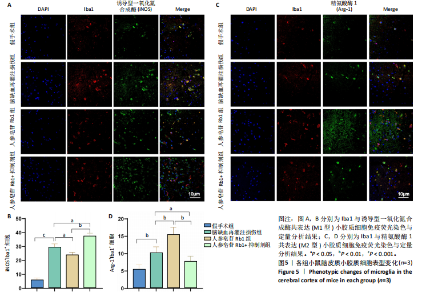
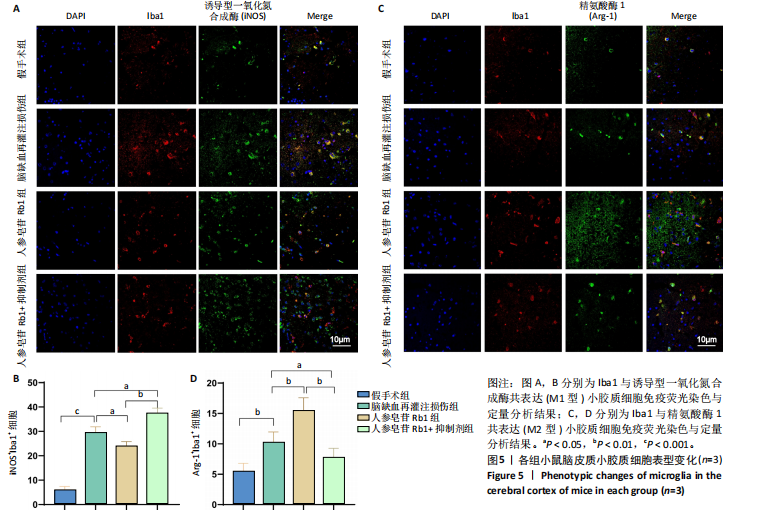
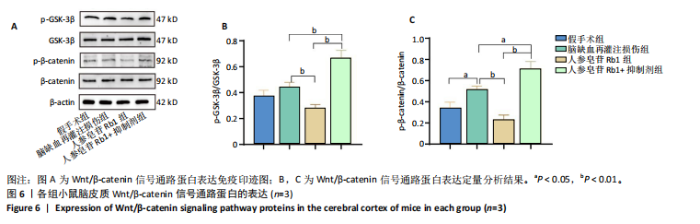
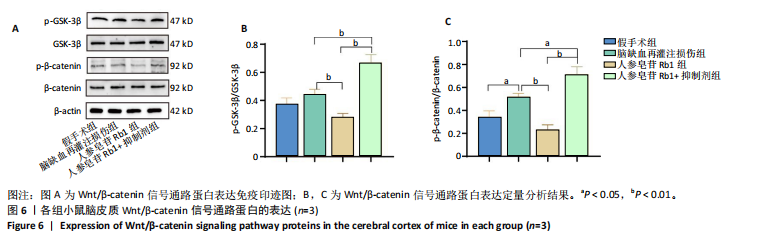
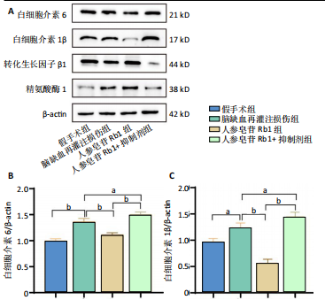
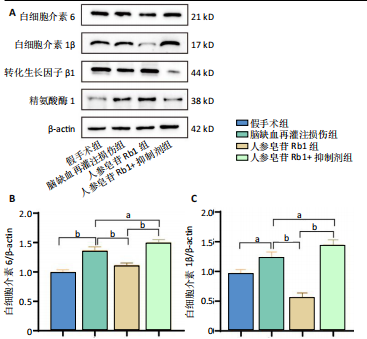
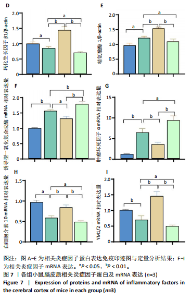
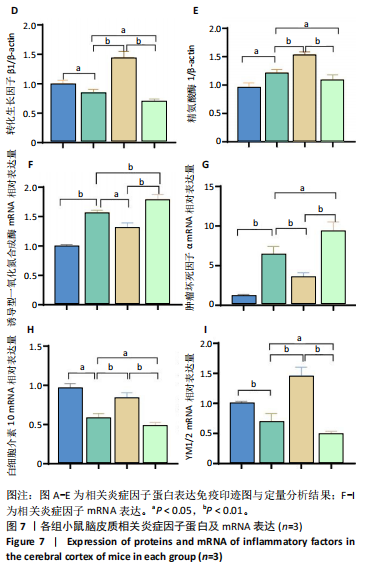
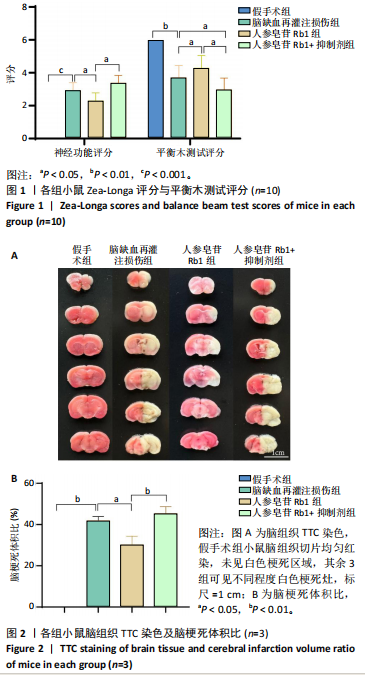
2.1 实验动物数量分析 100只小鼠全部进入结果分析。 2.2 人参皂苷Rb1改善脑缺血再灌注损伤后的神经功能缺损症状 各组小鼠Zea-Longa评分与平衡木测试得分见图1所示。假手术组小鼠未见神经功能缺损症状,提尾时四肢可自由伸展,行走正常无倾倒转圈;与假手术组相比,脑缺血再灌注损伤组评分显著升高(P < 0.001),小鼠出现偏瘫症状,行走时向健侧倾倒或转圈;与脑缺血再灌注损伤组相比,人参皂苷Rb1组小鼠Zea Longa评分显著降低(P < 0.05),神经损伤症状有所缓解;人参皂苷Rb1+抑制剂组小鼠Zea Longa评分与脑缺血再灌注损伤组相比无差异(P > 0.05),但高于人参皂苷Rb1组相比(P < 0.05)。 平衡木测试结果显示,假手术组小鼠均能平稳通过平衡木且无滑倒情况;与假手术组相比,脑缺血再灌注损伤组小鼠平衡木测试评分明显降低(P < 0.01);与脑缺血再灌注损伤相比,人参皂苷Rb1组平衡木测试评分升高(P < 0.05);人参皂苷Rb1+抑制剂组小鼠平衡木测试评分低于脑缺血再灌注损伤组、人参皂苷Rb1组(P < 0.05),说明抑制Wnt/β-catenin信号通路可逆转人参皂苷Rb1对于脑缺血再灌注损伤的神经保护作用。 2.3 人参皂苷Rb1改善脑缺血再灌注损伤后的脑梗死体积 各组小鼠脑组织TTC染色与脑梗死体积检测结果,见图2。假手术组小鼠脑组织切片均匀红染,未见白色梗死区域,其余3组可见不同程度白色梗死灶。与假手术组相比,脑缺血再灌注损伤组小鼠脑梗死体积比升高(P < 0.01);与脑缺血再灌注损伤组相比,人参皂苷Rb1组小鼠脑梗死体积比降低(P < 0.05);与人参皂苷Rb1组相比,人参皂苷Rb1+抑制剂组小鼠脑梗死体积比升高(P < 0.01)。 2.4 人参皂苷Rb1减轻脑缺血再灌注损伤后的脑水肿程度 各组小鼠水通道蛋白AQP4表达与脑组织含水量检测结果,见图3。与假手术组相比,脑缺血再灌注损伤组水通道蛋白AQP4表达及脑组织含水量均升高(P < 0.01);与脑缺血再灌注损伤组相比,人参皂苷Rb1组水通道蛋白AQP4表达与脑组织含水量均降低(P < 0.01,P < 0.05),人参皂苷Rb1+抑制剂组水通道蛋白AQP4表达与脑组织含水量均升高(P < 0.05);与人参皂苷Rb1组相比,人参皂苷Rb1+抑制剂组水通道蛋白AQP4表达与脑组织含水量均升高(P < 0.01,P < 0.05)。 2.5 人参皂苷Rb1减轻脑缺血再灌注损伤后的脑组织氧化应激反应 各组小鼠脑组织中氧化应激指标检测结果,见图4。与假手术组相比,脑缺血再灌注损伤组超氧化物歧化酶和谷胱甘肽过氧化物酶活性降低(P < 0.01),丙二醛浓度升高(P < 0.001);与脑缺血再灌注损伤组相比,人参皂苷Rb1组超氧化物歧化酶和谷胱甘肽过氧化物酶活性升高(P < 0.01)、丙二醛浓度降低(P < 0.01),人参皂苷Rb1+抑制剂XAV939组超氧化物歧化酶和谷胱甘肽过氧化物酶活性降低(P < 0.05,P < 0.01),丙二醛浓度升高(P < 0.01);与人参皂苷Rb1组相比,人参皂苷Rb1+XAV939组超氧化物歧化酶和谷胱甘肽过氧化物酶活性显著降低(P < 0.01),丙二醛浓度升高(P < 0.01)。 2.6 人参皂苷Rb1改变脑缺血再灌注损伤后脑皮质小胶质细胞表型变化 使用免疫荧光染色检测M1型小胶质细胞标志物诱导型一氧化氮合成酶、M2型小胶质细胞标志物精氨酸酶1与小胶质细胞标志物Iba1的共表达情况,见图5。与假手术组相比,脑缺血再灌注损伤组M1型小胶质细胞数量明显增多且细胞形态发生变化,由静息时的细长分支状变为胞体圆润且树突状分支减少甚至消失的“阿米巴样”激活态;与脑缺血再灌注损伤组相比,人参皂苷Rb1组M1型小胶质细胞数量减少且M2型小胶质细胞数量增多,人参皂苷Rb1+抑制剂组M1型小胶质细胞数量增多、M2型小胶质细胞数量减少;与人参皂苷Rb1组相比,人参皂苷Rb1+抑制剂组M1型小胶质细胞数量明显增加,M2型小胶质细胞数量减少。结果证明人参皂苷Rb1促进小胶质细胞向M2抗炎表型极化,但加入Wnt/β-catenin信号通路抑制剂XAV939后小胶质细胞向M1促炎表型极化。 2.7 人参皂苷Rb1改变脑缺血再灌注损伤后脑皮质Wnt/β-catenin信号通路蛋白的表达 如图6所示,与假手术组相比,脑缺血再灌注损伤组p-GSK-3β和p-β-catenin蛋白表达均升高,表明在脑缺血再灌注损伤发生后Wnt/β-catenin信号通路被抑制;与脑缺血再灌注损伤组相比,人参皂苷Rb1组p-GSK-3β和p-β-catenin蛋白表达均降低,说明人参皂苷Rb1活化了Wnt/β-catenin信号通路,人参皂苷Rb1+抑制剂组p-GSK-3β和p-β-catenin蛋白表达升高;与人参皂苷Rb1组相比,人参皂苷Rb1+抑制剂组 p-GSK-3β和p-β-catenin蛋白表达均升高(P < 0.01) ,表明在Wnt/β-catenin信号通路抑制剂XAV939作用下人参皂苷Rb1的对Wnt/β-catenin信号通路的活化作用被抑制。 2.8 人参皂苷Rb1改善脑缺血再灌注损伤后的神经炎症反应 Western Blot检测结果显示,与假手术组相比,脑缺血再灌注损伤组白细胞介素6、白细胞介素1β、精氨酸酶1蛋白表达均升高,转化生长因子β1蛋白表达降低;与脑缺血再灌注损伤组相比,人参皂苷Rb1组白细胞介素6、白细胞介素1β蛋白表达均降低以及精氨酸酶1、转化生长因子β1蛋白表达均升高,人参皂苷Rb1+抑制剂组白细胞介素6、白细胞介素1β蛋白表达均升高以及精氨酸酶1、转化生长因子β1蛋白表达均降低;与人参皂苷Rb1组相比,人参皂苷Rb1+抑制剂组白细胞介素6、白细胞介素1β蛋白表达均升高,精氨酸酶1、转化生长因子β1蛋白表达均降低,见图7A-E。"
| [1] TSAO CW, ADAY AW, ALMARZOOQ ZI, et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation. 2023;147(8):e593-e621. [2] GO AS, MOZAFFARIAN D, ROGER VL, et al. Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):399-410. [3] POWERS WJ, RABINSTEIN AA, ACKERSON T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. [4] HERPICH F, RINCON F. Management of Acute Ischemic Stroke. Crit Care Med. 2020;48(11):1654-1663. [5] MAJIDI S, SIMPKINS AN, LEIGH R. The Efficacy of IV Tissue Plasminogen Activator for Restoring Cerebral Blood Flow in the Hours Immediately after Administration in Patients with Acute Stroke. J Neuroimaging. 2019;29(2):206-210. [6] MOZAFFARIAN D, BENJAMIN EJ, GO AS, et al. Executive Summary: Heart Disease and Stroke Statistics--2016 Update: A Report From the American Heart Association. Circulation. 2016;133(4):447-454. [7] DING R, WU W, SUN Z, et al. AMP-activated protein kinase: An attractive therapeutic target for ischemia-reperfusion injury. Eur J Pharmacol. 2020;888:173484. [8] STOLL G, NIESWANDT B. Thrombo-inflammation in acute ischaemic stroke - implications for treatment. Nat Rev Neurol. 2019;15(8):473-481. [9] ZHANG Q, JIA M, WANG Y, et al. Cell Death Mechanisms in Cerebral Ischemia-Reperfusion Injury. Neurochem Res. 2022;47(12):3525-3542. [10] LI H, GUO Z, CHEN J, et al. Computational research of Belnacasan and new Caspase-1 inhibitor on cerebral ischemia reperfusion injury. Aging (Albany NY). 2022;14(4):1848-1864. [11] SONG Z, FENG J, ZHANG Q, et al. Tanshinone IIA Protects Against Cerebral Ischemia Reperfusion Injury by Regulating Microglial Activation and Polarization via NF-κB Pathway. Front Pharmacol. 2021;12:641848. [12] JURCAU A, SIMION A. Neuroinflammation in Cerebral Ischemia and Ischemia/Reperfusion Injuries: From Pathophysiology to Therapeutic Strategies. Int J Mol Sci. 2021;23(1):14. [13] ARIOZ BI, TASTAN B, TARAKCIOGLU E, et al. Melatonin Attenuates LPS-Induced Acute Depressive-Like Behaviors and Microglial NLRP3 Inflammasome Activation Through the SIRT1/Nrf2 Pathway. Front Immunol. 2019;10:1511. [14] JOLIVEL V, BICKER F, BINAMÉ F, et al. Perivascular microglia promote blood vessel disintegration in the ischemic penumbra. Acta Neuropathol. 2015;129(2):279-295. [15] LUO L, LIU M, FAN Y, et al. Intermittent theta-burst stimulation improves motor function by inhibiting neuronal pyroptosis and regulating microglial polarization via TLR4/NFκB/NLRP3 signaling pathway in cerebral ischemic mice. J Neuroinflammation. 2022;19(1):141. [16] GAO T, HUANG F, WANG W, et al. Interleukin-10 genetically modified clinical-grade mesenchymal stromal cells markedly reinforced functional recovery after spinal cord injury via directing alternative activation of macrophages. Cell Mol Biol Lett. 2022;27(1):27. [17] ZHANG Z, XU J, ZHANG X, et al. Protective Effect of SeMet on Liver Injury Induced by Ochratoxin A in Rabbits. Toxins (Basel). 2022; 14(9):628. [18] 赵晓华,田倩倩,薛伟,等.吴茱萸碱通过GSK-3β/β-catenin通路减轻大鼠脑缺血再灌注损伤[J]. 南开大学学报(自然科学版), 2023,56(5):23-28. [19] SONG D, ZHANG X, CHEN J, et al. Wnt canonical pathway activator TWS119 drives microglial anti-inflammatory activation and facilitates neurological recovery following experimental stroke. J Neuroinflammation. 2019;16(1):256. [20] JIANG N, ZHANG Y, YAO C, et al. Ginsenosides Rb1 Attenuates Chronic Social Defeat Stress-Induced Depressive Behavior via Regulation of SIRT1-NLRP3/Nrf2 Pathways. Front Nutr. 2022;9:868833. [21] NI XC, WANG HF, CAI YY, et al. Ginsenoside Rb1 inhibits astrocyte activation and promotes transfer of astrocytic mitochondria to neurons against ischemic stroke. Redox Biol. 2022;54:102363. [22] 周璐,陈珊,赵雪,等.人参皂苷Rb1改善局灶性CIRI小鼠模型神经损伤的调控机制研究[J].安徽医科大学学报,2023,58(2):252-258. [23] BELAYEV L, ALONSO OF, BUSTO R, et al. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27(9):1616-1622; discussion 1623. [24] ZHAO B, ZHU J, FEI Y, et al. JLX001 attenuates blood-brain barrier dysfunction in MCAO/R rats via activating the Wnt/β-catenin signaling pathway. Life Sci. 2020;260:118221. [25] ZHOU S, YU G, CHI L, et al. Neuroprotective effects of edaravone on cognitive deficit, oxidative stress and tau hyperphosphorylation induced by intracerebroventricular streptozotocin in rats. Neurotoxicology. 2013;38:136-145. [26] LIU K, LI L, LIU Z, et al. Acute Administration of Metformin Protects Against Neuronal Apoptosis Induced by Cerebral Ischemia-Reperfusion Injury via Regulation of the AMPK/CREB/BDNF Pathway. Front Pharmacol. 2022;13:832611. [27] ZHENG Y, HU Y, HAN Z, et al. Lomitapide ameliorates middle cerebral artery occlusion-induced cerebral ischemia/reperfusion injury by promoting neuronal autophagy and inhibiting microglial migration. CNS Neurosci Ther. 2022;28(12):2183-2194. [28] ZHANG Z, SONG Z, SHEN F, et al. Ginsenoside Rg1 Prevents PTSD-Like Behaviors in Mice Through Promoting Synaptic Proteins, Reducing Kir4.1 and TNF-α in the Hippocampus. Mol Neurobiol. 2021;58(4):1550-1563. [29] WEN S, ZOU ZR, CHENG S, et al. Ginsenoside Rb1 improves energy metabolism after spinal cord injury. Neural Regen Res. 2023;18(6): 1332-1338. [30] ZHOU T, ZU G, ZHANG X, et al. Neuroprotective effects of ginsenoside Rg1 through the Wnt/β-catenin signaling pathway in both in vivo and in vitro models of Parkinson’s disease. Neuropharmacology. 2016;101: 480-489. [31] CHEN H, FENG Z, MIN L, et al. Vagus Nerve Stimulation Reduces Neuroinflammation Through Microglia Polarization Regulation to Improve Functional Recovery After Spinal Cord Injury. Front Neurosci. 2022;16:813472. [32] KUMAR A, ALVAREZ-CRODA DM, STOICA BA, et al. Microglial/Macrophage Polarization Dynamics following Traumatic Brain Injury. J Neurotrauma. 2016;33(19):1732-1750. [33] AZEEM W, BAKKE RM, APPEL S, et al. Dual Pro- and Anti-Inflammatory Features of Monocyte-Derived Dendritic Cells. Front Immunol. 2020; 11:438. [34] WU Y, DU J, WU Q, et al. The osteogenesis of Ginsenoside Rb1 incorporated silk/micro-nano hydroxyapatite/sodium alginate composite scaffolds for calvarial defect. Int J Oral Sci. 2022;14(1):10. |
| [1] | Zhao Jiyu, Wang Shaowei. Forkhead box transcription factor O1 signaling pathway in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1923-1930. |
| [2] | Yin Lu, Jiang Chuanfeng, Chen Junjie, Yi Ming, Wang Zihe, Shi Houyin, Wang Guoyou, Shen Huarui. Effect of Complanatoside A on the apoptosis of articular chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1541-1547. |
| [3] | He Longcai, Song Wenxue, Ming Jiang, Chen Guangtang, Wang Junhao, Liao Yidong, Cui Junshuan, Xu Kaya. An experimental method for simultaneous extraction and culture of primary cortical neurons and microglial cells from SD rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1395-1400. |
| [4] | Chi Wenxin, Zhang Cunxin, Gao Kai, Lyu Chaoliang, Zhang Kefeng. Mechanism by which nobiletin inhibits inflammatory response of BV2 microglia [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1321-1327. |
| [5] | Zhao Ruihua, Chen Sixian, Guo Yang, Shi Lei, Wu Chengjie, Wu Mao, Yang Guanglu, Zhang Haoheng, Ma Yong. Wen-Shen-Tong-Du Decoction promoting spinal cord injury repair in mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1118-1126. |
| [6] | He Guanghui, Yuan Jie, Ke Yanqin, Qiu Xiaoting, Zhang Xiaoling. Hemin regulates mitochondrial pathway of oxidative stress in mouse chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1183-1191. |
| [7] | He Bo, Chen Wen, Ma Suilu, He Zhijun, Song Yuan, Li Jinpeng, Liu Tao, Wei Xiaotao, Wang Weiwei, Xie Jing . Pathogenesis and treatment progress of flap ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1230-1238. |
| [8] | Lang Mecuo, Zhang Yilin, Wang Li. MiR-338-3p affects proliferation and apoptosis of alveolar bone osteoblasts by targeting receptor activator of nuclear factor-kappaB ligand [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 899-907. |
| [9] | Lu Ranran, Zhou Xu, Zhang Lijie, Yang Xinling. Dimethyl fumarate alleviates nerve damage in a mouse model of Parkinson’s disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 989-994. |
| [10] | Zheng Yitong, Wang Yongxin, Liu Wen, Amujite, Qin Hu. Action mechanism of intrathecal transplantation of human umbilical cord mesenchymal stem cell-derived exosomes for repair of spinal cord injury under neuroendoscopy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7743-7751. |
| [11] | Sima Xinli, Liu Danping, Qi Hui. Effect and mechanism of metformin-modified bone marrow mesenchymal stem cell exosomes on regulating chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7728-7734. |
| [12] | Zhang Xiaoyu, Wei Shanwen, Fang Jiawei, Ni Li. Prussian blue nanoparticles restore mitochondrial function in nucleus pulposus cells through antioxidation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7318-7325. |
| [13] | Su Yongkun, Sun Hong, Liu Miao, Yang Hua, Li Qingsong. Development of novel antioxidants and antioxidant combination carried by nano-hydrogel systems in treatment of intervertebral disc degeneration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7376-7384. |
| [14] | Wu Qingyun, Su Qiang. Antioxidant nanomedicine-mediated targeted therapy for myocardial ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7431-7438. |
| [15] | Tian Yushi, Fu Qiang, Li Ji . Bioinformatics identification and validation of mitochondrial genes related to acute myocardial infarction [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6697-6707. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||