Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (25): 5469-5477.doi: 10.12307/2025.531
Previous Articles Next Articles
Major histocompatibility complex regulates immune responses in Parkinson’s disease
Guan Mengya1, 2, Ren Binbin2, Wang Jingying1
- 1School of Rehabilitation, Henan University of Chinese Medicine, Zhengzhou 450046, Henan Province, China; 2First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China
-
Received:2024-05-20Accepted:2024-07-08Online:2025-09-08Published:2024-12-30 -
Contact:Ren Binbin, MD, Master’s supervisor, Chief physician, First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China -
About author:Guan Mengya, Master candidate, School of Rehabilitation, Henan University of Chinese Medicine, Zhengzhou 450046, Henan Province, China; First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China -
Supported by:Traditional Chinese Medicine Inheritance and Innovation Talent Project (Zhongjing Project), No. CZ0325-10 (to RBB); 2022 Henan Province Traditional Chinese Medicine Top Talent Training Special Project, No. 2022ZYBJ09 (to RBB)
CLC Number:
Cite this article
Guan Mengya, Ren Binbin, Wang Jingying. Major histocompatibility complex regulates immune responses in Parkinson’s disease[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(25): 5469-5477.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
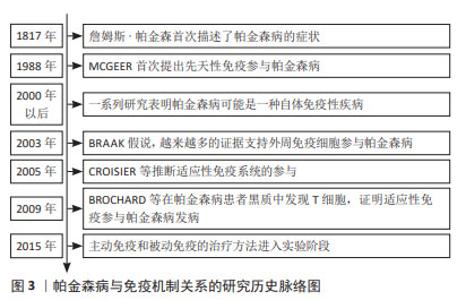
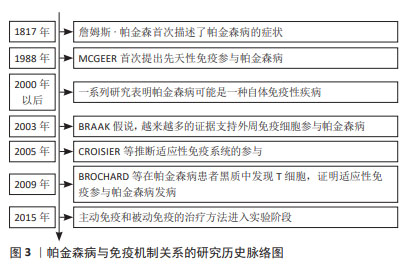
2.1 免疫反应与帕金森病关系研究的历程 1817年,詹姆斯·帕金森医生发表了《震颤麻痹》这篇医学论著,首次描述了帕金森病的症状,但当时并未认识到其与免疫反应的关系。直到1988年MCGEER及其同事在帕金森病患者的黑质中发现大量激活的小胶质细胞,并且人类白细胞抗原D相关表达增加[5],他们提出先天性免疫反应参与帕金森病的发生。后续的研究发现小胶质细胞增生不仅存在于中脑,还存在于纹状体、脑桥、皮质和海马体等其他区域[6],这支持免疫系统在帕金森病发病中的作用。另一个重大变化源于Braak假说,该假说提出α-突触核蛋白病理学始于中枢神经系统以外的周围神经系统(如肠道或鼻上皮),因此更多的学者认为对于帕金森病应该有一个全面的看法,这说明首先对α-突触核蛋白作出反应的可能是外周免疫细胞。2005年,CROISIER等[7]在α-突触核蛋白积聚的神经元附近发现了表达MHC-Ⅱ的小胶质细胞,推断适应性免疫反应与帕金森病的发病有关,在这个过程中小胶质细胞充当T细胞的抗原呈递细胞。2009年的一项研究报道在帕金森病患者黑质中发现T细胞,通过调控肠道和大脑的适应性免疫可以影响病情的发展[8]。近年来,帕金森病的免疫疗法受到广泛关注,许多研究机构研发出的疫苗在实验中表现出较好的治疗效果[9]。帕金森病与免疫机制联系的研究历程见图3。 "
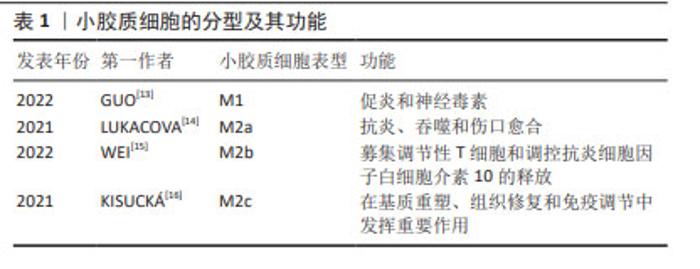
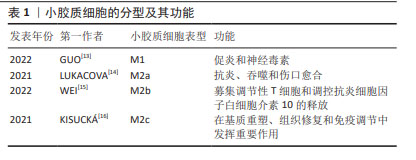
2.2 帕金森病中的免疫反应 2.2.1 先天性免疫 外周巨噬细胞能够对内源性刺激作出反应,发挥致病和保护功能,小胶质细胞也是如此。当暴露于内源性刺激时,小胶质细胞即被激活。活化的小胶质细胞释放出多种物质,包括促炎细胞因子、神经毒性蛋白、趋化因子、抗炎细胞因子和神经营养因子[10]。小胶质细胞有2种极性状态:M1表型与促炎和神经毒性反应相关,而M2表型主要介导抗炎和神经保护作用。M1小胶质细胞可以被错误折叠的蛋白质和环境毒素诱导,吞噬细胞,清除细胞碎片,以及释放促炎细胞因子:白细胞介素1β、白细胞介素6、白细胞介素12、白细胞介素17、白细胞介素18、白细胞介素23、肿瘤坏死因子α、干扰素γ和一氧化氮以及趋化因子CCL2[11]。M1小胶质细胞表型标记物包括诱导型一氧化氮合酶、环氧合酶2、MHC-Ⅱ和CD86等[12]。M2小胶质细胞的激活涉及多种机制,包括免疫调节、炎症抑制、修复和损伤消退,并且能产生一系列递质,如抗炎因子、细胞外基质蛋白、糖皮质激素和其他物质,见表1。 "
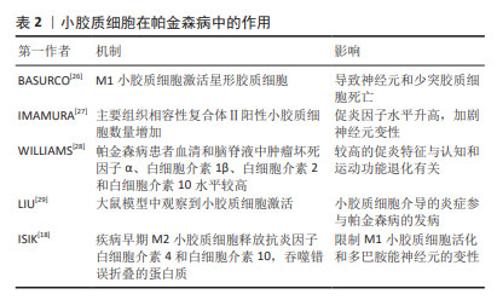
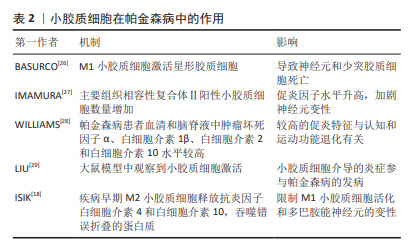
先天免疫参与帕金森病最早是由MCGEER等[5]提出的,他们发现帕金森病患者大脑显示出高水平的反应性小胶质细胞,并且在黑质和壳核中人类白细胞抗原D相关表达增加。GWAS表明人类白细胞抗原区域的变异与散发性帕金森病有关[17]。正电子发射断层扫描研究还发现,在不同的大脑区域,如脑干、基底节和额叶,小胶质细胞被广泛激活[6]。此外,在一些帕金森病小鼠模型中还观察到黑质和纹状体中的小胶质细胞增多[18]。不同脑区的反应性小胶质细胞伴随着较高水平的促炎细胞因子,如肿瘤坏死因子α、白细胞介素1β、白细胞介素6和干扰素γ,表明小胶质细胞参与了帕金森病发展过程中的神经炎症[19]。事实上,神经炎症被认为是一把“双刃剑”。活化的小胶质细胞具有神经保护性,有助于清除外来病原体和毒素。相反,慢性小胶质细胞增多症可导致帕金森病的细胞毒性和神经元丢失[20]。长期过度激活的小胶质细胞会加速细胞应激,影响记忆,并损害神经元的可塑性。随着帕金森病的进展,凋亡的神经细胞释放基质金属蛋白酶3、三磷酸腺苷和α-突触核蛋白,进一步激活小胶质细胞,导致多巴胺神经元变性。多项研究表明,帕金森病患者大脑中激活的小胶质细胞会加剧神经变性,而死亡多巴胺神经元释放的人类神经黑色素会引起趋化性并增加小胶质细胞培养物中的促炎物质[21-22]。BUTLER等[23]研究表明帕金森病患者中存在与M1激活相关的炎症标志物,例如MHC-Ⅱ、肿瘤坏死因子α和白细胞介素6。研究发现,帕金森病患者的脑脊液和纹状体样本中细胞因子水平升高,包括肿瘤坏死因子α、白细胞介素6和白细胞介素1β[24]。此外,通过免疫组化发现,在帕金森病患者黑质样本的阿米巴样小胶质细胞中,诱导型一氧化氮合酶、环氧合酶1和环氧合酶2的表达增加,同时黑质中细胞因子白细胞介素6、肿瘤坏死因子α水平升高[25],可见在小胶质细胞激活或增生时往往表现为M1促炎和M2抗炎两种不同的表型。促炎和抗炎的小胶质细胞可能在帕金森病脑中共存,小胶质细胞表型的复杂变化维持并加剧帕金森病的神经病理发展。小胶质细胞与多种神经退行性疾病有关,见表2。在阿尔茨海默病老年人脑中,已经报道了促炎和抗炎小胶质细胞表型的不平衡。因此,有理由推测帕金森病脑内存在一系列激活的小胶质细胞,它们聚集在一起,通过释放细胞因子来推动疾病的进展。"
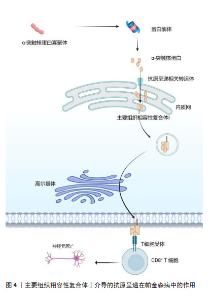
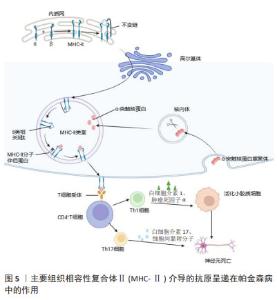
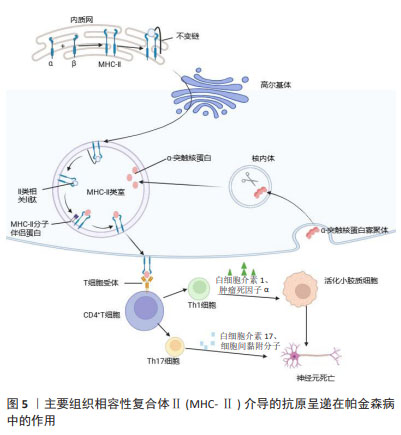
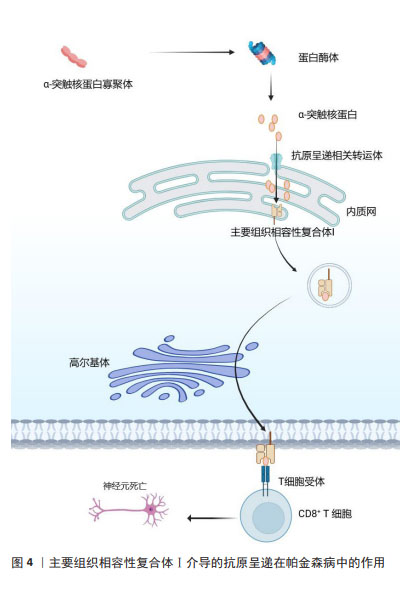
2.2.2 适应性免疫 外周T细胞由不同的亚群组成,当幼稚T细胞遇到树突状细胞呈递的抗原和共刺激配体时,引发免疫反应,调控白细胞介素2的产生,并增殖和分化为效应细胞,这些效应细胞迁移到不同的位点以促进病原体清除。一项关于死后帕金森病患者大脑的研究发现,在靠近血管和神经黑素阳性神经元的黑质中检测到了CD4+和CD8+ T细胞[30],这表明T细胞在帕金森病发病中具有重要作用。LAWTON等[31]通过观察帕金森病脑组织、血液和脑脊液发现,MHCⅡ+-APC之间的相互作用导致T细胞衍生的细胞因子表达升高,特别是干扰素γ和肿瘤坏死因子。SAUNDERS等[32]首次证明了人类帕金森病的运动障碍严重程度与T细胞的表型相关,研究还显示帕金森病中效应/记忆T细胞增加,CD4+CD45RA+静息/na?ve T细胞水平降低,这些改变与疾病进展有关。另一项研究表明在帕金森病小鼠中,CD4+和CD8+ T细胞的浸润程度明显升高[33],这一现象也可在帕金森病患者的大脑中发现。这些研究证明T细胞可能影响神经退行性变。因此,帕金森病进展与T细胞表型和功能有关这一观点被更多学者认可。调节适应性免疫应答可以影响帕金森病和其他神经退行性疾病的病理变化。 虽然多项研究显示B细胞能够通过外周作用引发中枢神经系统疾病,但迄今为止对B细胞在帕金森病中的研究还很有限[34]。B淋巴细胞是免疫系统的抗体分泌细胞,是体液免疫的关键递质,因为它们分泌的免疫球蛋白可以在远离实际分泌部位的组织或细胞产生作用[35]。鉴于帕金森病的慢性炎症性质,体液免疫可能在帕金森病的进展中发挥重要作用。在稳态条件下,B细胞少量存在于中枢神经系统薄壁组织(约0.1个细胞/cm2)和血管周围空间,并且这一B细胞亚群的数量和/或效应功能可以提高[36],这表明B细胞在免疫监视和抗原特异性记忆中发挥作用[34]。帕金森病患者的大脑中未检测到B细胞,但在黑质的多巴胺能神经元和中枢神经系统的路易小体上检测到IgG沉积[37]。已有研究证明帕金森病中自身抗体减少,B细胞通过清除细胞外病理性α-突触核蛋白的途径发挥神经保护作用[38]。α-突触核蛋白抗体水平在遗传性帕金森病、散发性帕金森病患者的血液和脑脊液中升高[39]。有学者对这些相互矛盾的结果进行了分析,认为队列、对照和技术方法的差异可能是造成结果不同的原因[40]。尽管目前尚不清楚这些衍生的α-突触核蛋白抗体是否具有神经保护作用,但体外实验表明,抗体有助于α-突触核蛋白清除[41],体内研究也支持这一观点。 2.3 MHC在免疫中的作用 在人类中,MHC也称为人类白细胞抗原,MHC位点分为3类:Ⅰ类(人类白细胞抗原A、B、C)、Ⅱ类(人类白细胞抗原DR、DP、DQ)和Ⅲ类[42]。几乎所有有核细胞都表达MHC-Ⅰ,而MHC-Ⅱ分子仅位于APC表面,包括B细胞、树突状细胞、小胶质细胞、巨噬细胞和胸腺上皮细胞[43]。MHC-Ⅰ将源自细胞质和细胞核的细胞内蛋白片段呈递给细胞毒性CD8+ T细胞或NK细胞[44]。这些细胞内蛋白在细胞质中被蛋白酶体降解为寡肽,并通过与抗原呈递相关的转运蛋白转位到内质网中以接触MHC-Ⅰ。MHC-Ⅰ和肽在内质网中组装后,被转运至高尔基体并根据分泌途径到达细胞表面[45]。MHC-Ⅰ介导的抗原呈递在帕金森病中的作用见图4。胞外肽通过MHC-Ⅱ分子呈递给CD4+ T细胞。与MHC-Ⅰ分子类似,MHC-Ⅱ被组装并分别运输到内质网和高尔基体,然后肽装载过程将在MHC-Ⅱ类室(pH 5.5)或细胞表面(pH 7.2)完成[46]。在低pH值条件下,不变链是一种伴侣蛋白,可保护MHC-Ⅱ的肽结合裂口不与细胞内肽结合,并促进MHC-Ⅱ转移至MⅡC,然后不变链被降解为一种小肽,称为Ⅱ类相关不变链肽。人类白细胞抗原DM是一种MHC-Ⅱ类伴侣蛋白,可去除CLIP/MHC复合物并促进抗原肽与MHC-Ⅱ的结合[47]。MHC-Ⅱ介导的抗原呈递在帕金森病中的作用见图5。MHC-Ⅲ编码不同的产物,包括热休克蛋白、肿瘤坏死因子家族蛋白(肿瘤坏死因子α、肿瘤坏死因子β)和不同过程中的补体成分[48]。 "
| [1] BI R, FANG Z, YOU M, et al. Microglia Phenotype and Intracerebral Hemorrhage: A Balance of Yin and Yang. Front Cell Neurosci. 2021; 15:765205. [2] CHU Y, HIRST WD, FEDEROFF HJ, et al. Nigrostriatal tau pathology in parkinsonism and Parkinson’s disease. Brain. 2024;147(2):444-457. [3] SONG Z, LI W, HAN Y, et al. Association of immune cell traits with Parkinson’s disease: a Mendelian randomization study. Front Aging Neurosci. 2024;16:1340110. [4] YANG Q, LV Z, WANG M, et al. LATS1/2 loss promote tumor immune evasion in endometrial cancer through downregulating MHC-I expression. J Exp Clin Cancer Res. 2024;43(1):54. [5] MCGEER PL, ITAGAKI S, BOYES BE, et al. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38(8):1285-1291. [6] HEIDARI A, YAZDANPANAH N, REZAEI N. The role of Toll-like receptors and neuroinflammation in Parkinson’s disease. J Neuroinflammation. 2022;19(1):135. [7] CROISIER E, MORAN LB, DEXTER DT, et al. Microglial inflammation in the parkinsonian substantia nigra: relationship to alpha-synuclein deposition. J Neuroinflammation. 2005;2:14. [8] BROCHARD V, COMBADIÈRE B, PRIGENT A, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119(1):182-192. [9] JANKOVIC J, GOODMAN I, SAFIRSTEIN B, et al. Safety and Tolerability of Multiple Ascending Doses of PRX002/RG7935, an Anti-α-Synuclein Monoclonal Antibody, in Patients With Parkinson Disease: A Randomized Clinical Trial. JAMA Neurol. 2018;75(10):1206-1214. [10] SEVENICH L. Brain-Resident Microglia and Blood-Borne Macrophages Orchestrate Central Nervous System Inflammation in Neurodegenerative Disorders and Brain Cancer. Front Immunol. 2018;9:697. [11] XUE Y, NIE D, WANG LJ, et al. Microglial Polarization: Novel Therapeutic Strategy against Ischemic Stroke. Aging Dis. 2021;12(2): 466-479. [12] WENDIMU MY, HOOKS SB. Microglia Phenotypes in Aging and Neurodegenerative Diseases. Cells. 2022;11(13):2091. [13] GUO S, WANG H, YIN Y. Microglia Polarization From M1 to M2 in Neurodegenerative Diseases. Front Aging Neurosci. 2022;14: 815347. [14] LUKACOVA N, KISUCKA A, KISS BIMBOVA K, et al. Glial-Neuronal Interactions in Pathogenesis and Treatment of Spinal Cord Injury. Int J Mol Sci. 2021;22(24):13577. [15] WEI Y, LI X. Different phenotypes of microglia in animal models of Alzheimer disease. Immun Ageing. 2022;19(1):44. [16] KISUCKÁ A, BIMBOVÁ K, BAČOVÁ M, et al. Activation of Neuroprotective Microglia and Astrocytes at the Lesion Site and in the Adjacent Segments Is Crucial for Spontaneous Locomotor Recovery after Spinal Cord Injury. Cells. 2021;10(8):1943. [17] HILL-BURNS EM, FACTOR SA, ZABETIAN CP, et al. Evidence for more than one Parkinson’s disease-associated variant within the HLA region. PLoS One. 2011;6(11):e27109. [18] ISIK S, YEMAN KIYAK B, AKBAYIR R, et al. Microglia Mediated Neuroinflammation in Parkinson’s Disease. Cells. 2023;12(7):1012. [19] ZHU R, LUO Y, LI S, et al. The role of microglial autophagy in Parkinson’s disease. Front Aging Neurosci. 2022;14:1039780. [20] LECOURS C, BORDELEAU M, CANTIN L, et al. Microglial Implication in Parkinson’s Disease: Loss of Beneficial Physiological Roles or Gain of Inflammatory Functions? Front Cell Neurosci. 2018;12:282. [21] SANDHU JK, KULKA M. Decoding Mast Cell-Microglia Communication in Neurodegenerative Diseases. Int J Mol Sci. 2021;22(3):1093. [22] WILMS H, ZECCA L, ROSENSTIEL P, et al. Inflammation in Parkinson’s diseases and other neurodegenerative diseases: cause and therapeutic implications. Curr Pharm Des. 2007;13(18):1925-1928. [23] BUTLER CA, POPESCU AS, KITCHENER EJA, et al. Microglial phagocytosis of neurons in neurodegeneration, and its regulation. J Neurochem. 2021;158(3):621-639. [24] LANZA M, CASILI G, CAMPOLO M, et al. Immunomodulatory Effect of Microglia-Released Cytokines in Gliomas. Brain Sci. 2021;11(4):466. [25] KNOTT C, STERN G, WILKIN GP. Inflammatory regulators in Parkinson’s disease: iNOS, lipocortin-1, and cyclooxygenases-1 and -2. Mol Cell Neurosci. 2000;16(6):724-739. [26] BASURCO L, ABELLANAS MA, AYERRA L, et al. Microglia and astrocyte activation is region-dependent in the α-synuclein mouse model of Parkinson’s disease. Glia. 2023;71(3):571-587. [27] IMAMURA K, HISHIKAWA N, SAWADA M, et al. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol. 2003;106(6): 518-526. [28] WILLIAMS-GRAY CH, WIJEYEKOON R, YARNALL AJ, et al. Serum immune markers and disease progression in an incident Parkinson’s disease cohort (ICICLE-PD). Mov Disord. 2016;31(7):995-1003. [29] LIU WW, WEI SZ, HUANG GD, et al. BMAL1 regulation of microglia-mediated neuroinflammation in MPTP-induced Parkinson’s disease mouse model. FASEB J. 2020;34(5):6570-6581. [30] SOMMER A, MARXREITER F, KRACH F, et al. Th17 Lymphocytes Induce Neuronal Cell Death in a Human iPSC-Based Model of Parkinson’s Disease. Cell Stem Cell. 2018;23(1):123-131.e6. [31] LAWTON M, BAIG F, TOULSON G, et al. Blood biomarkers with Parkinson’s disease clusters and prognosis: The oxford discovery cohort. Mov Disord. 2020;35(2):279-287. [32] SAUNDERS JA, ESTES KA, KOSLOSKI LM, et al. CD4+ regulatory and effector/memory T cell subsets profile motor dysfunction in Parkinson’s disease. J Neuroimmune Pharmacol. 2012;7(4):927-938. [33] SAPONJIC J, MEJÍAS R, NIKOLOVSKI N, et al. Experimental Models to Study Immune Dysfunction in the Pathogenesis of Parkinson’s Disease. Int J Mol Sci. 2024;25(8):4330. [34] SABATINO JJ JR, PRÖBSTEL AK, ZAMVIL SS. B cells in autoimmune and neurodegenerative central nervous system diseases. Nat Rev Neurosci. 2019;20(12):728-745. [35] NAKAGAWA R, CALADO DP. Positive Selection in the Light Zone of Germinal Centers. Front Immunol. 2021;12:661678. [36] MACHADO-SANTOS J, SAJI E, TRÖSCHER AR, et al. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain. 2018;141(7):2066-2082. [37] ORR CF, ROWE DB, MIZUNO Y, et al. A possible role for humoral immunity in the pathogenesis of Parkinson’s disease. Brain. 2005; 128(Pt 11):2665-2674. [38] BRUDEK T, WINGE K, FOLKE J, et al. Autoimmune antibody decline in Parkinson’s disease and Multiple System Atrophy; a step towards immunotherapeutic strategies. Mol Neurodegener. 2017;12(1):44. [39] AKHTAR RS, LICATA JP, LUK KC, et al. Measurements of auto-antibodies to α-synuclein in the serum and cerebral spinal fluids of patients with Parkinson’s disease. J Neurochem. 2018;145(6):489-503. [40] SCOTT KM, KOULI A, YEOH SL, et al. A Systematic Review and Meta-Analysis of Alpha Synuclein Auto-Antibodies in Parkinson’s Disease. Front Neurol. 2018;9:815. [41] BAE EJ, LEE HJ, ROCKENSTEIN E, et al. Antibody-aided clearance of extracellular α-synuclein prevents cell-to-cell aggregate transmission. J Neurosci. 2012;32(39):13454-13469. [42] ROSSJOHN J, GRAS S, MILES JJ, et al. T cell antigen receptor recognition of antigen-presenting molecules. Annu Rev Immunol. 2015;33:169-200. [43] ROCHE PA, FURUTA K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat Rev Immunol. 2015;15(4): 203-216. [44] KAUFMAN J. Unfinished Business: Evolution of the MHC and the Adaptive Immune System of Jawed Vertebrates. Annu Rev Immunol. 2018;36:383-409. [45] ZAITOUA AJ, KAUR A, RAGHAVAN M. Variations in MHC class I antigen presentation and immunopeptidome selection pathways. F1000Res. 2020;9:F1000 Faculty Rev-1177. [46] MÜNZ C. The Macroautophagy Machinery in MHC Restricted Antigen Presentation. Front Immunol. 2021;12:628429. [47] WIECZOREK M, ABUALROUS ET, STICHT J, et al. Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Front Immunol. 2017;8:292. [48] SZNARKOWSKA A, MIKAC S, PILCH M. MHC Class I Regulation: The Origin Perspective. Cancers (Basel). 2020;12(5):1155. [49] SCHONHOFF AM, WILLIAMS GP, WALLEN ZD, et al. Innate and adaptive immune responses in Parkinson’s disease. Prog Brain Res. 2020;252: 169-216. [50] KATIKANENI DS, JIN L. B cell MHC class II signaling: A story of life and death. Hum Immunol. 2019;80(1):37-43. [51] ROLAND MM, PEACOCK TE, HALL N, et al. B-cell-specific MhcII regulates microbiota composition in a primarily IgA-independent manner. Front Immunol. 2023;14:1253674. [52] CHIRMULE N, TAZELAAR J, WILSON JM. Th2-dependent B cell responses in the absence of CD40-CD40 ligand interactions. J Immunol. 2000;164(1):248-255. [53] JORDAN MB, MILLS DM, KAPPLER J, et al. Promotion of B cell immune responses via an alum-induced myeloid cell population. Science. 2004;304(5678):1808-1810. [54] FERREIRA SA, ROMERO-RAMOS M. Microglia Response During Parkinson’s Disease: Alpha-Synuclein Intervention. Front Cell Neurosci. 2018;12:247. [55] ALMOLDA B, GONZÁLEZ B, CASTELLANO B. Are Microglial Cells the Regulators of Lymphocyte Responses in the CNS? Front Cell Neurosci. 2015;9:440. [56] JIMENEZ-FERRER I, JEWETT M, TONTANAHAL A, et al. Allelic difference in Mhc2ta confers altered microglial activation and susceptibility to α-synuclein-induced dopaminergic neurodegeneration. Neurobiol Dis. 2017;106:279-290. [57] JIMENEZ-FERRER I, BÄCKSTRÖM F, DUEÑAS-REY A, et al. The MHC class II transactivator modulates seeded alpha-synuclein pathology and dopaminergic neurodegeneration in an in vivo rat model of Parkinson’s disease. Brain Behav Immun. 2021;91:369-382. [58] GOPINATH A, MACKIE PM, PHAN LT, et al. The complex role of inflammation and gliotransmitters in Parkinson’s disease. Neurobiol Dis. 2023;176:105940. [59] KANNARKAT GT, COOK DA, LEE JK, et al. Common Genetic Variant Association with Altered HLA Expression, Synergy with Pyrethroid Exposure, and Risk for Parkinson’s Disease: An Observational and Case-Control Study. NPJ Parkinsons Dis. 2015;1:15002. [60] DUFFY MF, COLLIER TJ, PATTERSON JR, et al. Lewy body-like alpha-synuclein inclusions trigger reactive microgliosis prior to nigral degeneration. J Neuroinflammation. 2018;15(1):129. [61] SULZER D, ALCALAY RN, GARRETTI F, et al. T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature. 2017; 546(7660):656-661. [62] HARMS AS, CAO S, ROWSE AL, et al. MHCII is required for α-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J Neurosci. 2013;33(23):9592-9600. [63] CEBRIÁN C, LOIKE JD, SULZER D. Neuronal MHC-I expression and its implications in synaptic function, axonal regeneration and Parkinson’s and other brain diseases. Front Neuroanat. 2014;8:114. [64] HOBSON BD, SULZER D. Neuronal Presentation of Antigen and Its Possible Role in Parkinson’s Disease. J Parkinsons Dis. 2022;12(s1): S137-S147. [65] WANG BY, YE YY, QIAN C, et al. Stress increases MHC-I expression in dopaminergic neurons and induces autoimmune activation in Parkinson’s disease. Neural Regen Res. 2021;16(12):2521-2527. [66] ALISEYCHIK MP, ANDREEVA TV, ROGAEV EI. Immunogenetic Factors of Neurodegenerative Diseases: The Role of HLA Class II. Biochemistry (Mosc). 2018;83(9):1104-1116. [67] XU S, LU J, SHAO A, et al. Glial Cells: Role of the Immune Response in Ischemic Stroke. Front Immunol. 2020;11:294. [68] HARLEY J, SANTOSA MM, NG CY, et al. Telomere shortening induces aging-associated phenotypes in hiPSC-derived neurons and astrocytes. Biogerontology. 2024;25(2):341-360. [69] QIAO C, NIU G, ZHAO W, et al. RIPK1-Induced A1 Reactive Astrocytes in Brain in MPTP-Treated Murine Model of Parkinson’s Disease. Brain Sci. 2023;13(5):733. [70] MAGNUSEN AF, HATTON SL, RANI R, et al. Genetic Defects and Pro-inflammatory Cytokines in Parkinson’s Disease. Front Neurol. 2021;12:636139. [71] DENG W, YI P, XIONG Y, et al. Gut Metabolites Acting on the Gut-Brain Axis: Regulating the Functional State of Microglia. Aging Dis. 2024; 15(2):480-502. [72] HEUBERGER C, POTT J, MALOY KJ. Why do intestinal epithelial cells express MHC class II? Immunology. 2021;162(4):357-367. [73] WOSEN JE, ILSTAD-MINNIHAN A, CO JY, et al. Human Intestinal Enteroids Model MHC-II in the Gut Epithelium. Front Immunol. 2019; 10:1970. [74] ABELLANAS MA, ZAMARBIDE M, BASURCO L, et al. Midbrain microglia mediate a specific immunosuppressive response under inflammatory conditions. J Neuroinflammation. 2019;16(1):233. [75] MARTIN HL, SANTORO M, MUSTAFA S, et al. Evidence for a role of adaptive immune response in the disease pathogenesis of the MPTP mouse model of Parkinson’s disease. Glia. 2016;64(3):386-395. [76] LIU TW, CHEN CM, CHANG KH. Biomarker of Neuroinflammation in Parkinson’s Disease. Int J Mol Sci. 2022;23(8):4148. [77] YU E, AMBATI A, ANDERSEN MS, et al. Fine mapping of the HLA locus in Parkinson’s disease in Europeans. NPJ Parkinsons Dis. 2021;7(1):84. [78] KAM TI, HINKLE JT, DAWSON TM, et al. Microglia and astrocyte dysfunction in parkinson’s disease. Neurobiol Dis. 2020;144:105028. [79] ROSTAMI J, FOTAKI G, SIROIS J, et al. Astrocytes have the capacity to act as antigen-presenting cells in the Parkinson’s disease brain. J Neuroinflammation. 2020;17(1):119. [80] WAISMAN A, JOHANN L. Antigen-presenting cell diversity for T cell reactivation in central nervous system autoimmunity. J Mol Med (Berl). 2018;96(12):1279-1292. [81] MASON HD, MCGAVERN DB. How the immune system shapes neurodegenerative diseases. Trends Neurosci. 2022;45(10):733-748. [82] GARRETTI F, AGALLIU D, LINDESTAM ARLEHAMN CS, et al. Autoimmunity in Parkinson’s Disease: The Role of α-Synuclein-Specific T Cells. Front Immunol. 2019;10:303. [83] KUSTRIMOVIC N, COMI C, MAGISTRELLI L, et al. Parkinson’s disease patients have a complex phenotypic and functional Th1 bias: cross-sectional studies of CD4+ Th1/Th2/T17 and Treg in drug-naïve and drug-treated patients. J Neuroinflammation. 2018;15(1):205. [84] SUBBARAYAN MS, HUDSON C, MOSS LD, et al. T cell infiltration and upregulation of MHCII in microglia leads to accelerated neuronal loss in an α-synuclein rat model of Parkinson’s disease. J Neuroinflammation. 2020;17(1):242. [85] HARMS AS, FERREIRA SA, ROMERO-RAMOS M. Periphery and brain, innate and adaptive immunity in Parkinson’s disease. Acta Neuropathol. 2021;141(4):527-545. [86] BADANJAK K, FIXEMER S, SMAJIĆ S, et al. The Contribution of Microglia to Neuroinflammation in Parkinson’s Disease. Int J Mol Sci. 2021; 22(9):4676. [87] ZHU B, YIN D, ZHAO H, et al. The immunology of Parkinson’s disease. Semin Immunopathol. 2022;44(5):659-672. [88] WILLIAMS GP, SCHONHOFF AM, JURKUVENAITE A, et al. Targeting of the class II transactivator attenuates inflammation and neurodegeneration in an alpha-synuclein model of Parkinson’s disease. J Neuroinflammation. 2018;15(1):244. [89] VENEZIA S, REFOLO V, POLISSIDIS A, et al. Toll-like receptor 4 stimulation with monophosphoryl lipid A ameliorates motor deficits and nigral neurodegeneration triggered by extraneuronal α-synucleinopathy. Mol Neurodegener. 2017;12(1):52. [90] VILLADIEGO J, LABRADOR-GARRIDO A, FRANCO JM, et al. Immunization with α-synuclein/Grp94 reshapes peripheral immunity and suppresses microgliosis in a chronic Parkinsonism model. Glia. 2018;66(1): 191-205. [91] CASTONGUAY AM, GRAVEL C, LÉVESQUE M. Treating Parkinson’s Disease with Antibodies: Previous Studies and Future Directions. J Parkinsons Dis. 2021;11(1):71-92. [92] ZHA J, LIU XM, ZHU J, et al. A scFv antibody targeting common oligomeric epitope has potential for treating several amyloidoses. Sci Rep. 2016;6:36631. |
| [1] | Yu Shuai, Liu Jiawei, Zhu Bin, Pan Tan, Li Xinglong, Sun Guangfeng, Yu Haiyang, Ding Ya, Wang Hongliang. Hot issues and application prospects of small molecule drugs in treatment of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1913-1922. |
| [2] | Sun Jiaqi, Bian Lu, Shi Wentao, Wu Xuechao, Lu Xiaojie. Mechanism of Piezo-type mechanosensitive ion channel component 1 in rat pressure injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1578-1584. |
| [3] | Li Kaiying, Wei Xiaoge, Song Fei, Yang Nan, Zhao Zhenning, Wang Yan, Mu Jing, Ma Huisheng. Mechanism of Lijin manipulation regulating scar formation in skeletal muscle injury repair in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1600-1608. |
| [4] | Chi Wenxin, Zhang Cunxin, Gao Kai, Lyu Chaoliang, Zhang Kefeng. Mechanism by which nobiletin inhibits inflammatory response of BV2 microglia [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1321-1327. |
| [5] | He Longcai, Song Wenxue, Ming Jiang, Chen Guangtang, Wang Junhao, Liao Yidong, Cui Junshuan, Xu Kaya. An experimental method for simultaneous extraction and culture of primary cortical neurons and microglial cells from SD rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1395-1400. |
| [6] | Zhao Ruihua, Chen Sixian, Guo Yang, Shi Lei, Wu Chengjie, Wu Mao, Yang Guanglu, Zhang Haoheng, Ma Yong. Wen-Shen-Tong-Du Decoction promoting spinal cord injury repair in mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1118-1126. |
| [7] | Bai Jing, Zhang Xue, Ren Yan, Li Yuehui, Tian Xiaoyu. Effect of lncRNA-TNFRSF13C on hypoxia-inducible factor 1alpha in periodontal cells by modulation of #br# miR-1246 #br# [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 928-935. |
| [8] | Zhi Fang, Zhu Manhua, Xiong Wei, Lin Xingzhen. Analgesic effect of acupuncture in a rat model of lumbar disc herniation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 936-941. |
| [9] | Lu Ranran, Zhou Xu, Zhang Lijie, Yang Xinling. Dimethyl fumarate alleviates nerve damage in a mouse model of Parkinson’s disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 989-994. |
| [10] | Wang Yuru, Li Siyuan, Xu Ye, Zhang Yumeng, Liu Yang, Hao Huiqin. Effects of wogonin on joint inflammation in collagen-induced arthritis rats via the endoplasmic reticulum stress pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1026-1035. |
| [11] | Yu Hui, Yang Yang, Wei Ting, Li Wenli, Luo Wenqian, Liu Bin. Gadd45b alleviates white matter damage in chronic ischemic rats by modulating astrocyte phenotype [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7797-7803. |
| [12] | Zheng Yitong, Wang Yongxin, Liu Wen, Amujite, Qin Hu. Action mechanism of intrathecal transplantation of human umbilical cord mesenchymal stem cell-derived exosomes for repair of spinal cord injury under neuroendoscopy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7743-7751. |
| [13] | Pan Chun, Fan Zhencheng, Hong Runyang, Shi Yujie, Chen Hao. Effect and mechanism of polystyrene microplastics on prostate in male mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7353-7361. |
| [14] | Su Yongkun, Sun Hong, Liu Miao, Yang Hua, Li Qingsong. Development of novel antioxidants and antioxidant combination carried by nano-hydrogel systems in treatment of intervertebral disc degeneration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7376-7384. |
| [15] | Tian Yushi, Fu Qiang, Li Ji . Bioinformatics identification and validation of mitochondrial genes related to acute myocardial infarction [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6697-6707. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||