Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (16): 3476-3485.doi: 10.12307/2025.413
Previous Articles Next Articles
Strategies and development of photothermal and photodynamic synergistic therapy of metal-organic frameworks
Chen Xiaoxuan, Pei Xibo, Gai Kuo, Wan Qianbing
- National Key Laboratory of Oral Disease Prevention and Control, National Center for Stomatology, National Clinical Medical Research Center for Oral Diseases, Department of Prosthodontics of West China School of Stomatology of Sichuan University, Chengdu 610041, Sichuan Province, China
-
Received:2024-03-22Accepted:2024-04-28Online:2025-06-08Published:2024-09-06 -
Contact:Wan Qianbing, MD, Professor, National Key Laboratory of Oral Disease Prevention and Control, National Center for Stomatology, National Clinical Medical Research Center for Oral Diseases, Department of Prosthodontics of West China School of Stomatology of Sichuan University, Chengdu 610041, Sichuan Province, China -
About author:Chen Xiaoxuan, National Key Laboratory of Oral Disease Prevention and Control, National Center for Stomatology, National Clinical Medical Research Center for Oral Diseases, Department of Prosthodontics of West China School of Stomatology of Sichuan University, Chengdu 610041, Sichuan Province, China -
Supported by:Sichuan Provincial Science and Technology Plan Project, No. 2023ZYD0109 (to PXB)
CLC Number:
Cite this article
Chen Xiaoxuan, Pei Xibo, Gai Kuo, Wan Qianbing. Strategies and development of photothermal and photodynamic synergistic therapy of metal-organic frameworks[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3476-3485.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
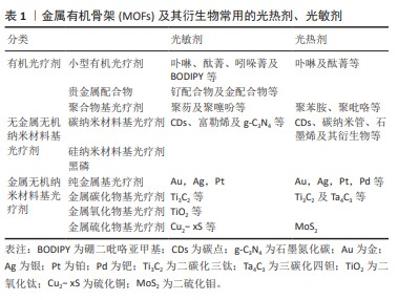
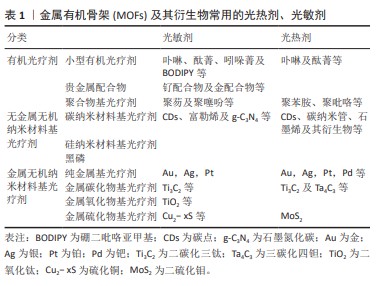
2.1 常见的光疗MOFs结构 光疗MOFs在结构上可以大体分为2种,一种是MOFs自身直接作为光热剂或光敏剂,即构成MOFs的金属节点或配体自身具有光动力或光热效应。例如普鲁士蓝基MOFs,在近红外光照下,其结构中的Fe(II)和Fe(III)间发生电荷转移产热 [32]。以光敏剂卟啉及其衍生物为配体的MOFs也是MOFs本体为光敏剂的典例[33];另一种是以没有光疗效应的MOFs作为载体,通过封装、形成核壳结构、原位还原及表面附着等改性方法将光敏剂或光热剂与MOFs结合,从而产生光疗效应。常见的载体MOFs包括ZIF-8[34]、MIL-100 [35]、UiO-66等,文章总结了常见的光疗剂[36-37],见表1。封装是在MOFs合成过程中或合成完毕后[38],通过MOFs配体与光疗剂特定的官能团结合或静电作用,将光疗剂封装于MOFs内部[21]。核壳结构主要是MOFs在光疗剂表面生长形成的结构,通常以金属纳米颗粒、上转纳米粒子或碳基纳米材料等为生长核心,外层MOFs起稳定、保护作用。原位还原主要是将光疗剂的金属离子引入MOFs结构的孔隙内,再将其原位还原形成纳米颗粒的方法。表面附着主要是通过表面修饰或共价修饰将光疗剂连接到MOFs外部,即将靶向药物或光疗剂附着在-NH2等官能团修饰的MOFs或MOFs的不饱和节点及配体上[39]。除此之外,还有一些比较特殊的改性方法,例如热解MOFs形成其衍生的碳材料等。以结构为立足点,可以通过不同的改性方式进行MOFs结构设计,赋予MOFs材料光热/光动力效应,实现联合治疗。"

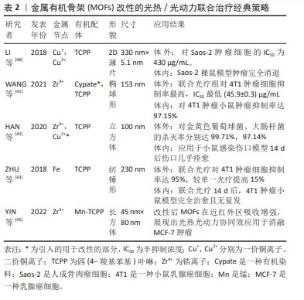
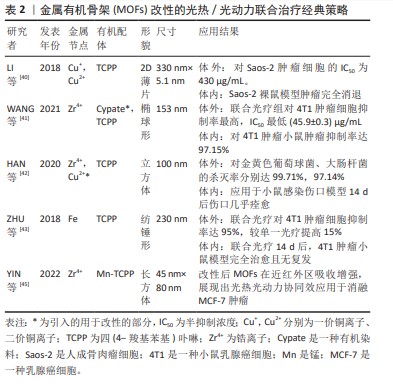
2.2 现有MOFs光热/光动力联合治疗策略 2.2.1 MOFs的改性 直接利用同时具有光动力/光热效应的物质作为有机配体,或引入可与配体形成中心配位的金属离子,或通过替换MOFs的部分金属节点或有机配体,使得MOFs自身即具光动力/光热效应,这种方法可不需要再搭载额外的光疗剂。 LI等[40]以溶剂热法合成了一种Cu为金属节点、卟啉衍生物四 (4-羧基苯基)卟啉(TCPP)为有机配体的超薄二维(2D)Cu-TCPP纳米片,薄片结构可提供更多的光响应位点,增加了光热转换效率和光动力效应。Cu-TCPP在体内外实验中显示出极少的Cu2+释放,具有良好的生物相容性。该材料中Cu(I)和Cu(II)共存,光热效应来自于Cu2+节点,光动力效应来自于TCPP自身在660 nm激光下产生的活性氧,且Cu2+捕获电子后将减少TCPP电子-空穴对的复合,增强其光催化性能。此外,Cu2+节点未配对的3d电子赋予其T1加权磁共振成像能力。体内外实验时,联合光疗组抗肿瘤效果均高于单独使用光热或光动力方法,表明光热/光动力联合治疗产生了协同作用 [40]。WANG等[41]以光热剂Cypate替代部分H2TCPP配体,与Zr4+簇通过一锅溶剂热法合成了卟啉/Cypate基MOFs,从而引入光热功能,并负载肿瘤靶向配体叶酸,在808 nm和660 nm双激光激发下,二者发挥协同作用显示出对4T1癌细胞高抑制率。HAN等[42]将Cu2+掺杂进Zr6和TCPP形成的MOFs PCN-224,研究表明Cu2+通过N-C键嵌入卟啉环形成中心配位,而并未显著改变MOFs的拓扑结构,其光疗效应机制类似Cu-TCPP;细胞相容性评估中,Zr几乎没有释放,Cu2+远低于毒性水平的缓释从而达到抗菌效果,结果表明该MOFs拥有良好的生物相容性。MOFs体内外实验表明10%Cu2+掺杂的MOFs具有强效、加速伤口愈合的功能。ZHU等[43]以Fe为金属节点,TCPP为有机配体,首先在660 nm较低功率密度光照10 min下诱导TCPP产生活性氧,接着在660 nm较高功率密度下照射5 min使TCPP转换光能产热。抗4T1肿瘤细胞实验显示,二者联合治疗下的肿瘤细胞杀灭率较单独使用光热或光动力疗法提高15%,显示出比单一疗法更好的协同抗肿瘤效应[43]。 直接改性MOFs赋予MOFs光热、光动力效应的办法,避免了额外负载。这种MOFs结构简单、稳定性高,尺寸更小,但也存在金属节点、有机配体选择有限的问题。例如能与卟啉的大环结构形成中心配位、同时能提供光疗效应的金属很少,代表金属为Cu。首先,Cu的离子半径、电子配置合适,保证了掺入Cu2+后MOFs的形貌、尺寸不会明显变化,且二者结合稳定;其次,Cu2+可以通过d-d能带跃迁增加近红外区吸收产热赋予MOFs光热效应,并捕获电子,抑制电子-空穴复合,提高MOFs载流能力从而增强其光动力效应[42-45]。因此,Cu2+是作为MOFs改性金属节点或中心配体的良好选择。另外,由于骨架改性MOFs组分较少,因此可以通过调控MOFs形貌来加强光疗效应。例如LI等[40]通过加入表面活性剂将MOFs制成二维薄片,通过增加光反应面积增强光疗效果。MOFs的改性方法的经典策略总结见表2。"


2.2.2 MOFs封装光疗剂 封装常通过MOFs配体与具有特定官能团(如-COOH,-SO3H)的光疗剂结合,或二者带不同电荷相互吸引实现,可在MOFs合成过程中或合成后进行。后者常在分别合成MOFs和光疗剂后,通过浸渍或研磨将光疗剂渗透进MOFs的孔隙中[46]。将光疗剂封装于MOFs内部可以有效防止光疗剂的聚集或自淬灭,提高材料的稳定性。另外,MOFs避免了光敏剂与氧气的提前接触失效,提高了其治疗的特异性[47]。YANG等[48]将Cypate引入金属节点为Fe3+的MOFs内部形成Cypate@MIL-53,外层再用PEG和转铁蛋白包裹,最终材料命名为CMNP,这种功能性MOFs构建方法属于在MOFs合成过程中负载光敏/光热剂,先让Cypate的羧基与Fe3+配位形成前体,再用有机配体1,4-苯二甲酸(H2BDC)将前体中的金属节点分离出来组成MOFs,这样Cypate被嵌入封装在单个MIL-53的内部空隙中。此法提高了Cypate的成药性和生物利用度,并可通过控制Cypate的浓度改变其装载率,从而调节MIL-53的粒径。在杀灭癌细胞时表现出光热光动力协同效应和核磁共振、光声成像等多种成像功能[48]。有研究通过相似的方法将ICG封装于ZIF-8中,用于抗肿瘤治疗。先让Zn2+与ICG磺酸基通过弱配位形成络合物,再加入有机配体2-甲基咪唑进一步合成ZIF-8,将ICG包裹在其内,最终合成的MOFs具有良好的稳定性和光疗性能 [49-50]。封装具有合成过程简便、耗时短等优势,符合未来临床转化的期盼方向。由于光疗剂被装载于MOFs内部,有效避免了光疗剂的提前聚集、预渗漏、提前遇氧后反应,保证了光疗剂的化学稳定性,提升了治疗的特异性。但如果采用在MOFs合成完毕后再渗透光疗剂的方法,则要求光疗剂的粒径与MOFs的孔径匹配。 2.2.3 光疗剂与MOFs形成核壳结构 在此核壳结构中,MOFs既可作为“壳”,亦可作为“核”;在一些核壳结构中,“壳”与“核”均为MOFs。但在光疗应用中,MOFs最常作为“壳”结构,最常通过其前体在活化后的核心层表面进行自组装的方法合成 [51]。由于可以合成多层外壳,核壳结构能够将多种MOFs和光疗剂的功能整合在一起,进一步提高了MOFs的负载 [52]。CAI等 [53]合成了一种简单的Au@MOFs的核壳结构,羧基修饰的金纳米棒为核充当光热剂,Fe3O(OAc)6(H2O)3+簇和TCPP在其表面自组装形成的MOFs作为壳产活性氧,通过金属节点和MOFs的配位反应,可获得不同厚度的MOFs外壳,以调节光热/光动力治疗的协同效应,用于抗肿瘤。有研究也用不同形貌的金纳米粒子和MOFs合成了核壳结构用于协同光疗抗肿瘤和抗菌[54-55]。LUO等[56]合成了一种双MOFs异质核壳结构,普鲁士蓝为核心提供光热效应,TCPP在不破坏原普鲁士蓝立方体结构的情况下被引入UiO-66的内部空隙中。普鲁士蓝可抑制电子-空穴对复合,核壳形成的异质界面可加快光生电子的转移速度,以增强光动力效应。外层UiO-66虽一定程度降低了普鲁士蓝的光热效应,但光热转换效率仍可达29.9%,避免温度过高导致的健康组织受损。在808 nm和660 nm双光照下,材料对金黄色葡萄球菌、大肠杆菌的抗菌效果分别为99.31%和98.68%。 同封装类似, MOFs“壳”可以保护内部的光疗剂“核”,形成稳定的结构,但在合成方法上较前者繁琐。首先,为了让“核”与“壳”形成稳定的结合,通常需要对“核”进行官能化;其次,为合成某一特定厚度的“壳”,需要循环多次、逐层包装。耗时复杂的材料合成过程,会在一定程度上限制其应用。 2.2.4 光疗剂在MOFs内原位还原 原位合成需要先合成MOFs结构,然后将其与金属离子接触,使金属离子引入MOFs内部或表面,最后通过化学还原及光还原等方法将金属离子还原为纳米粒 [46],最常见的是使用金纳米粒子作为光热剂,原位还原于具有光动力的MOFs结构中。ZHAO等[57]采用原位生长的方法合成了金纳米团簇和锆基卟啉MOFs,命名为AuNCs@PCN,并在外层包封水凝胶。不同于CAI等[53]合成的金纳米棒核,此MOFs中的金纳米团簇直径为2-5 nm,散在分布于PCN中。MOFs可在近红外光照射下有效抗耐药菌,通过划痕实验及免疫印迹验证其具有促进细胞生长、迁移和血管生成的作用,动物模型证明该MOFs能促进糖尿病大鼠伤口愈合。DU等[46]利用硼氢化钠和甲醛分别使Pt和Au在MOFs表面还原生长,额外赋予MOFs光热效应用于抗菌及促感染伤口愈合[58]。 原位还原中最常用的方法是化学还原法。合成难点在于,由于氧化还原反应时间短并大量产热,可能导致纳米粒子在MOFs内过度生长从而破坏MOFs 结构或还原产物在MOFs内不均匀分布 [59]。因此需要冰浴等条件控制氧化还原反应进程。"

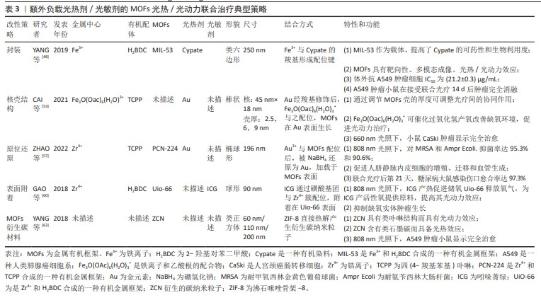
2.2.5 MOFs表面附着光疗剂 表面附着是指MOFs合成后,用-NH2、-COOH等官能团修饰,再通过共价键、配位键结合光疗剂。由于对负载物和MOFs官能团的要求,光疗剂表面附着于MOFs的例子较少。某些有机染料自身即兼具光热/光动力双重特性,例如近红外菁染料,包括吲哚菁绿(ICG)及其衍生物等,负载于MOFs材料上后,可赋予其双重光疗性能。GAO 等[60]将ICG在锆基MOFs UiO-66表面配位,再于外层涂覆红细胞膜以逃避免疫反应,内部存储O2,在808 nm近红外光激发下,ICG通过光动力效应原位产生1O2降解红细胞膜;同时,ICG的光热效应所产的热量导致1O2爆发性释放、促进内部O2通过UiO-66的多孔结构扩散,迅速改善肿瘤缺氧环境。在此策略中,ICG修饰后的MOFs孔径未发生明显改变 ,表明ICG仅附着于MOFs表面并未进入其内部,这为后来氧气分子进入UiO-66孔隙内提供了空间,这种策略通过提高肿瘤区域氧供应,充分放大了光热协同效应,具有临床应用优势 [60]。 表面附着结构方法未能如上述方法一般有效保护光疗剂,因此通常需额外覆盖其他材料以增强其稳定性。此外,由于对官能团具有特定需求,此方法的应用范围受到限制。 2.2.6 其他特殊改性方法 MOFs衍生的碳材料是由MOFs热解制备的,在热解过程中,金属节点被还原,有机配体碳化为碳载体,利于掺杂其他金属原子和杂原子 [61]。MOFs衍生碳材料同传统碳材料类似,具备光热转换能力。当有机配体含氮时,MOFs在某些热解条件下可以形成类似于卟啉的金属中心配位的M-N-C结构,使材料具备光动力能力。YANG等[62]将ZIF-8在氮气环境900 ℃下热解,形成ZIF-8衍生碳纳米粒子(ZCN),表面用Mpeg-DSPE 修饰以保持材料的稳定性,发现其具备光动力、光热双重光疗效应和光声成像能力。在808 nm激光下,粒径为200 nm的ZIF-8热解成的ZCN的光热转换率可达41.6%,远高于碳纳米点及金纳米壳等材料,其产活性氧产率甚至略高于ICG。体内实验显示,ZCN可以在低功率近红外激光的激发下达到97%的抗肿瘤有效率。有研究进一步将铂纳米酶整合到热解后的ZIF-8衍生碳框架表面。铂-碳纳米酶具备的类过氧化氢酶作用有利于缓解肿瘤缺氧微环境;铂内在的光热转换能力,增强了MOFs自身的光热效应[63]。表3总结了以MOFs为载体,通过不同合成方式搭载光疗剂的一些联合光疗的典型策略。"
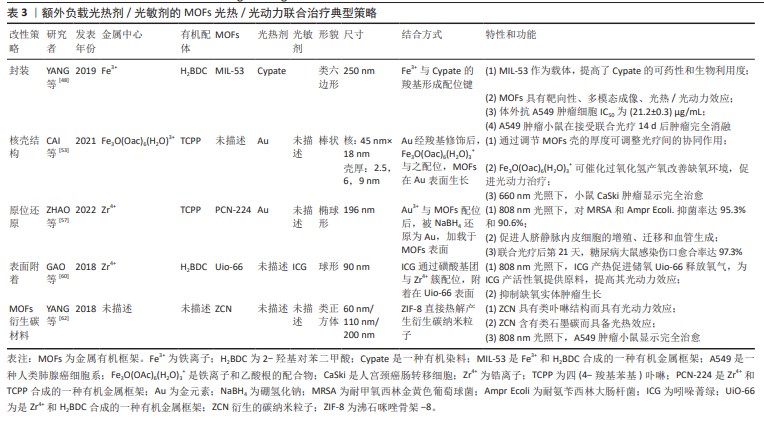

2.2.7 “多合一”策略 将上述的改性方法组合应用,可以将不同功能的光疗剂和MOFs整合在一起,形成多功能MOFs综合治疗平台。 黑磷量子点可同时产生单线态氧及进行光热转化。LIU等[64]采用原位生长法将黑磷量子点封装于第一层MIL内部,然后将过氧化氢酶引入第一层MIL外部,再用第二层MIL包裹,最终形成BQ-MIL@cat-MIL这一双层MOFs核壳结构,粒径约为140 nm,最后用PEG-FA和菁3-标记肽修饰。外层过氧化氢酶分解肿瘤微环境中的过氧化氢产生氧气,进入内层MIL为BQ的光动力效应提供原料,在660 nm和808 nm双光照下,肿瘤细胞凋亡率为75.6%,高于单独使用光动力(52.1%)或光热治疗(28.7%),表现出串联协同效应。YOU等[65]将铂(Pt)纳米粒子封装在-NH2修饰的UiO-66(UiO-66-NH2)内部,再通过静电作用将金纳米粒连接在-NH2上形成多孔金纳米外壳,形成的复合结构简称为PtMG;进一步修饰使PtMG带上-COOH官能团,通过共价连接负载人血清白蛋白(HAS)-钆(Gd)杂化物 (HGd);最后用ICG表面附着。此ICG-PtMGs@HGd平台,综合了封装、核壳结构及表面附着3种改性方法,将ICG的光动力效应、金纳米壳的光热效应、Pt的类过氧化氢酶活性及HGd的成像能力融合在一起。将ICG-PtMGs@HGd用于体内抗肿瘤时,观察到增强的光疗效应。可有效抑制肿瘤的生长和转移。LI等[66]将铝基MOFs MIL-121在440 ℃热处理,再通过氨解反应将光疗剂接枝于MOFs上。热解后的MIL-121形成的酸酐基团为光疗剂提供了反应位点,并且具有高比表面积和大孔径,有助于光动力产物活性氧的扩散,产生了具有良好的抗菌效果。有研究也通过“多合一”策略,搭建了光热、光动力联合治疗平台,并富集了成像及化疗等多种功能[67-70]。"


2.2.8 不同结构光疗MOFs的比较和选择 通过不同方法合成的MOFs结构,见图4。在结构稳定性方面,封装和核壳结构可以有效保护光疗剂,最大化生物可用性,再配合靶向标志物的负载,可以达到精准靶向。而对于封装和部分原位还原产生的MOFs结构,由于光疗剂位于MOFs载体表面,在应用过程中可能发生早接触氧后提前反应。但从另一方面讲,后者因为光疗剂没有占据MOFs内部空间,为活性氧或氧气等的转运提供了场所,加强了光热/光动力联合治疗的协同效应。从合成方法上讲,封装和表面附着的合成步骤最为简单,形成核壳结构、原位还原和热解MOFs的反应过程和条件较复杂。光疗MOFs的临床转化要求兼顾合成成本与产品稳定性,因此需要进一步探究大规模、简便可行的合成方法。 在具体的光疗MOFs的设计中,需要综合考虑多个元素。首先应从研究目的出发选择合适的光疗剂和MOFs。如MOFs要用于抗菌时,可选择能释放具有天然抗菌性的Cu2+和Zn2+的MOFs/光疗剂以增强抗感染效果。其次,通过二者的物理化学性质确定要形成的产物结构。比如选择Au为光热剂,就难以通过改变MOFs骨架的方法形成改性产物,因为Au难以在卟啉中形成中心配位 [45],普鲁士蓝可以与UiO-66形成稳定的核壳结构,但与PCN-224组装则会导致MOFs的结构崩解 [56]。部分光热剂和MOFs载体没有匹配的官能团,就需要对其进行官能化改性。因此,设计光疗MOFs时,应综合考虑成分选择、合成方法的适用性、成本效益、以及应用领域的特定需求。这种多维度的考量将有助于开发出既有效又经济的治疗方案。 "

| [1] NG KK, ZHENG G. Molecular interactions in organic nanoparticles for phototheranostic applications. Chem Rev. 2015;115(19):11012-11042. [2] BEIK J, ABED Z, GHOREISHI FS, et al. Nanotechnology in hyperthermia cancer therapy: from fundamental principles to advanced applications. J Control Release. 2016;235:205-221. [3] HOU YJ, YANG XX, LIU RQ, et al. Pathological mechanism of photodynamic therapy and photothermal therapy based on nanoparticles. Int J Nanomedicine. 2020;15:6827-6838. [4] DOLMANS DE, FUKUMURA D, JAIN RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3(5):380-387. [5] MøLLER KI, KONGSHOJ B, PHILIPSEN PA, et al. How finsen’s light cured lupus vulgaris. Photodermatol Photoimmunol Photomed. 2005;21(3):118-124. [6] DOUGHERTY TJ, GRINDEY GB, FIEL R, et al. Photoradiation therapy. II. Cure of animal tumors with hematoporphyrin and light. J Natl Cancer Inst. 1975;55(1):115-121. [7] LI X, LOVELL JF, YOON J, et al. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol. 2020;17(11):657-674. [8] CHEN F, CAI W. Nanomedicine for targeted photothermal cancer therapy: where are we now? Nanomedicine (Lond). 2015;10(1):1-3. [9] VOGL TJ, STRAUB R, EICHLER K, et al. Colorectal carcinoma metastases in liver: laser-induced interstitial thermotherapy--local tumor control rate and survival data. Radiology. 2004;230(2):450-458. [10] LI X, FERREL GL, GUERRA MC, et al. Preliminary safety and efficacy results of laser immunotherapy for the treatment of metastatic breast cancer patients. Photochem Photobiol Sci. 2011;10(5):817-821. [11] RASTINEHAD AR, ANASTOS H, WAJSWOL E, et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc Natl Acad Sci U S A. 2019;116(37):18590-18596. [12] ZHANG Z, XU W, KANG M, et al. An all-round athlete on the track of phototheranostics: subtly regulating the balance between radiative and nonradiative decays for multimodal imaging-guided synergistic therapy. Adv Mater. 2020;32(36):e2003210. [13] LI J, LIU X, TAN L, et al. Zinc-doped Prussian blue enhances photothermal clearance of Staphylococcus aureus and promotes tissue repair in infected wounds. Nat Commun. 2019;10(1):4490. [14] KWIATKOWSKI S, KNAP B, PRZYSTUPSKI D, et al. Photodynamic therapy-mechanisms, photosensitizers and combinations. Biomed Pharmacother. 2018;106:1098-1107. [15] LI Y, ZHAO L, LI XF. Hypoxia and the tumor microenvironment. Technol Cancer Res Treat. 2021;20:15330338211036304. [16] KOWALSKI CH, MORELLI KA, SCHULTZ D, et al. Fungal biofilm architecture produces hypoxic microenvironments that drive antifungal resistance. Proc Natl Acad Sci U S A. 2020;117(36):22473-22483. [17] TAN L, LI J, LIU X, et al. Rapid biofilm eradication on bone implants using red phosphorus and near-infrared light. Adv Mater. 2018;30(31): e1801808. [18] YANG X, WANG D, SHI Y, et al. Black phosphorus nanosheets immobilizing Ce6 for imaging-guided photothermal/photodynamic cancer therapy. ACS Appl Mater Interfaces. 2018;10(15):12431-12440. [19] ZHENG Q, LIU X, ZHENG Y, et al. The recent progress on metal-organic frameworks for phototherapy. Chem Soc Rev. 2021;50(8):5086-5125. [20] DUAN H, WANG F, XU W, et al. Recent advances in the nanoarchitectonics of metal-organic frameworks for light-activated tumor therapy. Dalton Trans. 2023;52(44):16085-16102. [21] WANG Y, YAN J, WEN N, et al. Metal-organic frameworks for stimuli-responsive drug delivery. Biomaterials. 2020;230:119619. [22] KARAMI A, MOHAMED O, AHMED A, et al. Recent advances in metal-organic frameworks as anticancer drug delivery systems: a review. Anticancer Agents Med Chem. 2021;21(18):2487-2504. [23] OSTERRIETH JWM, FAIREN-JIMENEZ D. Metal-organic framework composites for theragnostics and drug delivery applications. Biotechnol J. 2021;16(2):e2000005. [24] HAIDER J, SHAHZADI A, AKBAR MU, et al. A review of synthesis, fabrication, and emerging biomedical applications of metal-organic frameworks. Biomater Adv. 2022;140:213049. [25] SONG Y, WANG L, XIE Z. Metal-organic frameworks for photodynamic therapy: emerging synergistic cancer therapy. Biotechnol J. 2021;16(2): e1900382. [26] LIU J, HUANG J, ZHANG L, et al. Multifunctional metal-organic framework heterostructures for enhanced cancer therapy. Chem Soc Rev. 2021;50(2): 1188-1218. [27] WANG S, CHEN W, JIANG C, et al. Nanoscaled porphyrinic metal-organic framework for photodynamic/photothermal therapy of tumor. Electrophoresis. 2019;40(16-17):2204-2210. [28] 王刚,雷梦颖,周艳林,等.基于光热治疗和光动力治疗的光学疗法用于肿瘤治疗[J].化学试剂,2022,44(4):504-513. [29] 郭彩霞,马小杰,王博.金属有机框架基复合材料的制备及其光热性能研究[J].化学学报,2021,79(8):967-985. [30] LI R, CHEN T, PAN X. Metal-organic-framework-based materials for antimicrobial applications. ACS Nano. 2021;15(3):3808-3848. [31] SIKDER A, CHAUDHURI A, MONDAL S, et al. Recent advances on stimuli-responsive combination therapy against multidrug-resistant bacteria and biofilm. ACS Appl Bio Mater. 2021;4(6):4667-4683. [32] BUSQUETS MA, ESTELRICH J. Prussian blue nanoparticles: synthesis, surface modification, and biomedical applications. Drug Discov Today. 2020;25(8): 1431-1443. [33] YU W, ZHEN W, ZHANG Q, et al. Porphyrin-based metal-organic framework compounds as promising nanomedicines in photodynamic therapy. ChemMedChem. 2020;15(19):1766-1775. [34] KANG W, TIAN Y, ZHAO Y, et al. Applications of nanocomposites based on zeolitic imidazolate framework-8 in photodynamic and synergistic anti-tumor therapy. RSC Adv. 2022;12(26):16927-16941. [35] LIN Z, LIAO D, JIANG C, et al. Current status and prospects of MIL-based MOF materials for biomedicine applications. RSC Med Chem. 2023;14(10): 1914-1933. [36] LAN M, ZHAO S, LIU W, et al. Photosensitizers for Photodynamic Therapy. Adv Healthc Mater. 2019;8(13):e1900132. [37] LI C, CHENG Y, LI D, et al. Antitumor applications of photothermal agents and photothermal synergistic therapies. Int J Mol Sci. 2022;23(14):7909. [38] HE C, LIU D, LIN W. Nanomedicine applications of hybrid nanomaterials built from metal-ligand coordination bonds: nanoscale metal-organic frameworks and nanoscale coordination polymers. Chem Rev. 2015;115(19):11079-11108. [39] WANG S, MCGUIRK CM, D’AQUINO A, et al. Metal-organic framework nanoparticles. Adv Mater. 2018;30(37):e1800202. [40] LI B, WANG X, CHEN L, et al. Ultrathin Cu-TCPP MOF nanosheets: a new theragnostic nanoplatform with magnetic resonance/near-infrared thermal imaging for synergistic phototherapy of cancers. Theranostics. 2018;8(15): 4086-4096. [41] WANG C, XIONG C, LI Z, et al. Defect-engineered porphyrinic metal-organic framework nanoparticles for targeted multimodal cancer phototheranostics. Chem Commun (Camb). 2021;57(33):4035-4038. [42] HAN D, HAN Y, LI J, et al. Enhanced photocatalytic activity and photothermal effects of cu-doped metal-organic frameworks for rapid treatment of bacteria-infected wounds. Appl Catal B. 2020;261:118248. [43] ZHU W, LIU Y, YANG Z, et al. Albumin/sulfonamide stabilized iron porphyrin metal organic framework nanocomposites: targeting tumor hypoxia by carbonic anhydrase IX inhibition and T(1)-T(2) dual mode MRI guided photodynamic/photothermal therapy. J Mater Chem B. 2018;6(2):265-276. [44] ZHU X, XIONG J, WANG Z, et al. Metallic copper-containing composite photocatalysts: fundamental, materials design, and photoredox applications. Small Methods. 2022;6(2):e2101001. [45] YIN XB, ZHANG H. Mixed-ligand metal-organic frameworks for all-in-one theranostics with controlled drug delivery and enhanced photodynamic therapy. ACS Appli Mater Interfaces. 2022(23):14. [46] LELOUCHE SNK, BIGLIONE C, HORCAJADA P. Advances in plasmonic-based MOF composites, their bio-applications, and perspectives in this field. Expert Opin Drug Deliv. 2022;19(11):1417-1434. [47] YANG M, ZHANG J, SHI W, et al. Recent advances in metal-organic frameworks and their composites for the phototherapy of skin wounds. J Mater Chem B. 2022;10(25):4695-4713. [48] YANG P, MEN Y, TIAN Y, et al. Metal-organic framework nanoparticles with near-infrared dye for multimodal imaging and guided phototherapy. ACS Appl Mater Interfaces. 2019;11(12):11209-11219. [49] YANG C, XU J, YANG D, et al. ICG@ ZIF-8: One-step encapsulation of indocyanine green in ZIF-8 and use as a therapeutic nanoplatform. Chin Chem Lett. 2018;29(9):1421-1424. [50] WANG T, LI S, ZOU Z, et al. A zeolitic imidazolate framework-8-based indocyanine green theranostic agent for infrared fluorescence imaging and photothermal therapy. J Mater Chem B. 2018;6(23):3914-3921. [51] GE X, WONG R, ANISA A, et al. Recent development of metal-organic framework nanocomposites for biomedical applications. Biomaterials. 2022;281:121322. [52] ZENG Y, XU G, KONG X, et al. Recent advances of the core-shell MOFs in tumour therapy. Int J Pharm. 2022;627:122228. [53] CAI X, ZHAO Y, WANG L, et al. Synthesis of Au@MOF core-shell hybrids for enhanced photodynamic/photothermal therapy. J Mater Chem B. 2021;9(33):6646-6657. [54] LI RT, ZHU YD, LI WY, et al. Synergistic photothermal-photodynamic-chemotherapy toward breast cancer based on a liposome-coated core-shell AuNS@NMOFs nanocomposite encapsulated with gambogic acid. J Nanobiotechnology. 2022;20(1):212. [55] ZHOU Z, ZHAO J, DI Z, et al. Core-shell gold nanorod@mesoporous-MOF heterostructures for combinational phototherapy. Nanoscale. 2021;13(1): 131-137. [56] LUO Y, LI J, LIU X, et al. Dual metal-organic framework heterointerface. ACS Cent Sci. 2019;5(9):1591-1601. [57] ZHAO X, CHANG L, HU Y, et al. Preparation of photocatalytic and antibacterial MOF nanozyme used for infected diabetic wound healing. ACS Appl Mater Interfaces. 2022;14(16):18194-18208. [58] DU T, XIAO Z, ZHANG G, et al. An injectable multifunctional hydrogel for eradication of bacterial biofilms and wound healing. Acta Biomater. 2023;161:112-133. [59] WANG Q, WANG Z, LI Z, et al. Controlled growth and shape-directed self-assembly of gold nanoarrows. Sci Adv. 2017;3(10):e1701183. [60] GAO S, ZHENG P, LI Z, et al. Biomimetic O(2)-Evolving metal-organic framework nanoplatform for highly efficient photodynamic therapy against hypoxic tumor. Biomaterials. 2018;178:83-94. [61] XIANG H, FENG W, CHEN Y. Single-atom catalysts in catalytic biomedicine. Adv Mater. 2020;32(8):e1905994. [62] YANG P, TIAN Y, MEN Y, et al. Metal-organic frameworks-derived carbon nanoparticles for photoacoustic imaging-guided photothermal/photodynamic combined therapy. ACS Appl Mater Interfaces. 2018;10(49): 42039-42049. [63] YANG Y, ZHU D, LIU Y, et al. Platinum-carbon-integrated nanozymes for enhanced tumor photodynamic and photothermal therapy. Nanoscale. 2020;12(25):13548-13557. [64] LIU J, LIU T, DU P, et al. Metal-organic framework (MOF) hybrid as a tandem catalyst for enhanced therapy against hypoxic tumor cells. Angew Chem Int Ed Engl. 2019;58(23):7808-7812. [65] YOU Q, ZHANG K, LIU J, et al. Persistent regulation of tumor hypoxia microenvironment via a bioinspired Pt-based oxygen nanogenerator for multimodal imaging-guided synergistic phototherapy. Adv Sci (Weinh). 2020;7(17):1903341. [66] LI J, ZHANG Q, CHEN Z, et al. Postsynthetic modification of thermo-treated metal-organic framework for combined photothermal/photodynamic antibacterial therapy. ACS Appl Mater Interfaces. 2024;16(7):8459-8473. [67] FENG L, CHEN M, LI R, et al. Biodegradable oxygen-producing manganese-chelated metal organic frameworks for tumor-targeted synergistic chemo/photothermal/ photodynamic therapy. Acta Biomater. 2022;138:463-477. [68] FU W, LU Q, XING S, et al. Iron-doped metal-zinc-centered organic framework mesoporous carbon derivatives for single-wavelength NIR-activated photothermal/photodynamic synergistic therapy. Langmuir. 2023; 39(18):6505-6513. [69] DENG X, ZHAO R, SONG Q, et al. Synthesis of dual-stimuli responsive metal organic framework-coated iridium oxide nanocomposite functionalized with tumor targeting albumin-folate for synergistic photodynamic/photothermal cancer therapy. Drug Deliv. 2022;29(1):3142-3154. [70] HU X, LU Y, DONG C, et al. A Ru(II) polypyridyl alkyne complex based metal-organic frameworks for combined photodynamic/photothermal/chemotherapy. Chemistry. 2020;26(7):1668-1675. [71] ZHANG F, LIU Y, LEI J, et al. Metal-organic-framework-derived carbon nanostructures for site-specific dual-modality photothermal/photodynamic thrombus therapy. Adv Sci (Weinh). 2019;6(17):1901378. [72] HOSHYAR N, GRAY S, HAN H, et al. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine (Lond). 2016;11(6):673-692. [73] MARSHALL CR, STAUDHAMMER SA, BROZEK CK. Size control over metal-organic framework porous nanocrystals. Chem Sci. 2019;10(41): 9396-9408. [74] LINNANE E, HADDAD S, MELLE F, et al. The uptake of metal-organic frameworks: a journey into the cell. Chem Soc Rev. 2022;51(14):6065-6086. [75] LIN J, WANG S, HUANG P, et al. Photosensitizer-loaded gold vesicles with strong plasmonic coupling effect for imaging-guided photothermal/photodynamic therapy. ACS Nano. 2013;7(6):5320-5329. [76] CHEN YZ, WANG ZU, WANG H, et al. Singlet oxygen-engaged selective photo-oxidation over Pt nanocrystals/porphyrinic MOF: the roles of photothermal effect and Pt electronic state. J Am Chem Soc. 2017;139(5):2035-2044. |
| [1] | Chen Yaodong, Ren Jiayi, Cao Jingwei, Fan Wenwen, Chen Wu. Near-infrared photoresponsive h-PCuNF nanoparticles mediate multimodal therapeutics against malignant tumors [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 780-788. |
| [2] | Gui Bin, Jiang Nan, Huang Xin, Zhong Fanglu, Wang Zhiwen, Liu Qianhui, Guo Yuxin, Chen Yueying, Pu Huan, Deng Qing. Application of nanoprobe based on aggregation-induced luminescence in photothermal diagnosis and treatment of prostate cancer [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3400-3409. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||