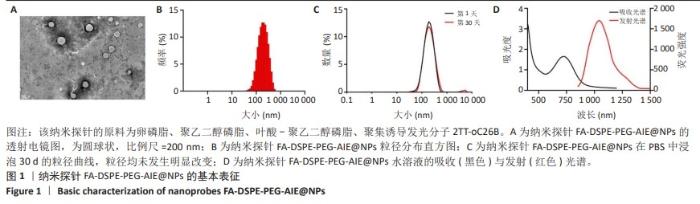
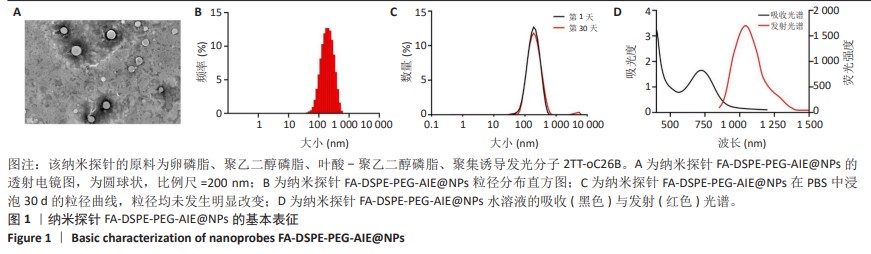
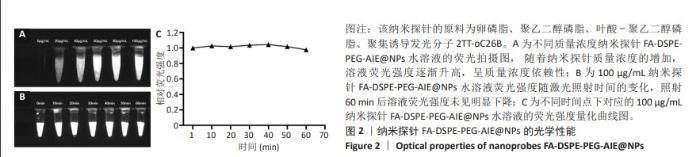
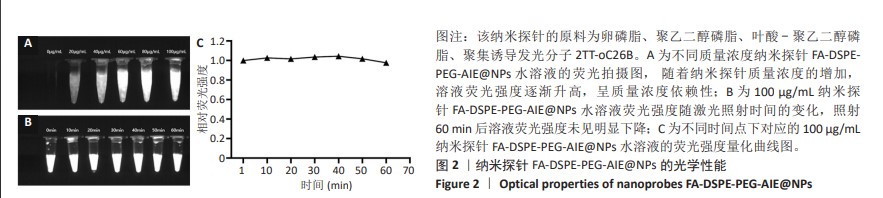
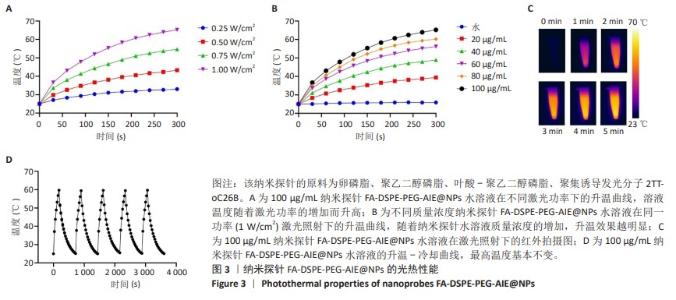
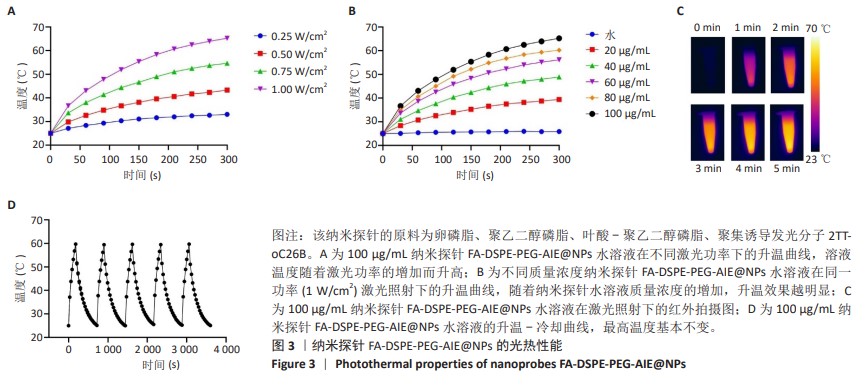
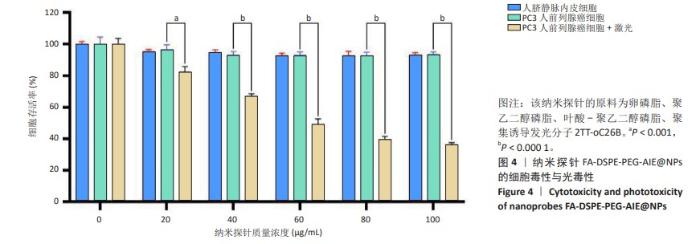
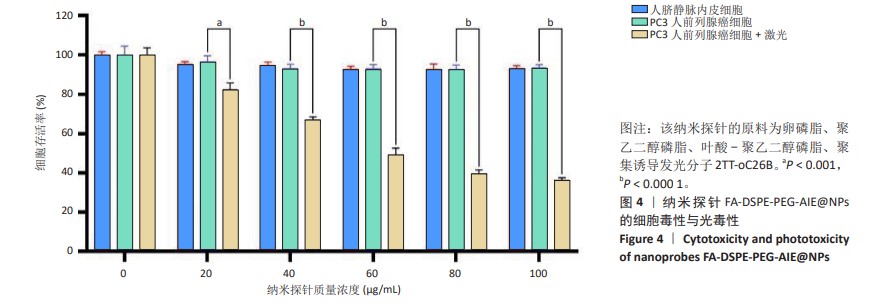
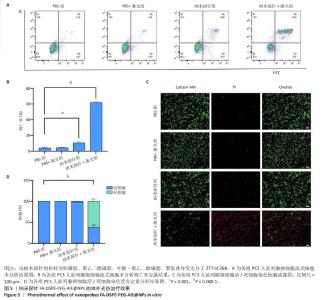
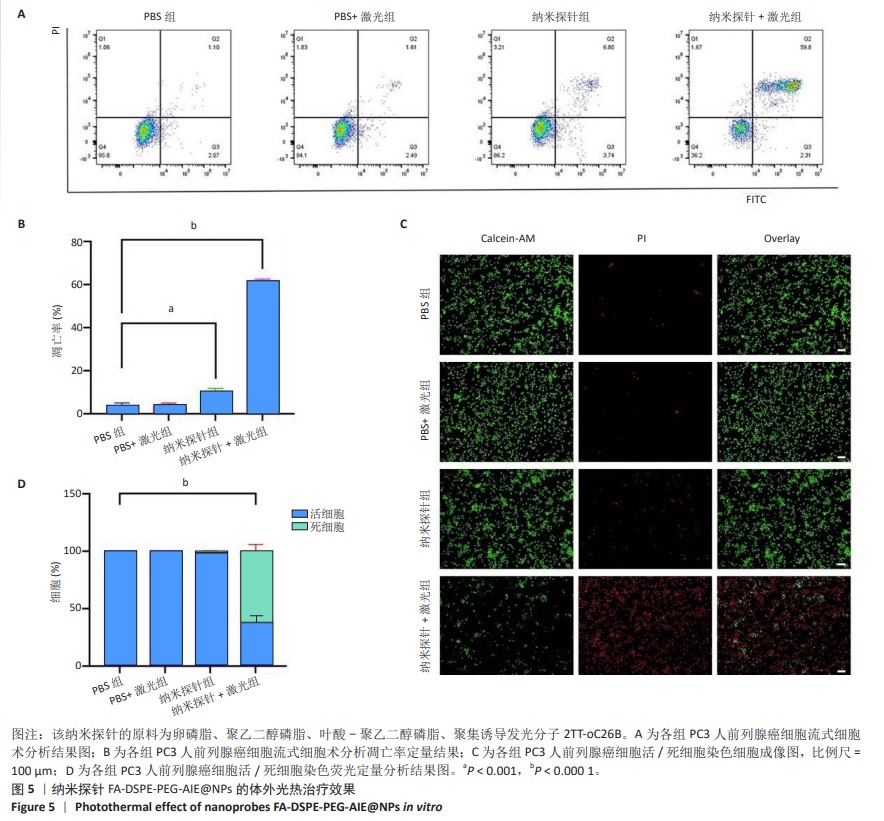
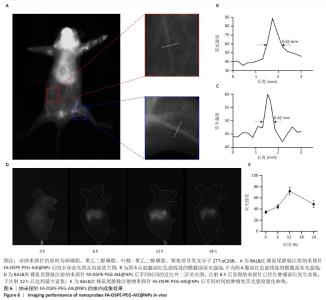
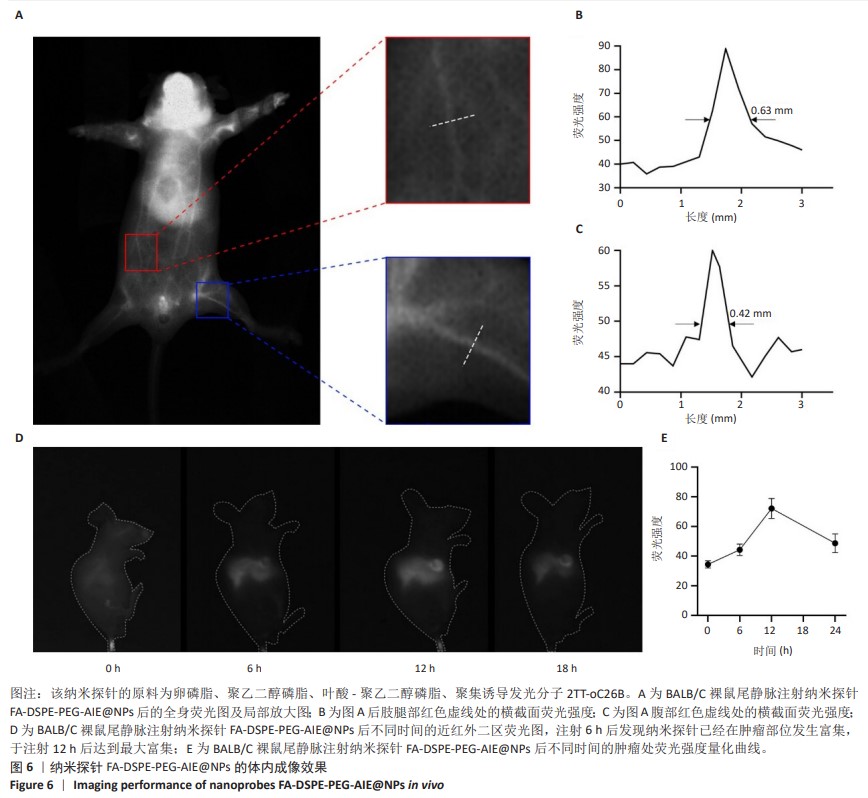
[1] SUNG H, FERLAY J, SIEGEL RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249.
[2] 赫捷,陈万青,李霓,等.中国前列腺癌筛查与早诊早治指南(2022,北京)[J].中华肿瘤杂志,2022,44(1):29-53.
[3] FENG RM, ZONG YN, CAO SM, et al. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond). 2019;39(1):22.
[4] 吴炯,胡嘉华,施美芳,等.前列腺癌生物标志物研究进展[J].检验医学,2023,38(2):190-195.
[5] MOTTET N, VAN DEN BERGH RCN, BRIERS E, et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2021;79(2):243-262.
[6] 孟小丽,康飞,全志永,等.多参数MRI联合68Ga-PSMA PET/CT诊断临床显著性前列腺癌[J].中华核医学与分子影像杂志,2024,44(1): 25-29.
[7] 岳丽娜,贺雅萍,樊佳,等.多参数磁共振成像技术诊断PCa研究进展[J].医学影像学杂志,2024,34(2):128-131.
[8] HEESAKKERS J, FARAG F, BAUER RM, et al. Pathophysiology and Contributing Factors in Postprostatectomy Incontinence: A Review. Eur Urol. 2017;71(6):936-944.
[9] LEI Z, ZHANG F. Molecular Engineering of NIR-II Fluorophores for Improved Biomedical Detection. Angew Chem Int Ed Engl. 2021;60(30): 16294-16308.
[10] LI B, ZHAO M, FENG L, et al. Organic NIR-II molecule with long blood half-life for in vivo dynamic vascular imaging. Nat Commun. 2020;11(1): 3102.
[11] MEI J, HUANG Y, TIAN H . Progress and Trends in AIE-Based Bioprobes: A Brief Overview. ACS Appl Mater Interfaces. 2018;10(15):12217-12261.
[12] LUO J, XIE Z, LAM JW, et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem Commun (Camb). 2001;(18):1740-1741.
[13] HONG Y, LAM JW, TANG BZ. Aggregation-induced emission: phenomenon, mechanism and applications. Chem Commun (Camb). 2009;(29):4332-4353.
[14] MEI J, LEUNG NL, KWOK RT, et al. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem Rev. 2015;115(21): 11718-11940.
[15] LIN X, LI W, WEN Y, et al. Aggregation-induced emission (AIE)-Based nanocomposites for intracellular biological process monitoring and photodynamic therapy. Biomaterials. 2022;287:121603.
[16] LI Y, HE D, ZHENG Q, et al. Single-Component Photochemical Afterglow Near-Infrared Luminescent Nano-Photosensitizers: Bioimaging and Photodynamic Therapy. Adv Healthc Mater. 2024:13(13):e2304392.
[17] HE X, YU J, YIN R, et al. An AIEgen and Iodine Double-Ornamented Platinum(II) Complex for Bimodal Imaging-Guided Chemo-Photodynamic Combination Therapy. Small. 2024;20(23):e2309894.
[18] 何玉芳,唐文洁,江新青.具有聚集诱导发光特性的纳米材料在肿瘤诊断和治疗中的研究进展[J].中华核医学与分子影像杂志, 2020,40(6):373-377.
[19] SONG S, ZHAO Y, KANG M, et al. An NIR-II Excitable AIE Small Molecule with Multimodal Phototheranostic Features for Orthotopic Breast Cancer Treatment. Adv Mater. 2024;36(14):e2309748.
[20] CUI M, TANG D, WANG B, et al. Bioorthogonal Guided Activation of cGAS-STING by AIE Photosensitizer Nanoparticles for Targeted Tumor Therapy and Imaging. Adv Mater. 2023;35(52):e2305668.
[21] LIU S, LI Y, KWOK RTK, et al. Structural and process controls of AIEgens for NIR-II theranostics. Chem Sci. 2020;12(10):3427-3436.
[22] LI Y, CAI Z, LIU S, et al. Design of AIEgens for near-infrared IIb imaging through structural modulation at molecular and morphological levels. Nat Commun. 2020;11(1):1255.
[23] CUI S, FAN S, TAN H, et al. Ultra-homogeneous NIR-II fluorescent self-assembled nanoprobe with AIE properties for photothermal therapy of prostate cancer. Nanoscale. 2021;13(37):15569-15575.
[24] 李文兰,王文渊,任文秀,等.制备近红外光响应性仿生纳米探针及在乳腺癌光热诊疗中的应用[J]. 中国组织工程研究,2024,28(5): 669-675.
[25] GENG B, HU J, LI Y, et al. Near-infrared phosphorescent carbon dots for sonodynamic precision tumor therapy. Nat Commun. 2022;13(1):5735.
[26] LIANG S, HU D, LI G, et al. NIR-II fluorescence visualization of ultrasound-induced blood-brain barrier opening for enhanced photothermal therapy against glioblastoma using indocyanine green microbubbles. Sci Bull (Beijing). 2022;67(22):2316-2326.
[27] HU Z, FANG C, LI B, et al. First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows. Nat Biomed Eng. 2020;4(3):259-271.
[28] WU GL, LIU F, LI N, et al. Tumor Microenvironment-Responsive One-for-All Molecular-Engineered Nanoplatform Enables NIR-II Fluorescence Imaging-Guided Combinational Cancer Therapy. Anal Chem. 2023; 95(47):17372-17383.
[29] WANG Y, FENG M, LIN B, et al. MET-targeted NIR II luminescence diagnosis and up-conversion guided photodynamic therapy for triple-negative breast cancer based on a lanthanide nanoprobe. Nanoscale. 2021;13(43):18125-18133.
[30] XIE Q, LIU J, CHEN B, et al. NIR-II Fluorescent Activatable Drug Delivery Nanoplatform for Cancer-Targeted Combined Photodynamic and Chemotherapy. ACS Appl Bio Mater. 2022;5(2):711-722.
[31] MOURATIDIS PX, RIVENS I, TER HAAR G. A study of thermal dose-induced autophagy, apoptosis and necroptosis in colon cancer cells. Int J Hyperthermia. 2015;31(5):476-488.
[32] SPYRATOU E, MAKROPOULOU M, EFSTATHOPOULOS EP, et al. Recent Advances in Cancer Therapy Based on Dual Mode Gold Nanoparticles. Cancers (Basel). 2017;9(12):173.
[33] ASSARAF YG, LEAMON C, PREDDY JA. The folate receptor as a rational therapeutic target for personalized cancer treatment. Drug Resist Updat. 2014;17(4-6):89-95.
[34] MANDAL AK, WU X, FERREIRA JS, et al. Fluorescent sp(3) Defect-Tailored Carbon Nanotubes Enable NIR-II Single Particle Imaging in Live Brain Slices at Ultra-Low Excitation Doses. Sci Rep. 2020;10(1):5286.
[35] DAHAL D, RAY P, PAN D. Unlocking the power of optical imaging in the second biological window: Structuring near-infrared II materials from organic molecules to nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021;13(6):e1734.
[36] LI B, WANG G, TONG Y, et al. Noninvasive Gastrointestinal Tract Imaging Using BSA-Ag(2)Te Quantum Dots as a CT/NIR-II Fluorescence Dual-Modal Imaging Probe in Vivo. ACS Biomater Sci Eng. 2023;9(1):449-457.
[37] CHEN HJ, QIN Y, WANG ZG, et al. An Activatable and Reversible Virus-Mimicking NIR-II Nanoprobe for Monitoring the Progression of Viral Encephalitis. Angew Chem Int Ed Engl. 2022;61(39):e202210285.
[38] CHEN LL, ZHAO L, WANG ZG, et al. Near-Infrared-II Quantum Dots for In Vivo Imaging and Cancer Therapy. Small. 2022;18(8):e2104567.
[39] ZHANG X, HE S, DING B, et al. Synergistic strategy of rare-earth doped nanoparticles for NIR-II biomedical imaging. J Mater Chem B. 2021;9(44):9116-9122.
[40] WEI Z, DUAN G, HUANG B, et al. Rapidly liver-clearable rare-earth core-shell nanoprobe for dual-modal breast cancer imaging in the second near-infrared window. J Nanobiotechnology. 2021;19(1):369.
[41] ZHU H, DING X, WANG C, et al. Preparation of rare earth-doped nano-fluorescent materials in the second near-infrared region and their application in biological imaging. J Mater Chem B. 2024;12(8):1947-1972.
[42] LIU Y, LI Y, KOO S, et al. Versatile Types of Inorganic/Organic NIR-IIa/IIb Fluorophores: From Strategic Design toward Molecular Imaging and Theranostics. Chem Rev. 2022;122(1):209-268.
[43] WANG Z, WANG X, WAN JB, et al. Optical Imaging in the Second Near Infrared Window for Vascular Bioimaging. Small. 2021;17(43): e2103780.
[44] WANG Q, TIAN L, XU J, et al. Multifunctional supramolecular vesicles for combined photothermal/photodynamic/hypoxia-activated chemotherapy. Chem Commun (Camb). 2018;54(73):10328-10331. |