Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (1): 128-135.doi: 10.12307/2024.732
Previous Articles Next Articles
Effect of bone marrow fat on bone metastasis and quantitative evaluation by magnetic resonance imaging
Xie Qinglin, Zhang Xiaodong
- Department of Imaging, Third Affiliated Hospital of Southern Medical University (Guangdong Orthopedics Research Institute), Guangzhou 510630, Guangdong Province, China
-
Received:2023-11-02Accepted:2023-11-27Online:2025-01-08Published:2024-05-18 -
Contact:Zhang Xiaodong, MD, Chief physician, Department of Imaging, Third Affiliated Hospital of Southern Medical University (Guangdong Orthopedics Research Institute), Guangzhou 510630, Guangdong Province, China -
About author:Xie Qinglin, Department of Imaging, Third Affiliated Hospital of Southern Medical University (Guangdong Orthopedics Research Institute), Guangzhou 510630, Guangdong Province, China
CLC Number:
Cite this article
Xie Qinglin, Zhang Xiaodong. Effect of bone marrow fat on bone metastasis and quantitative evaluation by magnetic resonance imaging[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(1): 128-135.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
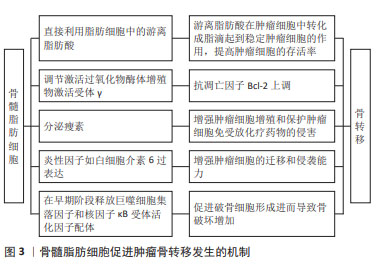
2.2 骨髓微环境在骨转移的发生中发挥重要作用 骨髓微环境是骨骼的微观结构,是骨构塑和骨重构发生的重要部位,主要由造血干细胞、间充质干细胞、内皮细胞、脂肪细胞、骨髓基质细胞、成骨细胞、破骨细胞及多种可溶性因子组成[13]。已有研究表明,骨髓中的脂肪细胞及其代谢产物能够在髓系恶性肿瘤发生中发挥重要作用[14-17]。 既往研究已经证实骨髓脂肪细胞能够促进髓系恶性肿瘤的发生,目前人们逐渐将关注点放到了骨髓脂肪细胞是否能够在骨转移瘤发生中发挥作用。DIRAT等[18]将4T1肿瘤细胞在体外与脂肪细胞共培养4 d,对照组为无脂肪细胞单独培养的4T1肿瘤细胞,将其分别注入到BALB/c小鼠体内,结果显示注射与脂肪细胞共培养的4T1肿瘤细胞的小鼠肺转移灶数量增多,结节数量和大小均显著增加,从而证实了脂肪细胞能够促进肿瘤细胞发生转移。 已经证实骨髓脂肪细胞能通过多种机制促进骨转移的发生。其一,肿瘤细胞能够利用骨髓脂肪细胞为自身提供能量。除了从头合成游离脂肪酸外,肿瘤细胞还能直接利用脂肪细胞中的游离脂肪酸[19]。游离脂肪酸能在肿瘤细胞中转化成脂滴起到稳定肿瘤细胞的作用,提高肿瘤细胞的存活率,且仅有脂肪细胞能为肿瘤细胞提供额外的游离脂肪酸作为能量来源,这种额外的能量来源对肿瘤细胞的存活而言起着至关重要的作用。同时脂肪细胞的分解机制在骨转移的发生中发挥重要作用。SCHELLER等[20]将骨髓脂肪细胞区分为“调节骨髓脂肪细胞”(regulated bone marrow adipocytes,rBMAs)和“组成性骨髓脂肪细胞”(constitutive bone marrow adipocytes,cBMAs)。一般来说,肿瘤细胞会更容易转移到富含具有较小且稳定性较差的节骨髓脂肪细胞区域(股骨近端、髋部和腰椎等)。其二,骨髓脂肪细胞能够促进肿瘤细胞增殖和增强对放、化疗的抵抗力。骨髓间充质干细胞主要分化为成骨细胞和脂肪细胞,维持其分化平衡在保持骨骼稳定性中具有重要作用。过氧化物酶体增殖物激活受体γ(peroxisome proliferator-activated receptor-γ,PPARγ)是促进脂肪分化的关键转录因子,受到骨髓脂肪细胞的调节,主要由游离脂肪酸及其代谢产物激活,在间充质干细胞分化成脂肪细胞的2个阶段均升高,在成脂肪分化过程中起到重要作用[21]。同时,PPARγ能够与通过CD36和FABP4转运到细胞核中的游离脂肪酸结合,使抗凋亡因子Bcl-2上调[17]。 除此之外,成熟的骨髓脂肪细胞能够分泌瘦素调节间充质干细胞的分化,以牺牲成骨为代价促进分化为脂肪细胞[22]。同时,已经证实骨髓脂肪细胞分泌的瘦素能够激活PI3K/Akt和MEK/ERK信号通路,促进肿瘤细胞增殖以及保护肿瘤细胞免受放化疗药物的侵害[23]。其三,骨髓脂肪细胞产生的炎性因子也会影响骨转移的发生。研究表明,白细胞介素6在肿瘤周围脂肪细胞中的过表达能够增加ER阴性乳腺癌细胞的迁移和侵袭能力[24],DIRAT等[18]研究表明,与单独培养的肿瘤细胞对照组相比,与脂肪细胞共培养的肿瘤细胞中白细胞介素6 mRNA水平增加了5倍。其四,骨髓脂肪细胞能够促进破骨细胞形成进而导致骨破坏增加。正常情况下,成骨细胞和破骨细胞精确协调,以维持骨代谢的动态平衡,然而,有研究称骨髓脂肪细胞前体能够在早期阶段释放巨噬细胞集落刺激因子和核因子κB受体活化因子配体,这2个因子可以在共培养条件下诱导破骨细胞的形成,从而使骨破坏增加[25]。 骨髓脂肪细胞促进骨转移发生的机制见图3。"
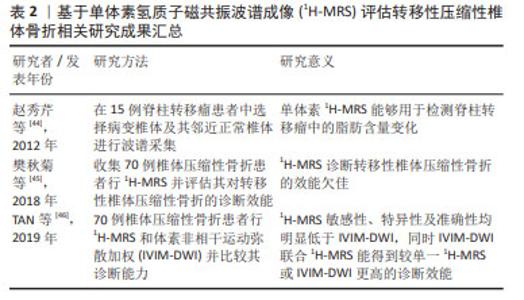
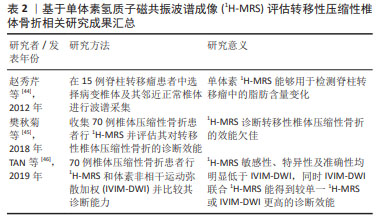
2.3 骨转移MRI诊断方法新进展 2.3.1 MRI定量成像技术 骨髓脂肪细胞在骨转移中发挥的作用已经被体外细胞实验所证实[18],如何在临床中通过检测骨髓脂肪含量的差异对骨转移患者进行诊断乃至预测成为了一个值得关注的问题。而MRI对发生骨转移时肿瘤细胞浸润骨髓微环境导致的骨髓内脂肪组织和水分子含量发生的改变非常敏感,在早期就能检测到信号改变。MRI和18FDG PET在诊断骨转移方面具有可比性,且均明显高于CT和骨闪烁显像[26-32],从而被广泛用于骨转移的诊断中。更重要的是,相比于其他骨转移的检查方法,目前仅有MRI能够做到定量脂肪含量。随着MRI技术不断地提高与发展,MRI诊断骨转移不再仅局限于解剖学诊断上,功能性MRI被越来越多地运用到骨转移的诊断中[33-39]。由于MRI常规序列的诊断往往具有一定的主观性,并且受到硬件设备以及技术因素的干扰,在这种情况下,磁共振定量成像技术在一定程度上使得获得的参数具有更高的稳定性和可信度[40],现阶段,一些新技术如磁共振波谱成像(magnetic resonance spectroscopy,MRS)和基于化学移位编码的水脂肪成像(chemical shift encoding-based water-fat imaging,CSE-MRI)也逐渐被应用于骨转移相关的研究中。 2.3.2 MRS 临床应用的磁共振波谱成像既可以是单体素采集也可以是多体素采集,现有的基于MRS定量骨髓脂肪的研究大多选择的是单体素氢质子磁共振波谱(1H-MRS)。骨髓由红骨髓和黄骨髓组成,骨髓1H-MRS谱线中水峰的峰值信号强度主要来源于红骨髓,而脂峰的峰值信号强度主要来源于黄骨髓。MRS技术能够通过高光谱分辨率从指定的局部感兴趣区域(VOI)获得数据,继而可以通过其共振频率对水和脂肪进行量化。MRS在采集方面主要采用点分辨光谱(PRESS)和受激回波采集模式(STEAM)序列。在评估脂肪方面,STEAM序列较PRESS而言具有明显的优势。首先,与STEAM序列相比,PRESS低估了脂肪的T2值,高估了脂肪分数,并且提供了不太一致的脂肪分数估计值,这可能是由于PRESS较STEAM序列对脂肪峰J耦合效应敏感性更高所导致的[41]。其次,由于骨髓通常具有很强的水信号[42],因此具有较短的T2水峰值,尽管PRESS 较STEAM序列得到的MRS具有更高的信噪比,但相比之下STEAM序列可以实现更短的TE值,可以减少T2加权信号损失,用于较短的T2水峰值[43]。 迄今为止,已存在几项基于1H-MRS定量骨髓脂肪分数值的研究,研究多集中于探讨1H-MRS在脊柱转移瘤中的应用价值。赵秀芹等[44]在15例脊柱转移瘤患者中选择弥漫性病变椎体及其邻近正常椎体进行波谱采集,将感兴趣区放在相应椎体的前中1/3,感兴趣区容积确定为1 cm×1 cm×1 cm-2 cm×2 cm×2 cm,测量两者的水峰和脂峰的高度并计算椎体脂肪与水的比值和脂肪分数,结果显示病变椎体的脂峰、脂肪与水的比值及脂肪分数较正常椎体降低,提示1H-MRS能够用于检测脊柱转移瘤中的脂肪含量变化。尽管此研究说明了1H-MRS能够定量椎体中的脂肪含量,但其对椎体恶性转移性病变的诊断效能目前仍被质疑。樊秋菊等[45]使用1H-MRS鉴别骨质疏松和转移性椎体压缩性骨折,选取的感兴趣区尽量在包含整个病灶的同时避开囊变及坏死区域,平均感兴趣区容积确定为(2.5±0.5) cm3,同样对两组的水峰和脂峰的高度进行测量并得出脂肪与水的比值和脂肪分数,结果显示1H-MRS诊断转移性椎体压缩性骨折的曲线下面积为0.73,其敏感性、特异性及准确性分别为87.50%,57.89%,71.43%。TAN等[46]对比1H-MRS和体素非相干运动弥散加权(intravoxel incoherent motion diffusion-weighted MR imaging,IVIM-DWI)MR成像在鉴别椎体良恶性骨折方面的效能,同样在避开坏死及囊变区域的同时将1H-MRS体素放置在病变椎体的STIR高信号中心并得出相应的脂肪与水的比值和脂肪分数,在IVIM参数图上则直接显示与1H-MRS体素对应的最大病灶面积,研究显示1H-MRS敏感性、特异性及准确性均明显低于IVIM-DWI,同时IVIM-DWI联合1H-MRS能得到较单一1H-MRS或IVIM-DWI更高的诊断效能。以上结果表明,尽管1H-MRS曾被认为能够用于提高椎体转移性病变的准确性,但在现有技术无法攻克的情况下,1H-MRS单独评估转移性压缩性椎体骨折的诊断效能仍较低,需要结合其他检查进行联合评估,在临床上通常只能作为一种辅助手段进行鉴别诊断。 目前国内外基于1H-MRS评估转移性压缩性椎体骨折的相关研究进展见表2。 "
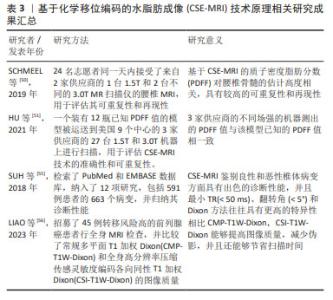
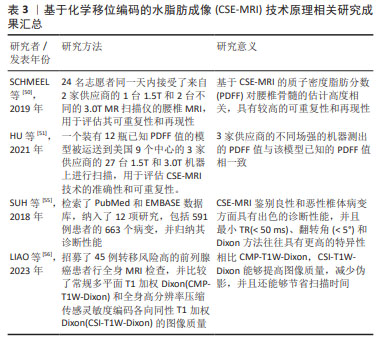
MRS由于其安全、准确、无创的特点目前被广泛用于定量检测活体组织中的骨髓脂肪及其代谢产物,已经成为最常用的骨髓定量方法。但MRS有其局限性,首先椎体中的松质骨骨小梁会导致水和脂肪的T2时间缩短,导致波谱图上水峰和脂峰的宽度增大,从而使脂峰与其周围的水峰难以辨别,造成定量的误差;其次MRS极大地受到所选取的感兴趣区容积的影响,椎体内的脂肪含量分布不均,既往研究证实在L3椎体内选择的感兴趣区容积位置不同,所测得的脂肪分数差异超过30%[47];与此同时还具有技术难度大、一次扫描仅能获得较小局部感兴趣区域的代谢物信息、扫描时间长患者难以配合、容易受到运动伪影的干扰以及后处理繁琐耗时的问题,因此在临床推行中存在着一定的困难。 2.3.3 CSE-MRI 由于MRS存在上述局限性,现在多使用CSE-MRI尤其是多回波校正Dixon(modified Dixon,mDixon)技术进行替代。Dixon技术是一种多梯度回波技术,完成一次图像采集能获得6个回波,同时结合7峰值脂肪模型,使得水和脂肪信号得以在正相位和反相位相分离,相比于传统脂肪测定技术更加简便快捷且无创。Dixon技术需要满足主磁场B0均匀,不会出现磁敏感伪影。在早期使用过程中,由于当时的磁共振技术局限导致此条件不能被满足,很容易出现伪影。随着现代磁体设计和匀场技术的改进,目前已经能够达到Dixon技术所需要的条件[48]。现在常使用两点式及多点式Dixon方法,能够通过一次扫描即可获得水相(Water)、同相位(In-Phase,IP)、反相位(Out-of-Phase,OP)、脂相(Fat)4组图像,不仅使得扫描速度明显加快,而且伪影减少,图像质量得到了提升[48-49]。尽管可以使用多种核磁共振生物标志物进行脂肪定量,但在2012年ISMRM脂水分离研讨会中已经就这个问题达成共识,即质子密度脂肪分数(proton density fat fraction,PDFF)是目前来说最有意义和价值的核磁共振生物标志物[43]。不同于MRS在后处理中对操作者相关技术水平有较高的要求,CSE-MRI扫描后得到的PDFF在后处理上具有简便和易操作的特性,能够直接从PDFF图上获取PDFF值,更容易被临床医生理解接受,并且CSE-MRI获得的是3D的PDFF图,能够获得任意一个层面的一定面积进行脂肪定量,考虑到了椎体脂肪空间分布不均匀的问题。从方法原理上,CSE-MRI具有比MRS更精确的脂肪定量能力。 近年来,CSE-MRI技术逐步发展为GE的“IDEAL-IQ”技术、飞利浦的“mDixon-Quant”技术及西门子的“Liver Lab”技术,针对在不同供应商技术及场强下获得的PDFF值是否具有可重复性这一问题,目前已有大量的研究者对其进行了验证。SCHMEEL等[50]研究证实基CSE-MRI的椎体骨髓定量PDFF在不同观察者、场强以及成像平台中具有高度的准确性和可重复性。这项研究招募了24名成年志愿者在同一天接受了腰椎MRI检查,使用来自GE和飞利浦两家供应商的1台1.5T和2台不同的3.0T MR扫描仪,结果表明CSE-MRI在不同设备采集到的高质量PDFF图具有高度准确性和可重复性。HU等[51]将一个装有已知PDFF值的模型运送到美国的9个中心,在3家供应商的27台1.5T和3.0T机器上进行了160次MRI检查,结果显示3家供应商的不同场强设备测出的PDFF值与该模型已知的PDFF值是相符的,提示CSE-MRI技术具有精确定量脂肪的能力,能够反映骨髓脂肪含量[49],在临床上可用于解决评估骨髓病变的问题,区分骨髓浸润性和非浸润性病变。 然而,现阶段CSE-MRI技术仍然存在缺陷,目前已知T1偏倚和T2*效应等多种混杂因素均会影响同相/对相MRI量化骨髓脂肪含量的能力。首先,骨髓中水和脂肪成分的T1弛豫时间具有较大的差异[52];其次,椎骨骨髓的特点是T2*弛豫时间短,而水和脂肪成分的T2*弛豫时间并不相同,尤其在高场强的情况下会混淆骨髓脂肪的定量[53];再者,尽管实际上脂肪通常存在多个光谱峰,但多数传统的化学位移编码MRI技术通常假设脂肪只具有单个光谱峰,这将会导致部分脂肪信号被误认为是由于水所导致的,从而出现明显的定量误差[54]。通过改变TR及翻转角等参数可以在一定程度上纠正这些误差。SUH等[55]检索了PubMed和EMBASE数据库用以比较传统梯度回波CSE-MRI或Dixon方法区分良性和恶性椎体骨髓病变的诊断准确度,对收集到的数据进行Meta分析,结果显示最小TR(< 50 ms)、翻转角(< 5°)和Dixon方法往往具有更高的特异性。近期LIAO等[56]的研究结果表明,相比常规多平面T1加权Dixon(CMP-T1W-Dixon),全身高分辨率压缩传感灵敏度编码各向同性T1加权Dixon(CSI-T1W-Dixon)能够提供更好的定量对比度噪声比(CNR),在检测病变时具有更高的灵敏度,有望未来取代CMP-T1W-Dixon在临床上评估骨转移。 作者总结了目前CSE-MRI技术原理相关研究成果见表3。 "
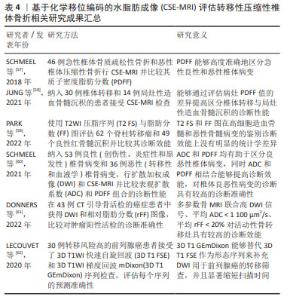
近年来有诸多研究探讨基于CSE-MRI评估椎体恶性病变的诊断效能。在常规的形态学MRI上,椎体恶性病变的信号特征难以和许多椎体良性病变相鉴别,一旦发生恶性病变的患者被误诊,将极大地影响患者的治疗和预后,显著降低患者的生存率。因此,能否通过定量病变椎体PDFF值提高诊断椎体恶性病变的准确性成为了目前的热点。首先,对急性椎体骨质疏松性骨折和恶性椎体压缩性骨折进行鉴别在临床上十分常见,对治疗和预后都具有相当大的临床意义。然而使用常规磁共振成像进行检查时,急性期骨质疏松性骨折导致的反应性炎症和骨髓水肿的信号与恶性肿瘤中观察到的信号改变类似,因此常常难以进行区分,在这种情况下很多患者不得不行侵入性骨活检才能最终确诊。但近些年已经有不少学者关注到了基于CSE-MRI使用PDFF图像安全无创地通过评估椎骨骨髓脂肪含量对良恶性椎体骨折进行鉴别。在SCHMEEL等[57]的研究中,研究者选取每个患者在形态学MRI上具有最大病变直径的单个层面绘制感兴趣区域,并将其复制到相应的PDFF图上,在PDFF图上自动生成相应病变部位的PDFF值,结果表明,椎体恶性病变的PDFF值显著低于良性病变,将PDFF < 9%作为判定恶性肿瘤的临界值,鉴别良恶性椎体骨折的敏感性、特异性及准确性均大于90%。发生恶性病变时PDFF值降低,是由于恶性肿瘤倾向于完全取代正常的脂肪骨髓成分,导致骨髓脂肪细胞显著降低。其次,当发生严重造血骨髓增生时,造血骨髓在T1WI上的信号强度难以与恶性骨髓病变区分开来。但最近一项研究招募了30例椎体转移患者和14例局灶性造血骨髓沉积患者,尽可能地将PDFF图上患者的整个病变部位包含在感兴趣区内进行分析,结果显示CSE-MRI能够通过评估病灶PDFF值的差异,提高区分椎体转移与局灶性造血骨髓沉积的诊断性能[58],显著改善了不同诊断医师之间观察的一致性。另一项研究将T2WI压脂序列(fat-suppressed T2-weighted imaging,T2 FS)与脂肪分数图进行对比,勾画病灶的边界绘制感兴趣区,若有多个病灶则选取图像中最大的病灶进行绘制并分析,结果显示这两者在高细胞造血骨髓和恶性骨髓病变的鉴别诊断效能上没有明显的统计学差异[59]。T2WI压脂序列的定量参数与脂肪分数图类似,均能反映骨髓中的脂肪含量,由此也能印证骨髓脂肪含量对骨转移的诊断有极大的价值。现有研究显示,CSE-MRI由于其精确定量脂肪含量的能力,在技术原理上已经实现了在临床应用的前提条件,而PDFF值在提高椎体恶性病变的诊断效能方面展现出了卓越的能力,在临床制定相应的治疗决策、监测疗效及评估预后上具有巨大的发展潜能。 磁共振弥散加权成像(diffusion weighted imaging,DWI)是一种广泛应用于临床诊断的成熟序列,已经成为多参数MRI(multi-parametric MRI,mp-MRI)临床应用中常见的功能序列。近来人们发现DWI和CSE-MRI联合使用时诊断准确率会提高。基于先前的研究结果,SCHMEEL等[60]进一步应用DWI和CSE-MRI对椎体良恶性病变的诊断性能进行比较和结合,在具有最大病变直径的DWI图像层面上手动勾画高信号病变区域,并将其复制到相应的ADC和PDFF图上,比较两组间ADC和PDFF值是否存在差异,结果表明ADC和PDFF均有助于区分良恶性椎体病变,同时ADC和PDFF相结合能够提高诊断效能,对椎体良恶性病变的诊断具有高达96.6%的准确性[57]。另外,有学者提出多参数骨MRI (multiparametric bone MRI,mpBMRI),观察DWI信号、ADC和相对脂肪分数的组合是否可以识别活动性骨转移,进而能否更有信心地选择癌症患者CT引导下骨活检的目标病变。在43例CT引导骨活检的癌症患者中,同样首先在DWI图像上对病灶进行勾画,再将其复制到ADC和PDFF图上得到相应的值并进行分析对比。经过小样本的研究表明mpBMRI联合高DWI信号、平均ADC < 1 100 μm2/s、平均相对脂肪分数< 20%对肿瘤阳性活检的诊断敏感性为82%,特异性为80%,阳性预测值为93%,与单一MRI参数相比,mpBMRI对活动性骨转移灶具有较高的诊断效能,证实其有助于提高CT引导下的骨活检成功率[61]。然而,多参数MRI有检查时间长的固有缺陷,这限制了其临床应用。但近来LECOUVET等[62]经过研究得出,在全身磁共振成像(Whole-Body Magnetic Resonance Imaging,WB-MRI)中,3DT1WI梯度回波mDixon(3D T1-weighted gradient echo mDixon,3D T1 GEmDixon)可替代3D T1WI快速自旋回波(3D T1-weighted fast spin echo,3D T1 FSE)作为形态序列来补充DWI用于前列腺癌的转移筛查,能够使序列的采集时间减少10 min以上,极大地缩短了扫描时间,并且还能够用于检测淋巴结转移。以上研究说明联合DWI进行的多参数MRI检查不仅能提高椎体恶性病变的诊断效能,还能够显著缩短扫描时间,在临床应用上,基于CSE-MRI诊断椎体恶性病变以及联合DWI进行多参数MRI检查显现了更多的可能性。这一技术为临床医生提供了一种准确高效的诊断工具,有助于更早地发现病变,并制定针对性的治疗方案,提高治疗的效果。 目前国内外基于CSE-MRI评估转移性压缩性椎体骨折相关研究进展见表4。 "

| [1] LV T, LI Z, WANG D, et al. Role of exosomes in prostate cancer bone metastasis. Arch Biochem Biophys. 2023;748:109784. [2] XU S, LI X, DU Y, et al. Decoding the past and future of breast cancer bone metastasis: A bibliometric analysis from 2003 to 2022. Asian J Surg. 2023:S1015-9584(23)01400-8. doi: 10.1016/j.asjsur.2023.08.231. [3] TSENG YD. Radiation Therapy for Painful Bone Metastases: Fractionation, Recalcification, and Symptom Control. Semin Radiat Oncol. 2023;33(2):139-147. [4] CHOY E, COTE GM, MICHAELSON MD, et al. Phase II Study of Cabozantinib in Patients With Bone Metastasis. Oncologist. 2022;27(7):600-606. [5] NISHIMURA K. Management of bone metastasis in prostate cancer. J Bone Miner Metab. 2023;41(3):317-326. [6] DAI X, LIU B, HOU Q, et al. Global and local fat effects on bone mass and quality in obesity. Bone Joint Res. 2023;12(9):580-589. [7] LI J, LU L, LIU L, et al. The unique role of bone marrow adipose tissue in ovariectomy-induced bone loss in mice. Endocrine. 2023 Sep 8. doi: 10.1007/s12020-023-03504-6. [8] ROSEN CJ, HOROWITZ MC. Nutrient regulation of bone marrow adipose tissue: skeletal implications of weight loss. Nat Rev Endocrinol. 2023; 19(11):626-638. [9] TODOSENKO N, KHAZIAKHMATOVA O, MALASHCHENKO V, et al. Adipocyte- and Monocyte-Mediated Vicious Circle of Inflammation and Obesity (Review of Cellular and Molecular Mechanisms). Int J Mol Sci. 2023;24(15):12259. [10] CHENG F, HE J, YANG J. Bone marrow microenvironment: roles and therapeutic implications in obesity-associated cancer. Trends Cancer. 2023; 9(7):566-577. [11] LUO G, HE Y, YU X. Bone Marrow Adipocyte: An Intimate Partner With Tumor Cells in Bone Metastasis. Front Endocrinol (Lausanne). 2018;9:339. [12] SALAMANNA F, CONTARTESE D, ERRANI C, et al. Role of bone marrow adipocytes in bone metastasis development and progression: a systematic review. Front Endocrinol (Lausanne). 2023;14:1207416. [13] WITKOWSKI MT, KOUSTENI S, AIFANTIS I. Mapping and targeting of the leukemic microenvironment. J Exp Med. 2020;217(2):e20190589. [14] GALÁN-DÍEZ M, CUESTA-DOMÍNGUEZ Á, KOUSTENI S. The Bone Marrow Microenvironment in Health and Myeloid Malignancy. Cold Spring Harb Perspect Med. 2018;8(7):a031328. [15] SABBAH R, SAADI S, SHAHAR-GABAY T, et al. Abnormal adipogenic signaling in the bone marrow mesenchymal stem cells contributes to supportive microenvironment for leukemia development. Cell Commun Signal. 2023; 21(1):277. [16] LWIN ST, OLECHNOWICZ SW, FOWLER JA, et al. Diet-induced obesity promotes a myeloma-like condition in vivo. Leukemia. 2015;29(2):507-510. [17] TABE Y, YAMAMOTO S, SAITOH K, et al. Bone Marrow Adipocytes Facilitate Fatty Acid Oxidation Activating AMPK and a Transcriptional Network Supporting Survival of Acute Monocytic Leukemia Cells. Cancer Res. 2017; 77(6):1453-1464. [18] DIRAT B, BOCHET L, DABEK M, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71(7):2455-2465. [19] FAIRFIELD H, FALANK C, FARRELL M, et al. Development of a 3D bone marrow adipose tissue model. Bone. 2019;118:77-88. [20] SCHELLER EL, DOUCETTE CR, LEARMAN BS, et al. Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun. 2015;6:7808. [21] 张纯希,李想,周钰翔,等.骨骼微环境中谁决定间充质干细胞的分化命运[J].中国组织工程研究,2021,25(25):4045-4052. [22] YUE R, ZHOU BO, SHIMADA IS, et al. Leptin Receptor Promotes Adipogenesis and Reduces Osteogenesis by Regulating Mesenchymal Stromal Cells in Adult Bone Marrow. Cell Stem Cell. 2016;18(6):782-796. [23] CHI M, CHEN J, YE Y, et al. Adipocytes contribute to resistance of human melanoma cells to chemotherapy and targeted therapy. Curr Med Chem. 2014; 21(10):1255-1267. [24] WALTER M, LIANG S, GHOSH S, et al. Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene. 2009;28(30):2745-2755. [25] HOLT V, CAPLAN AI, HAYNESWORTH SE. Identification of a subpopulation of marrow MSC-derived medullary adipocytes that express osteoclast-regulating molecules: marrow adipocytes express osteoclast mediators. PLoS One. 2014; 9(10):e108920. [26] YANG HL, LIU T, WANG XM, et al. Diagnosis of bone metastases: a meta-analysis comparing ¹⁸FDG PET, CT, MRI and bone scintigraphy. Eur Radiol. 2011;21(12): 2604-2617. [27] HOTTAT NA, BADR DA, BEN GHANEM M, et al. Assessment of whole-body MRI including diffusion-weighted sequences in the initial staging of breast cancer patients at high risk of metastases in comparison with PET-CT: a prospective cohort study. Eur Radiol. 2023 Aug 9. doi: 10.1007/s00330-023-10060-0. [28] MONTOYA-BORDÓN J, ELVIRA-RUIZ P, CARRIAZO-JIMÉNEZ B, et al. Imaging diagnosis of vertebral metastasis. Rev Esp Cir Ortop Traumatol. 2023;67(6): 511-522. [29] MORAWITZ J, BRUCKMANN NM, JANNUSCH K, et al. Conventional Imaging, MRI and 18F-FDG PET/MRI for N and M Staging in Patients with Newly Diagnosed Breast Cancer. Cancers (Basel). 2023;15(14):3646. [30] RUAN D, SUN L. Diagnostic Performance of PET/MRI in Breast Cancer: A Systematic Review and Bayesian Bivariate Meta-analysis. Clin Breast Cancer. 2023;23(2):108-124. [31] ZHAN Y, ZHANG G, LI M, et al. Whole-Body MRI vs. PET/CT for the Detection of Bone Metastases in Patients With Prostate Cancer: A Systematic Review and Meta-Analysis. Front Oncol. 2021;11:633833. [32] BRUCKMANN NM, KIRCHNER J, UMUTLU L, et al. Prospective comparison of the diagnostic accuracy of 18F-FDG PET/MRI, MRI, CT, and bone scintigraphy for the detection of bone metastases in the initial staging of primary breast cancer patients. Eur Radiol. 2021;31(11):8714-8724. [33] BHALUDIN BN, TUNARIU N, KOH DM, et al. A review on the added value of whole-body MRI in metastatic lobular breast cancer. Eur Radiol. 2022;32(9):6514-6525. [34] NAKANISHI K, TANAKA J, NAKAYA Y, et al. Whole-body MRI: detecting bone metastases from prostate cancer. Jpn J Radiol. 2022;40(3):229-244. [35] CHEN R, YANG Q, CHEN W, et al. Whole-body MRI-based multivariate prediction model in the assessment of bone metastasis in prostate cancer. World J Urol. 2021;39(8):2937-2943. [36] GONG XQ, TAO YY, WANG R, et al. Application of Diffusion Weighted Imaging in Prostate Cancer Bone Metastasis: Detection and Therapy Evaluation. Anticancer Agents Med Chem. 2021;21(15):1950-1956. [37] VAN NIEUWENHOVE S, VAN DAMME J, PADHANI AR, et al. Whole-body magnetic resonance imaging for prostate cancer assessment: Current status and future directions. J Magn Reson Imaging. 2022;55(3):653-680. [38] CERANKA J, WUTS J, CHIABAI O, et al. Computer-aided diagnosis of skeletal metastases in multi-parametric whole-body MRI. Comput Methods Programs Biomed. 2023;242:107811. [39] RAYA JG, DUARTE A, WANG N, et al. Applications of Diffusion-Weighted MRI to the Musculoskeletal System. J Magn Reson Imaging. 2023 Jul 21. doi: 10.1002/jmri.28870. [40] 朱柳红,刘豪,周建军.磁共振T2 mapping技术在体部恶性肿瘤中的研究进展[J].磁共振成像,2020,11(5):398-400. [41] HAMILTON G, MIDDLETON MS, BYDDER M, et al. Effect of PRESS and STEAM sequences on magnetic resonance spectroscopic liver fat quantification. J Magn Reson Imaging. 2009;30(1):145-152. [42] BYDDER M, GIRARD O, HAMILTON G. Mapping the double bonds in triglycerides. Magn Reson Imaging. 2011;29(8):1041-1046. [43] REEDER SB, HU HH, SIRLIN CB. Proton density fat-fraction: a standardized MR-based biomarker of tissue fat concentration. J Magn Reson Imaging. 2012;36(5): 1011-1014. [44] 赵秀芹,狄玉进,徐金法,等.单体素氢质子磁共振波谱在脊柱转移瘤中的初步应用研究[J].医学影像学杂志,2012,22(4):624-626. [45] 樊秋菊,谭辉,于楠,等.IVIM-DWI联合MRS鉴别诊断骨质疏松与转移性椎体压缩性骨折[J].中国医学影像技术,2018,34(2):297-301. [46] TAN H, XU H, LUO F, et al. Combined intravoxel incoherent motion diffusion-weighted MR imaging and magnetic resonance spectroscopy in differentiation between osteoporotic and metastatic vertebral compression fractures. J Orthop Surg Res. 2019;14(1):299. [47] 刘斌,董国礼,郭静,等.椎体~1H-MRS的临床应用研究进展[J].国际医学放射学杂志,2012,35(6):553-556. [48] MA J. Dixon techniques for water and fat imaging. J Magn Reson Imaging. 2008; 28(3):543-558. [49] VAN VUCHT N, SANTIAGO R, LOTTMANN B, et al. The Dixon technique for MRI of the bone marrow. Skeletal Radiol. 2019;48(12):1861-1874. [50] SCHMEEL FC, VOMWEG T, TRÄBER F, et al. Proton density fat fraction MRI of vertebral bone marrow: Accuracy, repeatability, and reproducibility among readers, field strengths, and imaging platforms. J Magn Reson Imaging. 2019; 50(6):1762-1772. [51] HU HH, YOKOO T, BASHIR MR, et al. Linearity and Bias of Proton Density Fat Fraction as a Quantitative Imaging Biomarker: A Multicenter, Multiplatform, Multivendor Phantom Study. Radiology. 2021;298(3):640-651. [52] BAUM T, YAP SP, DIECKMEYER M, et al. Assessment of whole spine vertebral bone marrow fat using chemical shift-encoding based water-fat MRI. J Magn Reson Imaging. 2015;42(4):1018-1023. [53] KARAMPINOS DC, RUSCHKE S, DIECKMEYER M, et al. Modeling of T2* decay in vertebral bone marrow fat quantification. NMR Biomed. 2015; 28(11): 1535-1542. [54] REEDER SB, ROBSON PM, YU H, et al. Quantification of hepatic steatosis with MRI: the effects of accurate fat spectral modeling. J Magn Reson Imaging. 2009; 29(6):1332-1339. [55] SUH CH, YUN SJ, JIN W, et al. Diagnostic Performance of In-Phase and Opposed-Phase Chemical-Shift Imaging for Differentiating Benign and Malignant Vertebral Marrow Lesions: A Meta-Analysis. AJR Am J Roentgenol. 2018;211(4): W188-W197. [56] LIAO Z, LIU G, MING B, et al. Evaluating prostate cancer bone metastasis using accelerated whole-body isotropic 3D T1-weighted Dixon MRI with compressed SENSE: a feasibility study. Eur Radiol. 2023;33(3):1719-1728. [57] SCHMEEL FC, LUETKENS JA, ENKIRCH SJ, et al. Proton density fat fraction (PDFF) MR imaging for differentiation of acute benign and neoplastic compression fractures of the spine. Eur Radiol. 2018;28(12):5001-5009. [58] JUNG Y, JEON SW, KWACK KS, et al. Differentiation of Vertebral Metastases From Focal Hematopoietic Marrow Depositions on MRI: Added Value of Proton Density Fat Fraction. AJR Am J Roentgenol. 2021;216(3):734-741. [59] PARK S, DO HUH J. Differentiation of bone metastases from benign red marrow depositions of the spine: the role of fat-suppressed T2-weighted imaging compared to fat fraction map. Eur Radiol. 2022;32(10):6730-6738. [60] SCHMEEL FC, ENKIRCH SJ, LUETKENS JA, et al. Diagnostic Accuracy of Quantitative Imaging Biomarkers in the Differentiation of Benign and Malignant Vertebral Lesions : Combination of Diffusion-Weighted and Proton Density Fat Fraction Spine MRI. Clin Neuroradiol. 2021;31(4):1059-1070. [61] DONNERS R, FIGUEIREDO I, TUNARIU N, et al. Multiparametric bone MRI can improve CT-guided bone biopsy target selection in cancer patients and increase diagnostic yield and feasibility of next-generation tumour sequencing. Eur Radiol. 2022;32(7):4647-4656. [62] LECOUVET FE, PASOGLOU V, VAN NIEUWENHOVE S, et al. Shortening the acquisition time of whole-body MRI: 3D T1 gradient echo Dixon vs fast spin echo for metastatic screening in prostate cancer. Eur Radiol. 2020; 30(6):3083-3093. [63] 罗云,高新.2021版欧洲泌尿外科学会前列腺癌诊疗指南更新要点解读[J].中华腔镜泌尿外科杂志(电子版),2022,16(2):97-100. [64] COOK GJR. Imaging of Bone Metastases in Breast Cancer. Semin Nucl Med. 2022;52(5):531-541. [65] COOK GJR, GOH V. Molecular Imaging of Bone Metastases and Their Response to Therapy. J Nucl Med. 2020;61(6):799-806. [66] OPREA-LAGER DE, CYSOUW MCF, BOELLAARD R, et al. Bone Metastases Are Measurable: The Role of Whole-Body MRI and Positron Emission Tomography. Front Oncol. 2021;11:772530. [67] 李利,谢莎,敬宗林,等.影像学检查技术在前列腺癌骨转移诊断中的应用进展[J].山东医药,2021,61(36):105-109. |
| [1] | Yu Shuai, Liu Jiawei, Zhu Bin, Pan Tan, Li Xinglong, Sun Guangfeng, Yu Haiyang, Ding Ya, Wang Hongliang. Hot issues and application prospects of small molecule drugs in treatment of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1913-1922. |
| [2] | Yu Jingbang, Wu Yayun. Regulatory effect of non-coding RNA in pulmonary fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1659-1666. |
| [3] | Wang Qiuyue, Jin Pan, Pu Rui . Exercise intervention and the role of pyroptosis in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1667-1675. |
| [4] | Yuan Weibo, Liu Chan, Yu Limei. Potential application of liver organoids in liver disease models and transplantation therapy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1684-1692. |
| [5] | Peng Hongcheng, Peng Guoxuan, Lei Anyi, Lin Yuan, Sun Hong, Ning Xu, Shang Xianwen, Deng Jin, Huang Mingzhi . Role and mechanism of platelet-derived growth factor BB in repair of growth plate injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1497-1503. |
| [6] | Liu Haoyang, Xie Qiang, Shen Mengran, Ren Yansong, Ma Jinhui, Wang Bailiang, Yue Debo, Wang Weiguo . Application, research hotspots, and shortcomings of degradable zinc-based alloys in bone defect repair and reconstruction [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 839-845. |
| [7] | Guo Zhao, Zhuang Haoyan, Shi Xuewen. Role of exosomes derived from mesenchymal stem cells in treatment of colorectal cancer [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7872-7879. |
| [8] | Huang Haina, Yu Yanrong, Bi Jian, Huang Miao, Peng Weijie. Epigenetic characteristics of hepatogenic differentiation of mesenchymal stem cells in three-dimensional culture [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7848-7855. |
| [9] | Liu Lu, Zhong Chang, Yu Xin, Ren Chenyuan, Gong Yangyang, Zhou Ping, Wang Yingbin. Academic progress and clinical application of in vitro synthetic microenvironment to promote maturation of human pluripotent stem cell-derived cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7856-7862. |
| [10] | Ji Long, Chen Ziyang, , Jin Pan, Kong Xiangkui, Pu Rui, . Lipophagy, exercise intervention and prevention and treatment of nonalcoholic fatty liver disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7611-7619. |
| [11] | Yan Jing, Qin Qiujun, Li Fen, Zhou Jun, Ding Yuanyuan, Jin Chunlin. A systematic review of osteoporosis-specific quality-of-life scales [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7649-7655. |
| [12] | Li Tangbo, Song Diyu, Hao Guobing, Zhang Shuming, Zhu Zexing. Quantitative analysis on effect of dimethyl sulfoxide penetration in cryopreservation of rabbits’ severed hindlimb [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7326-7332. |
| [13] | Su Yongkun, Sun Hong, Liu Miao, Yang Hua, Li Qingsong. Development of novel antioxidants and antioxidant combination carried by nano-hydrogel systems in treatment of intervertebral disc degeneration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7376-7384. |
| [14] | Yi Xiaoding, Zhang Di, Guo Hong, Qing Liang, Zhao Tianyu. Decellularized tendon scaffold: a biomedical material for tendon injury repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7385-7392. |
| [15] | Li Zhongzheng, Chen Zhenghao, Tang Ziyou, Lou Kaiyang, Zhang Rui, Liu Qi, Zhao Na, Yang Kun. Effects of scaffold materials combined with biological factors on biological characteristics of dental follicle cell proliferation and osteogenic differentiation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7405-7414. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||