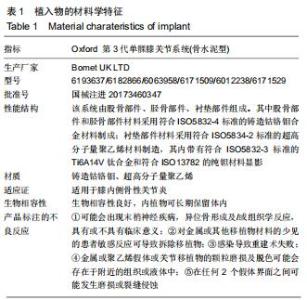
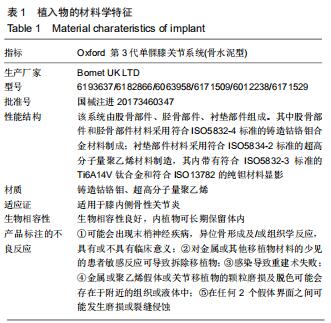
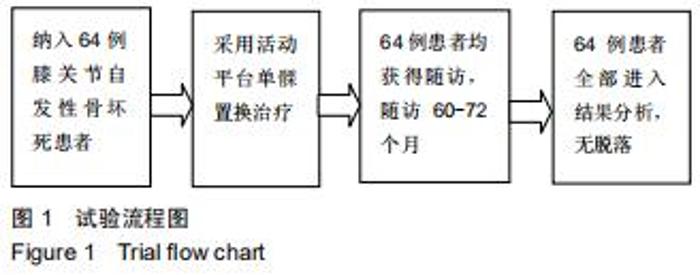
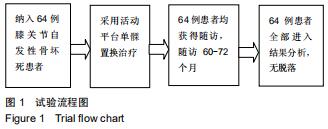
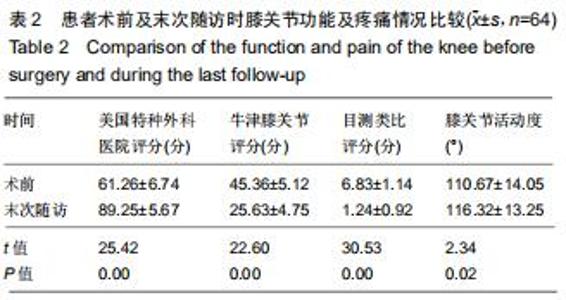
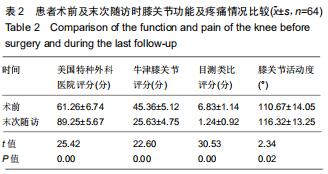
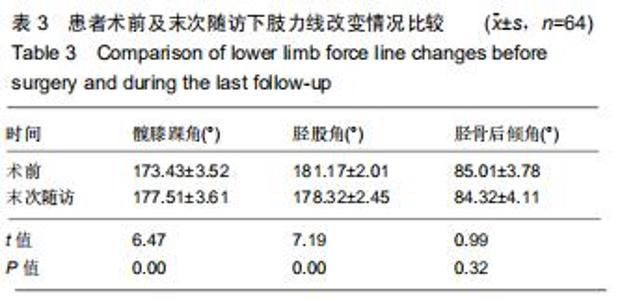
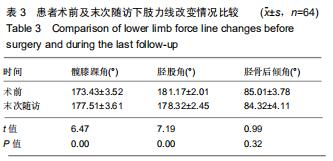
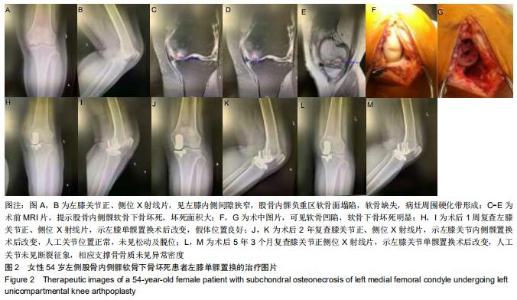
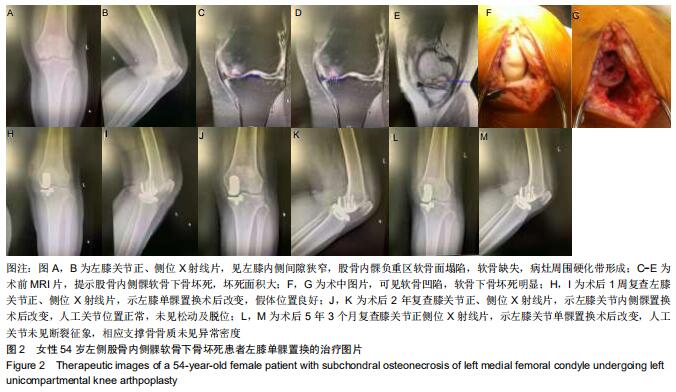
|
[1] AHLBACK S, BAUER GC, BOHNE WH. Spontaneous osteonecrosis of the knee. Arthritis Rheum. 1968;11(6):705-733.
[2] PAPE D, SEIL R, FRITSCH E, et al. Prevalence of spontaneous osteonecrosis of the medial femoral condyle in elderly patients. Knee Surg Sports Traumatol Arthrosc. 2002;10(4):233-240.
[3] KARIM AR, CHERIAN JJ, JAUREGUI JJ, et al. Osteonecrosis of the knee: review. Ann Transl Med. 2015;3(6):1-11.
[4] WILMOT AS, RUUTIAINEN AT, BAKHRU PT, et al. Subchondral insufficiency fracture of the knee: A recognizable associated soft tissue edema pattern and a similar distribution among men and women. Eur J Radiol. 2016;85(11):2096-2103.
[5] 刘新光,郭万首.膝关节自发性骨坏死的病因学研究进展[J].中华骨与关节外科杂志,2016,9(6):536-540.
[6] PRICE AJ, SVARD U. A second decade lifetable survival analysis of the Oxford unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2011;469(1): 174 -179.
[7] 王冰,于秀淳,孙海宁,等.牛津人工单髁关节置换假体生存分析及并发症处理策略[J].中华关节外科杂志(电子版),2018,12(5):631-637.
[8] MA T, TU Y, MEI J, et al. Unicompartmental knee arthroplasty for spontaneous osteonecrosis. J Orthop Surg. 2017;25(1):1-5.
[9] KOSHINO T. The treatment of spontaneous osteonecrosis of the knee by high tibial osteotomy with and without bone-grafting or drilling of the lesion. J Bone Joint Surg Am. 1982;64(1):47-58.
[10] 卢明峰,李泽晖,朱东平,等.单髁置换术治疗膝关节自发性骨坏死的近期疗效研究[J]. 中华关节外科杂志(电子版), 2017,11(5):477-483.
[11] KARIM AR, CHERIAN JJ, JAUREGUI JJ, et al. Osteonecrosis of the knee: Review. Ann Trans Med. 2015;3(1):6.
[12] 杨伟铭,曹学伟.膝关节自发性骨坏死的研究进展[J].中国中医骨伤科杂志, 2017,25(2):83-86.
[13] SIBILSKA A, GÓRALCZYK A, HERMANOWICZ K, et al. Spontaneous osteonecrosis of the knee: what do we know so far? A literature review. J Bone Joint Surg Am. 2020;4(2):103-109.
[14] YANG WM, ZHAO CQ, LU ZY, et al. Clinical characteristics and treatment of spontaneous osteonecrosis of medial tibial plateau: A retrospective case study. Chin Med J (Engl). 2018;131(21): 2544-2550.
[15] 王竣,黄永明,曹振武,等.唑来膦酸治疗早期膝关节自发性骨坏死疗效分析[J].广东医学,2019,40(9):1221-1224.
[16] SOUCACOS PN, XENAKIS TH, BERIS AE, et al. Idiopathic osteonecrosis of the medial femoral condyle. Classification and treatment. Clin Orthop Relat Res. 1997;3(41): 82-89.
[17] AGLIETTI P, INSALL JN, BUZZI R, et al.Idiopathic osteonecrosis of the knee. Aetiology,prognosis and treatment. J Bone JointSurg Br. 1983; 65(5):588-597.
[18] STEMPIN R, STEMPIN K, KACZMAREK W. Medium-term outcome of cementless, mobile-bearing, unicompartmental knee arthroplasty. Ann Transl Med. 2019; 7(3): 41.
[19] MOHAMMAD HR, STRICKLAND L, HAMILTON TW, et al. Long-term outcomes of over 8,000 medial Oxford Phase 3 Unicompartmental Knees-a systematic review. Acta Orthopaedica. 2017;89(3):1-7.
[20] PARRATTE S, OLLIVIER M, LUNEBOURG A, et al. Long-term results of compartmental arthroplasties of the knee: Long term results of partial knee arthroplasty. Bone Joint J. 2015;97-B (10_Supple_A):9-15.
[21] YOON C, CHANG MJ, CHANG CB, et al. Does unicompartmental knee arthroplasty have worse outcomes in spontaneous osteonecrosis of the knee than in medial compartment osteoarthritis? A systematic review and meta-analysis.Arch Orthop Trauma Surg.2019;139(3): 393-403.
[22] FUKUOKA S, FUKUNAGA K, TANIURA K, et al.Medium-term clinical results of unicompartmental knee arthroplasty for the treatment for spontaneous osteonecrosis of the knee with four to 15 years of follow-up. Knee. 2019;26(5):1111-1116.
[23] CHALMERS BP, MEHROTRA KG, SIERRA RJ, et al. Reliable outcomes and survivorship of unicompartmental knee arthroplasty for isolated compartment osteonecrosis. Bone Joint J. 2018; 100-B(4):450-454.
[24] KANEKO T, KONO N, SUNAKAWA T, et al. Reliable patient-reported outcome measure and survivorship of UKA for primary spontaneous osteonecrosis. Eur J Orthop Surg Traumatol. 2019;29(1):119-124.
[25] JAUREGUI JJ, BLUM CL, SARDESAI N, et al. Unicompartmental knee arthroplasty for spontaneous osteonecrosis of the knee: A metaanalysis. J Orthop Surg (Hong Kong). 2018; 26(2): 1-6.
[26] 郭万首,张启栋,刘朝晖,等.膝关节单髁置换术治疗晚期膝关节自发性骨坏死[J].中华骨科杂志,2014,34(6):631-637.
[27] 胡德庆,黄子达,张文明,等.单髁关节置换治疗膝关节自发性骨坏死的疗效分析[J].中国修复重建外科杂志,2019,33(1):20-24.
[28] 陈睿,宋恒平,倪凤民,等.手术治疗股骨髁上自发性骨坏死1例[J]. 中国骨与关节损伤杂志,2014,29(10):1017.
[29] GUO WS, ZHANG QD, LIU ZH, et al. Minimally invasive unicompartmental knee arthroplasty for spontaneous osteonecrosis of the knee. Orthop Surg. 2015;7(2): 119-124.
|