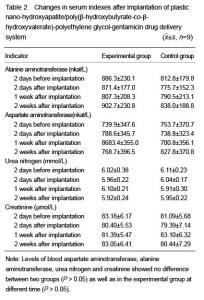
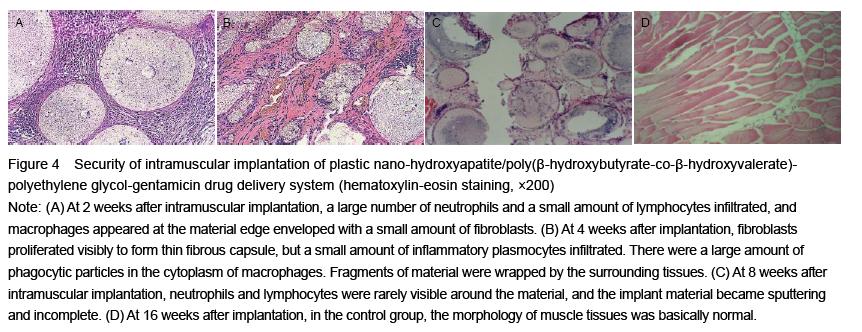
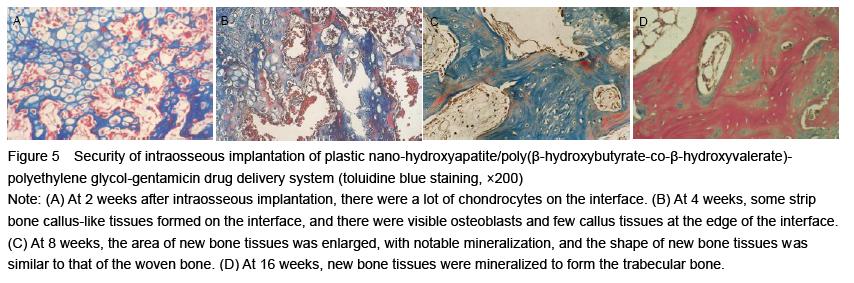
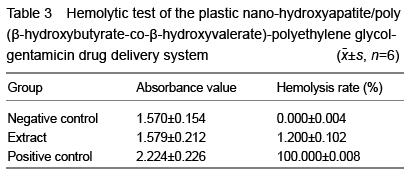
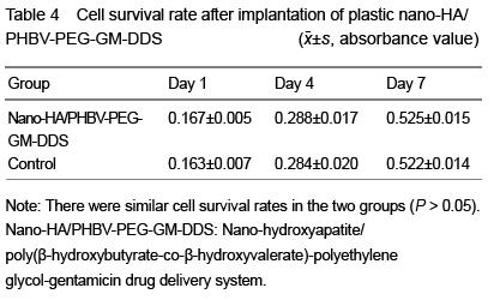
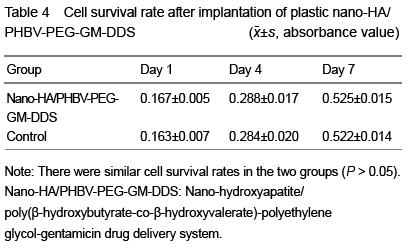
| [1] Joosten U, Joist A, Gosheger G, et al. Effectiveness of hydroxyapatite-vancomycin bone cement in the treatment of Staphylococcus aureus induced chronic osteomyelitis. Biomaterials. 2005;26(25):5251-5258.
[2] Buranapanitkit B, Srinilta V, Ingviga N, et al. The efficacy of a hydroxyapatite composite as a biodegradable antibiotic delivery system. Clin Orthop Relat Res. 2004; (424):244-252.
[3] Mendel V, Simanowski HJ, Scholz HCh. Synergy of HBO2 and a local antibiotic carrier for experimental osteomyelitis due to Staphylococcus aureus in rats. Undersea Hyperb Med. 2004;31(4):407-416.
[4] Wang G, Liu SJ, Ueng SW, et al. The release of cefazolin and gentamicin from biodegradable PLA/PGA beads. Int J Pharm. 2004;273(1-2):203-212.
[5] Sun L, Hu YY, PanY. Preparation release of rbx gentamycin local drug delivery system and its release effect in vivo. Jiefangjun Yixue Zazhi. 2003;28(5): 456-57.
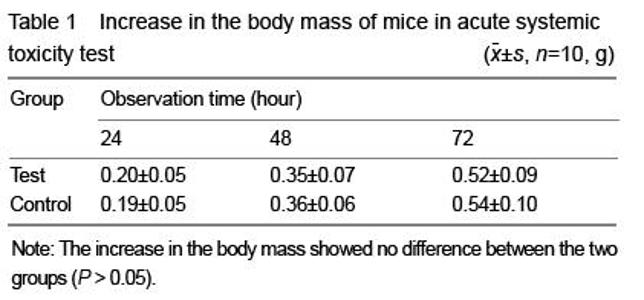
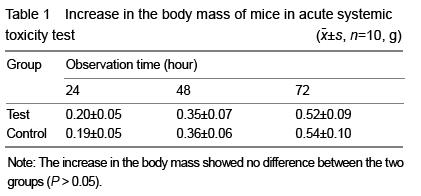
[6] Hao HP. Guidelines for the Implementation of Biological Evaluation Standards for Medical Devices. Beijing: China Standards Press. 2002:81-135.
[7] Wang YF, Jin AM, Wei K, et al. Development of an anti-infection nano-hydroxypatite drug delivery microsphere and its drug-release in vitro. Nanfang Yike Daxue Xuebao. 2006;26(6):754-756.
[8] Hench LL. Bioceramics: from concept to clinic. J Am Ceram Soc. 1991;74(7):1487-1510.
[9] Li SP. Introdution of Biomedical Materials. Wuhan: Wuhan University of Technology Press. 2000:88-123.
[10] Kano S, Yamazaki A, Otsuka R, et al. Application of hydroxyapatite-sol as drug carrier. Biomed Mater Eng. 1994;4(4):283-290.
[11] Liu F, Chen ZH, Chen P, et al. Studies on BG/PHBV co-combined with cultured osteoblasts in vitro, liufeng. Shandong Daxue Xuebao: Yixue Ban. 2004;42(1):63-66.
[12] Saito N, Murakami N, Takahashi J, et al. Synthetic biodegradable polymers as drug delivery systems for bone morphogenetic proteins. Adv Drug Deliv Rev. 2005; 57(7):1037-1048. [13] Haidar ZS, Hamdy RC, Tabrizian M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part A: Current challenges in BMP delivery. Biotechnol Lett. 2009;31(12):1817-1824.
[14] Haidar ZS, Hamdy RC, Tabrizian M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part B: Delivery systems for BMPs in orthopaedic and craniofacial tissue engineering. Biotechnol Lett. 2009;31(12):1825-1835.
[15] Issa JP, Bentley MV, Iyomasa MM, et al. Sustained release carriers used to delivery bone morphogenetic proteins in the bone healing process. Anat Histol Embryol. 2008;37(3):181-187.
[16] Wikesjö UM, Qahash M, Huang YH, et al. Bone morphogenetic proteins for periodontal and alveolar indications; biological observations - clinical implications. Orthod Craniofac Res. 2009;12(3):263-270.
[17] Saito N, Takaoka K. New synthetic biodegradable polymers as BMP carriers for bone tissue engineering. Biomaterials. 2003;24(13):2287-2293.
[18] Bessa PC, Casal M, Reis RL. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery). J Tissue Eng Regen Med. 2008;2(2-3):81-96.
[19] Avgoustakis K. Pegylated poly(lactide) and poly(lactide-co-glycolide) nanoparticles: preparation, properties and possible applications in drug delivery. Curr Drug Deliv. 2004;1(4):321-333. |