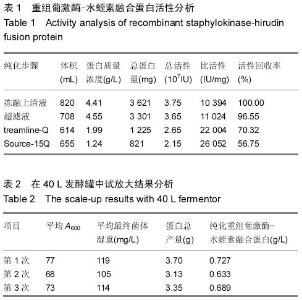
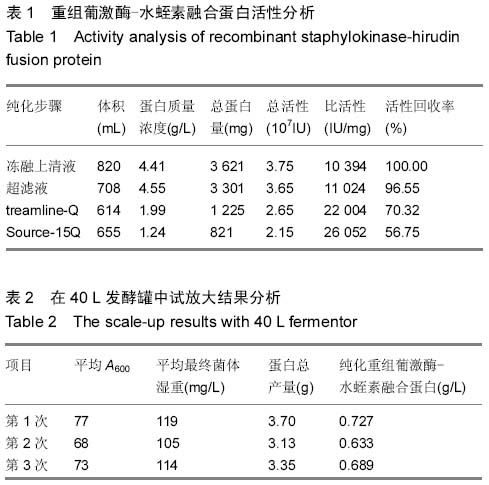
| [1] 张光满,于爱平,秦宜德.重组葡激酶-水蛭素融合蛋白的分离纯化工艺建立[J].安徽医科大学学报,2010,45(4):567-569.
[2] 江良梁,秦宜德,刘滋康,等.乳源抗菌-免疫调节融合蛋白原核表达和纯化[J].安徽医科大学学报,2008,43(3):260-263.
[3] 杨可,杨震,周建中,等.重组葡激酶的表达纯化及纤溶活性鉴定[J].重庆医科大学学报,2012,37(3):232-235.
[4] 张毅,周淑艳,陈锐,等.EB病毒融合蛋白ZtaN-p23在大肠杆菌中的表达、纯化及活性鉴定[J].细胞与分子免疫学杂志,2012,28(2): 120-123.
[5] 王航,许静,谭树华.水蛭素的分子改造与结构修饰研究进展[J].西北药学杂志,2011,26(5):385-387.
[6] 丁有学,韩春梅,李响,等.重组葡激酶-水蛭素融合蛋白质控方法和质量标准的建立[J].中国生物制品学杂志,2014,27(8): 1024-1034.
[7] 王贵鑫,吕莉.溶栓药物的药理学研究进展[J].血栓与止血学, 2011, 17(5):234-236.
[8] 于敏,黄一农,莫炜.水蛭素及其衍生物研究进展及展望[J].药物生物技术,2011,18(4):351-354.
[9] 孙玮佳.新型合成水蛭肽Hirulog-s抗动脉血栓和抗弥散性血管内凝血作用的实验研究[D].大连医科大学,2011.
[10] 李冰宁,欧阳杰,刘玲玲,等.水蛭素检测方法研究进展[J].药物分析杂志,2010,30(4):755-760.
[11] 戴淑芳,邹向阳,段东升,等.重组水蛭素对角叉菜胶致小鼠尾部血栓的抗凝效应分析[J].中国误诊学杂志,2010,10(25):6081- 6082.
[12] 瞿新艳.水蛭的抗凝血作用研究[J].现代中西医结合杂志,2010, 19(13):1582-1583.
[13] 国家药典委员会.中国药典(三部)[S].北京:中国医药科技出版社,2010:附录 60-61,89-92,95-98.
[14] 孙倩倩.水蛭纤溶活性蛋白的分离纯化及性质研究[D].山东大学, 2014.
[15] Wang X,Zhang G,Wang L,et al. A fusion protein with improved thrombolytic effect and low bleeding risk.Thromb Haemost. 2009;102(6):1194-203.
[16] 何钱.《本经》水蛭功效释义[S].中国中医药报,2014-03-21.
[17] 任青华,牟忠祥,耿延君,等.中药水蛭素对荷瘤鼠Ki-67与VEGF表达的影响[J].医学研究杂志,2010,39(6):46-48.
[18] Chen HY ,Qi XH, Geng X, et al. Separation and purification of recombinant hirudin variant 3 from Bacillus subtilis.Agri Basic Sci Method.2009;10(8):15-19.
[19] 钟山,杨得坡,崔征,等.水蛭抗凝血活性成分的研究[J].中国中药杂志,2008,33 (23): 2781.
[20] 万明.宽体金线蛭抗凝血活性寡肽研究[D].博士学位论文,2012.
[21] 荆玲,周继红,范炎峰.抗血栓药的研究进展[J].医学理论与实践, 2012,25(16):1985-1986.
[22] 吴丹明,张立魁.溶栓药物分类及合理应用[J].中国实用外科杂志, 2011,31(12): 1136-1137.
[23] Yao TC, Xu YL, The advances of Thrombolytics Drugs.Strait Pharmaceutical J.2010;22(16):217-219.
[24] 王芳芳,王金平.溶栓药物的临床研究新进展[J].承德医学院学报, 2011,28(3):311-313.
[25] 王红颖,王倩,黄瑾.蛋白质分离纯化方法的研究进展[J].现代生物医学进展,2011,11(24):5168-5170.
[26] 施琛.凝血及纤溶实验室检验的进展及临床应用[J].现代诊断与治疗,2013,24(5):1168-1170.
[27] 鲁碧楠,许丽娜,韩旭,等.蛋白质分离分析中的色谱技术新进展[J].中国现代应用要学杂志,2009,26(13):1116-1121.
[28] 苟冶然,周建中.葡激酶研究进展[J].心血管病学进展,2014,35(6): 695-699.
[29] Prasad B,Salunkhe SS,Padmanabhan S.Novel self-cleavage activity of Staphylokinase fusion proteins: an interesting finding and its possible applications. Protein Expr Purif. 2010; 69(2):191-197.
[30] Armstrong PW,Burton J,Pakola S,et al. Collaborative angiographic patency trial of recombinant staphylokinase ( Captors Ⅱ). Am Heart J.2009;146(3):484-488.
[31] Muthukrishnan S,Puri M,Lefevre C. Support vector machine( SVM) based multiclass prediction with basic statistical analysis of plasminogen activators.BMC Res Notes. 2014;7:63.
[32] Furuya H,Ikeda R.Interaction of triosephosphate isomerase from Staphylococcus aureus with plasminogen. Microbiol Immunol.2011;55(12):855-862.
[33] Xu RG, He JT, Jia K, et al. Simultaneous removal of T and B cell epitope in recombinant staphylokinase by structure-based mutagenesis of immuno-dominant Arg77 and Glu80 residue.Wei Sheng Wu Xue Bao.2011;51(5):692-703.
[34] Xue X,Li D,Yu J,et al. Phenyl linker-induced dense PEG conformation improves the efficacy of C-terminally monoPEGylated staphylokinase. Biomacromolecules.2013; 14(2):331-341.
[35] Wang J,Hu T,Liu Y,et al. Kinetic and stoichiometric analysis of the modification process for N-terminal PEGylation of staphylokinase. Anal Biochem.2011;412(1): 114-116.
[36] Ruyan L,Dongxia L,Jun W,et al.Preparation,characterization and in vitrbioactivity of N-terminally PEGylated staphylokinase dimmers.Proc Biochem.2012;1(47): 41-46.
[37] 王改珍,贺进田,周志涛,等.聚乙烯醇与葡激酶的相互作用及其对葡激酶二级结构的影响[J].高等学校化学学报,2009,8(30): 1581-1585.
[38] 苏晓明,费瑜,苏东影.葡激酶纳米脂质体的制备及靶向溶栓的实验研究[J].中国老年学杂志,2008,12(28):2429-2430.
[39] 狄静芳,贺进田,徐瑞光,等. N-末端引入RGD序列的葡激酶突变体表达、纯化及性质研究[J].高技术通讯,2009,19(1):99-104.
[40] 房永祥,冯海燕,莫斯科,等.细胞因子融合蛋白技术及其应用前景[J].细胞与分子免疫学杂志,2009,25(9):856-859. |