Chinese Journal of Tissue Engineering Research
Previous Articles Next Articles
Mechanism underlying mesenchymal stem cells to regulate autoimmune thrombosis of systemic lupus erythematosus
Ren Min-min, Gu Jian
- Clinical Medical College of Yangzhou University, Yangzhou 225001, Jiangsu Province, China
-
Revised:2013-08-25Online:2013-11-05Published:2013-11-05 -
Contact:Gu Jian, Professor, Master’s supervisor, Clinical Medical College of Yangzhou University, Yangzhou 225001, Jiangsu Province, China maolujiu918@163.com -
About author:Ren Min-min★, Studying for master’s degree, Clinical Medical College of Yangzhou University, Yangzhou 225001, Jiangsu Province, China renminmin2010@163.com -
Supported by:the National Natural Science Foundation of China, No. 81270590*; the Health Bureau of Jiangsu Province, No. H201048*
CLC Number:
Cite this article
Ren Min-min, Gu Jian. Mechanism underlying mesenchymal stem cells to regulate autoimmune thrombosis of systemic lupus erythematosus[J]. Chinese Journal of Tissue Engineering Research, doi: 10.3969/j.issn.2095-4344.2013.45.022.
share this article

2.1 T、B细胞功能失调对系统性红斑狼疮的致病作用 T淋巴细胞异常活跃是系统性红斑狼疮的重要致病机制之一。系统性红斑狼疮患者的T淋巴细胞在某些反应途径具有无反应性(即对某些免疫反应途径不能应答),使它们产生黏附分子及共刺激分子的能力增加,如CD44,CD40L[2]。 初始T细胞的完全活化需有两种活化信号的协同参与。第一信号由T细胞受体(T cell receptor,TCR)识别抗原产生,经CD3分子将信号转导至细胞内;第二信号(又称协同刺激信号)则由抗原提呈细胞(antigen presenting cells,APC)或靶细胞表面的协同刺激分子与T细胞表面的相应的协同刺激分子受体相互作用而产生。在正常免疫反应过程中,细胞活化需要双信号的完整性。表达于抗原提呈细胞表面的B7分子与T细胞相应受体CD28结合,共同组成B7-CD28/CTLA-4共刺激通路,调节T细胞的活化、分化及增殖,参与B细胞Ig的类型转换和生发中心的形成,通过调节T细胞对凋亡的易感性,清除非特异性激活的T细胞,保护机体不受异常免疫功能的攻击,见图1。"

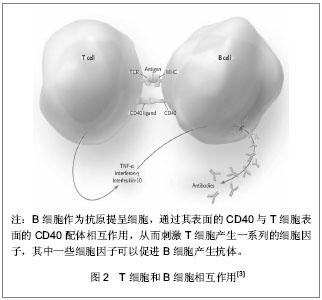
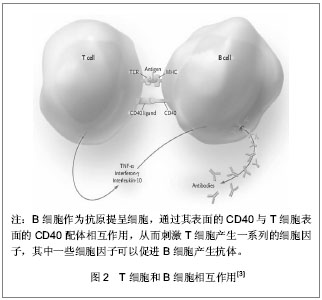
系统性红斑狼疮患者体内T细胞活化后驱使B细胞产生自身抗体,已有动物实验证实阻断CD28与B7分子之间的交联可明显降低血清中自身抗体的滴度。而系统性红斑狼疮患者自身抗体导致的血管内壁损伤,使内皮下胶原暴露,激活内源性的凝血途径,血管壁损伤促进组织因子(tissue factor,TF)产生增加并激活外源性凝血途径。凝血系统的异常激活导致纤溶酶原激活,抗凝血系统功能减弱,组织纤溶酶原活性降低,纤维蛋白沉积。当这种变化持续存在时,患者处于易栓状态,进而发生血栓,组织局部微血栓形成,诱发各种严重血栓形成并发症,如动静脉血栓、流产、多发性脑梗死性痴呆、肺动脉高压等。 ICOS(inducible co-stimulator)是Hutloff等[4]在激活的人体外周血T细胞首次发现,它与CD28结构相似、基因位点相近,而且为T细胞活化提供了共刺激信号,故将其归为CD28家族新成员。ICOS能增强T细胞对B细胞的辅助功能,促进体液免疫应答。ICOS受体(B7h)诱导T细胞和B细胞的增殖,刺激B细胞分化为浆细胞,使免疫球蛋白产生增加[5]。有研究发现ICOS对免疫球蛋白的类别转换至关重要。ICOS基因敲除导致由T细胞特异性抗原引起的IgG1、IgE的水平下降。研究发现系统性红斑狼疮患者的ICOS水平是增高的,由ICOS介导而产生的自身抗体导致血管内皮细胞的损伤[6]。 活化的T细胞表达CD40L,它可与B细胞表面的CD40结合形成CD40-CD40L。活动性系统性红斑狼疮患者T细胞表面的CD40L表达增加。而过度活化的B细胞刺激T细胞,使其表面的CD40L表达增加。其能与内皮细胞表达的CD40相互作用,上调黏附分子的表达,从而增加炎症反应,见图2。"
| [1] Bonfield TL, Nolan Koloze MT, Lennon DP,et al. Defining human mesenchymal stem cell efficacy in vivo. J Inflamm (Lond). 2010 ;7:51. [2] Moulton VR, Tsokos GC. Abnormalities of T cell signaling in systemic lupus erythematosus. Arthritis Res Ther. 2011;13(2): 207. [3] Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358(9):929-939. [4] Hutloff A, Dittrich AM, Beier KC,et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397(6716):263-266. [5] Simpson TR, Quezada SA, Allison JP. Regulation of CD4 T cell activation and effector function by inducible costimulator (ICOS). Curr Opin Immunol. 2010;22(3):326-332. [6] Odegard JM, DiPlacido LD, Greenwald L,et al. ICOS controls effector function but not trafficking receptor expression of kidney-infiltrating effector T cells in murine lupus. J Immunol. 2009;182(7):4076-4084. [7] Garrett-Sinha LA, John S, Gaffen SL. IL-17 and the Th17 lineage in systemic lupus erythematosus. Curr Opin Rheumatol. 2008;20(5):519-525. [8] Wong CK, Lit LC, Tam LS,et al. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for Th17-mediated inflammation in auto-immunity. Clin Immunol. 2008;127(3):385-393. [9] Henriques A, Inês L, Pais ML,et al.Th17 cells in systemic lupus erythematosus share functional features with Th17 cells from normal bone marrow and peripheral tissues. Clin Rheumatol. 2012 ;31(3):483-491. [10] Breitfeld D, Ohl L, Kremmer E,et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000; 192(11):1545-1552. [11] Dong W, Zhu P, Wang Y,et al. Follicular helper T cells in systemic lupus erythematosus: a potential therapeutic target. Autoimmun Rev. 2011;10(6):299-304. [12] Craft JE. Follicular helper T cells in immunity and systemic autoimmunity. Nat Rev Rheumatol. 2012 ;8(6):337-347. [13] Simpson N, Gatenby PA, Wilson A,et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010;62(1):234-244. [14] Pierangeli SS, Chen PP, Raschi E,et al. Antiphospholipid antibodies and the antiphospholipid syndrome: pathogenic mechanisms. Semin Thromb Hemost. 2008 ;34(3):236-250. [15] Pierangeli SS, Chen PP, González EB. Antiphospholipid antibodies and the antiphospholipid syndrome: an update on treatment and pathogenic mechanisms. Curr Opin Hematol. 2006;13(5):366-375. [16] Mackay F, Figgett WA, Saulep D,et al. B-cell stage and context-dependent requirements for survival signals from BAFF and the B-cell receptor. Immunol Rev. 2010;237(1): 205-225. [17] Davidson A.The rationale for BAFF inhibition in systemic lupus erythematosus. Curr Rheumatol Rep. 2012;14(4):295- 302. [18] Ota M, Duong BH, Torkamani A,et al. Regulation of the B cell receptor repertoire and self-reactivity by BAFF. J Immunol. 2010;185(7):4128-4136. [19] Shirota Y, Yarboro C, Fischer R,et al. Impact of anti-interleukin-6 receptor blockade on circulating T and B cell subsets in patients with systemic lupus erythematosus. Ann Rheum Dis. 2013;72(1):118-128. [20] Kyttaris VC, Tsokos GC.Targeting lymphocyte signaling pathways as a therapeutic approach to systemic lupus erythematosus. Curr Opin Rheumatol. 2011;23(5):449-453. [21] Chung SW, Park JW, Lee SA,et al. Thrombin promotes proinflammatory phenotype in human vascular smooth muscle cell. Biochem Biophys Res Commun. 2010;396(3): 748-754. [22] Yuan W, DiMartino SJ, Redecha PB,et al. Systemic lupus erythematosus monocytes are less responsive to interleukin-10 in the presence of immune complexes. Arthritis Rheum. 2011;63(1):212-218. [23] Weckerle CE, Mangale D, Franek BS,et al. Large-scale analysis of tumor necrosis factor α levels in systemic lupus erythematosus. Arthritis Rheum. 2012;64(9):2947-2952. [24] Shirinsky I, Polovnikova O, Kalinovskaya N,et al. The effects of fenofibrate on inflammation and cardiovascular markers in patients with active rheumatoid arthritis: a pilot study. Rheumatol Int. 2012. [Epub ahead of print] [25] Soleymaninejadian E, Pramanik K, Samadian E. Immunomodulatory properties of mesenchymal stem cells: cytokines and factors. Am J Reprod Immunol. 2012;67(1): 1-8. [26] 张培培,刘慧纯,王世民,等.脑缺血再灌注损伤大鼠尾静脉移植脐带间充质干细胞的安全性[J].中国组织工程研究,2012,16(14): 2581-2584. [27] Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010; 28(3):585-596. [28] Mariño E, Grey ST. B cells as effectors and regulators of autoimmunity. Autoimmunity. 2012;45(5):377-387. [29] Traggiai E, Volpi S, Schena F,et al. Bone marrow-derived mesenchymal stem cells induce both polyclonal expansion and differentiation of B cells isolated from healthy donors and systemic lupus erythematosus patients. Stem Cells. 2008; 26(2):562-569. [30] Asari S, Itakura S, Ferreri K,et al. Mesenchymal stem cells suppress B-cell terminal differentiation. Exp Hematol. 2009; 37(5):604-615. [31] Schena F, Gambini C, Gregorio A,et al. Interferon-γ- dependent inhibition of B cell activation by bone marrow-derived mesenchymal stem cells in a murine model of systemic lupus erythematosus. Arthritis Rheum. 2010; 62(9):2776-2786. [32] Asari S, Itakura S, Ferreri K,et al. Mesenchymal stem cells suppress B-cell terminal differentiation. Exp Hematol. 2009; 37(5):604-615. [33] Ma X, Che N, Gu Z,et al. Allogenic mesenchymal stem cell transplantation ameliorates nephritis in lupus mice via inhibition of B-cell activation. Cell Transplant. 2012. [Epub ahead of print] [34] Najar M, Raicevic G, Boufker HI,et al. Mesenchymal stromal cells use PGE2 to modulate activation and proliferation of lymphocyte subsets: Combined comparison of adipose tissue, Wharton's Jelly and bone marrow sources. Cell Immunol. 2010;264(2):171-179. [35] Yagi H, Soto-Gutierrez A, Parekkadan B,et al. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant. 2010;19(6):667-679. [36] Fu QL, Chow YY, Sun SJ,et al. Mesenchymal stem cells derived from human induced pluripotent stem cells modulate T-cell phenotypes in allergic rhinitis. Allergy. 2012;67(10): 1215-1222. [37] Sun YQ, Deng MX, He J,et al. Human pluripotent stem cell-derived mesenchymal stem cells prevent allergic airway inflammation in mice. Stem Cells. 2012;30(12):2692-2699. [38] Hemeda H, Jakob M, Ludwig AK,et al. Interferon-gamma and tumor necrosis factor-alpha differentially affect cytokine expression and migration properties of mesenchymal stem cells. Stem Cells Dev. 2010;19(5):693-706. [39] Yang SH, Park MJ, Yoon IH,et al. Soluble mediators from mesenchymal stem cells suppress T cell proliferation by inducing IL-10. Exp Mol Med. 2009;41(5):315-324. [40] Yagi H, Soto-Gutierrez A, Parekkadan B,et al. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant. 2010;19(6):667-679. [41] English K.Mechanisms of mesenchymal stromal cell immunomodulation.Immunol Cell Biol. 2013;91(1):19-26. [42] Duffy MM, Pindjakova J, Hanley SA,et al. Mesenchymal stem cell inhibition of T-helper 17 cell- differentiation is triggered by cell-cell contact and mediated by prostaglandin E2 via the EP4 receptor. Eur J Immunol. 2011;41(10):2840-2851. [43] Griffin MD, Ritter T, Mahon BP. Immunological aspects of allogeneic mesenchymal stem cell therapies. Hum Gene Ther. 2010;21(12):1641-1655. [44] Sun L, Akiyama K, Zhang H,et al. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans.Stem Cells. 2009; 27(6):1421-1432. [45] Choi H, Lee RH, Bazhanov N,et al. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood. 2011; 118(2):330-338. [46] François M, Romieu-Mourez R, Li M,et al. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20(1):187-195. [47] Zhang QZ, Su WR, Shi SH,et al. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28(10):1856-1868. [48] Ylöstalo JH, Bartosh TJ, Coble K,et al. Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells. 2012;30(10):2283-2296. |
| [1] | Kong Desheng, He Jingjing, Feng Baofeng, Guo Ruiyun, Asiamah Ernest Amponsah, Lü Fei, Zhang Shuhan, Zhang Xiaolin, Ma Jun, Cui Huixian. Efficacy of mesenchymal stem cells in the spinal cord injury of large animal models: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(在线): 3-. |
| [2] | Zhou Qi, Gao Yi, Wei Kang, Li Jun, Xu Jianda, Jiang Yang, Qu Yuxing. Total knee arthroplasty for rheumatoid arthritis: knee function and biochemical index changes [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(9): 1337-1341. |
| [3] | Liu Chundong, Shen Xiaoqing, Zhang Yanli, Zhang Xiaogen, Wu Buling. Effects of strontium-modified titanium surfaces on adhesion, migration and proliferation of bone marrow mesenchymal stem cells and expression of bone formation-related genes [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(7): 1009-1015. |
| [4] | Lin Ming, Pan Jinyong, Zhang Huirong. Knockout of NIPBL gene down-regulates the abilities of proliferation and osteogenic differentiation in mouse bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(7): 1002-1008. |
| [5] | Zhang Wen, Lei Kun, Gao Lei, Li Kuanxin. Neuronal differentiation of rat bone marrow mesenchymal stem cells via lentivirus-mediated bone morphogenetic protein 7 transfection [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(7): 985-990. |
| [6] | Wu Zhifeng, Luo Min. Biomechanical analysis of chemical acellular nerve allograft combined with bone marrow mesenchymal stem cell transplantation for repairing sciatic nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(7): 991-995. |
| [7] | Huang Yongming, Huang Qiming, Liu Yanjie, Wang Jun, Cao Zhenwu, Tian Zhenjiang, Chen Bojian, Mai Xiujun, Feng Enhui. Proliferation and apoptosis of chondrocytes co-cultured with TDP43 lentivirus transfected-human umbilical cord mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(7): 1016-1022. |
| [8] | Qin Xinyu, Zhang Yan, Zhang Ningkun, Gao Lianru, Cheng Tao, Wang Ze, Tong Shanshan, Chen Yu. Elabela promotes differentiation of Wharton’s jelly-derived mesenchymal stem cells into cardiomyocyte-like cells [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(7): 1046-1051. |
| [9] | Liu Mengting, Rao Wei, Han Bing, Xiao Cuihong, Wu Dongcheng. Immunomodulatory characteristics of human umbilical cord mesenchymal stem cells in vitro [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(7): 1063-1068. |
| [10] | Huang Cheng, Liu Yuanbing, Dai Yongping, Wang Liangliang, Cui Yihua, Yang Jiandong. Transplantation of bone marrow mesenchymal stem cells overexpressing glial cell line derived neurotrophic factor gene for spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(7): 1037-1045. |
| [11] |
Wang Tiantian, Wang Jianzhong.
Application and prospect of bone marrow mesenchymal stem cells in the
treatment of early femoral head necrosis |
| [12] | Wang Zhangling, Yu Limei, Zhao Chunhua. Tissue repair using mesenchymal stem cells via mitochondrial transfer [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(7): 1123-1129. |
| [13] | Deng Junhao, Li Miao, Zhang Licheng, Tang Peifu. Three-dimensional hanging-drop culture of mesenchymal stem cells in the treatment of tissue injury [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(7): 1101-1106. |
| [14] | Huang Wenwen, Li Shuo, Hou Zongliu, Wang Wenju. Pathogenesis of inflammatory bowel disease and mesenchymal stem cell therapy: therapeutic application and existing problems [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(7): 1138-1143. |
| [15] | Zhang Shuang, Xu Xiaomei, Zeng Yang, Yuan Xiaoping, Lin Fuwei. Rev-erbα’s effect on osteoblastogenesis of mouse bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(31): 4921-4926. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||