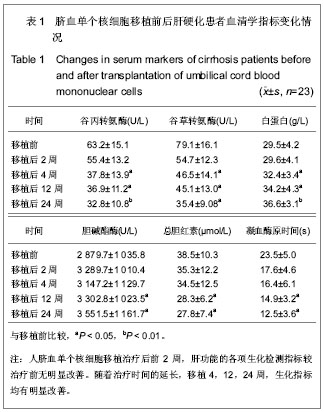
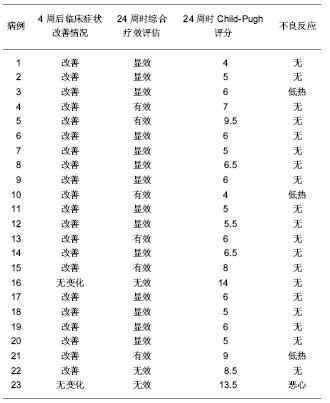
| [1] 胡祥.干细胞与肝脏病治疗研究进展[J].中国继续医学教育,2011, 3(6):27-34.
[2] 王吉耀.内科学(供8年制及7年制临床医学等专业用)[M].北京:人民卫生出版社,2005:520.
[3] 张耿林,彭亮,高志良.干细胞移植在乙肝肝衰竭中的研究及应用[J].内科急危重症杂志,2011,17(6):333-336.
[4] 吴祖泽,贺福初,裴雪涛.造血调控[M].上海:上海医科大学出版社, 2000:245.
[5] 张巧玲,覃莉珍,解继胜,等.脐带间充质干细胞研究进展及应用前景[J]. 右江民族医学院学报,2005,27(1):101-103.
[6] Hong SH, Gang EJ, Jeong JA,et al. In vitro differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocyte-like cells. Biochem Biophys Res Commun. 2005;330(4):1153-1161.
[7] Kang XQ, Zang WJ, Bao LJ,et al. Fibroblast growth factor-4 and hepatocyte growth factor induce differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocytes.World J Gastroenterol. 2005;11(47):7461-7465.
[8] Oh SH, Miyazaki M, Kouchi H,et al. Hepatocyte growth factor induces differentiation of adult rat bone marrow cells into a hepatocyte lineage in vitro.Biochem Biophys Res Commun. 2000;279(2):500-504.
[9] Lyra AC, Soares MB, da Silva LF,et al. Feasibility and safety of autologous bone marrow mononuclear cell transplantation in patients with advanced chronic liver disease. World J Gastroenterol. 2007;13(7):1067-1073.
[10] Kakinuma S, Tanaka Y, Chinzei R,et al. Human umbilical cord blood as a source of transplantable hepatic progenitor cells. Stem Cells. 2003;21(2):217-227.
[11] Levicar N, Pai M, Habib NA,et al. Long-term clinical results of autologous infusion of mobilized adult bone marrow derived CD34+ cells in patients with chronic liver disease. Cell Prolif. 2008;41 Suppl 1:115-125.
[12] Zhang YN, Lie PC, Wei X. Differentiation of mesenchymal stromal cells derived from umbilical cord Wharton's jelly into hepatocyte-like cells. Cytotherapy. 2009;11(5):548-558.
[13] Tsai PC, Fu TW, Chen YM,et al. The therapeutic potential of human umbilical mesenchymal stem cells from Wharton's jelly in the treatment of rat liver fibrosis.Liver Transpl. 2009; 15(5):484-495.
[14] Lee MW, Jang IK, Yoo KH,et al. Stem and progenitor cells in human umbilical cord blood. Int J Hematol. 2010;92(1):45-51.
[15] Harris DT. Non-haematological uses of cord blood stem cells. Br J Haematol. 2009;147(2):177-184.
[16] Zhao Q, Ren H, Zhu D,et al. Stem/progenitor cells in liver injury repair and regeneration. Biol Cell. 2009;101(10): 557-571.
[17] Jung KH, Shin HP, Lee S,et al. Effect of human umbilical cord blood-derived mesenchymal stem cells in a cirrhotic rat model. Liver Int. 2009;29(6):898-909.
[18] 孙嫣,段芳龄,陈香宇,等.人骨髓间充质干细胞体外分化为肝细胞样细胞[J].胃肠病学和肝病学杂志,2004,13(3):244-248.
[19] Schwartz RE, Reyes M, Koodie L,et al. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109(10):1291-1302.
[20] Theise ND, Badve S, Saxena R,et al. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000;31(1): 235-240.
[21] Petersen BE, Bowen WC, Patrene KD,et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284 (5417):1168-1170.
[22] 韩翠萍,刘吉勇,高蕾,等.人脐血间充质干细胞向类肝细胞分化:人肝细胞共培养诱导法的可行性[J].中国组织工程研究与临床康复,2010,14(1):48-52.
[23] 詹三华,张鲁峰,姚卫民,等.人脐血间充质干细胞移植治疗大鼠肝硬化模型[J].中国组织工程研究,2013,17(19):3461-3466.
[24] 杜玲,罗国君,袁雅红,等.脐血干细胞静脉输注治疗失代偿期肝硬化1 例并文献复习[J].现代生物医学进展,2009,9(18):3511- 3513.
[25] 张丽欣,邢利和,张丽丽,等.脐血干细胞移植治疗失代偿期肝硬化的临床效果研究[J].中国全科医学,2010,13(24):2680-2682. |