Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (20): 5214-5228.doi: 10.12307/2026.691
Previous Articles Next Articles
Vancomycin-containing porcine skin acellular extracellular matrix hydrogel promotes wound healing in skin infections
Xu Yixuan1, Yao Jun2, Liu Xulu1, Li Xinlian1, Liu Zhixiong1, Zhang Zhihong1
- 1Department of General Medicine, Affiliated Hospital of Southwest Medical University, Luzhou 646000, Sichuan Province, China; 2Department of Spine Surgery, Shaoxing Central Hospital, Shaoxing 312000, Zhejiang Province, China
-
Accepted:2025-05-28Online:2026-07-18Published:2025-11-27 -
Contact:Zhang Zhihong, MD, Associate professor, Department of General Medicine, Affiliated Hospital of Southwest Medical University, Luzhou 646000, Sichuan Province, China -
About author:Xu Yixuan, Master candidate, Department of General Medicine, Affiliated Hospital of Southwest Medical University, Luzhou 646000, Sichuan Province, China
CLC Number:
Cite this article
Xu Yixuan, Yao Jun, Liu Xulu, Li Xinlian, Liu Zhixiong, Zhang Zhihong. Vancomycin-containing porcine skin acellular extracellular matrix hydrogel promotes wound healing in skin infections[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5214-5228.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
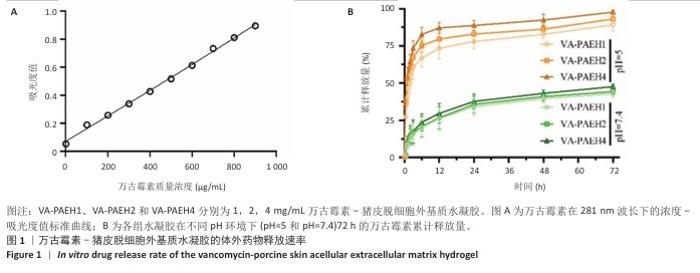
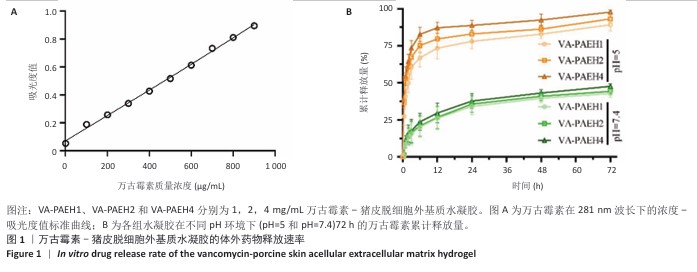
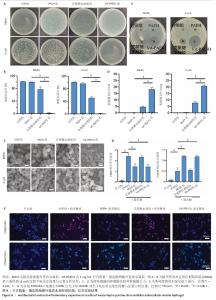
2.1 性能优异水凝胶筛选结果 2.1.1 体外药物释放速率 VA-PAEH1、VA-PAEH2和VA-PAEH4水凝胶的药物包封率分别为98.34%,98.15%和97.68%。如图1所示,万古霉素的质量浓度与281 nm波长下吸光度值之间呈线性关系,这一结果与CAI等[30]的研究一致。如图1B所示,VA-PAEH1、VA-PAEH2和VA-PAEH4水凝胶在pH=5和pH=7.4环境下前12 h内均快速释放万古霉素,其中VA-PAEH1水凝胶的万古霉素累计释放量分别为72.22%和20.33%,在模拟感染创面的酸性环境下水凝胶的快速释放有利于杀菌[31],但是VA-PAEH4水凝胶在pH=5环境下12 h的万古霉素累积释放量为86.67%,明显高于VA-PAEH1、VA-PAEH2水凝胶,这可能导致VA-PAEH4水凝胶存在安全风险;在pH=7.4环境下72 h后,VA-PAEH1、VA-PAEH2和VA-PAEH4水凝胶的万古霉素累计释放量分别为42.67%,44.33%和47.67%。当皮肤感染控制后创面pH值变成中性[26]。结果表明各组水凝胶均具有良好的缓释效果。 "
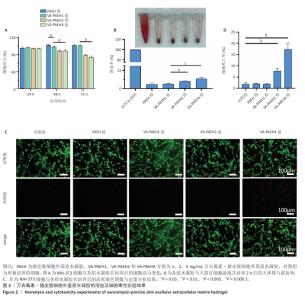
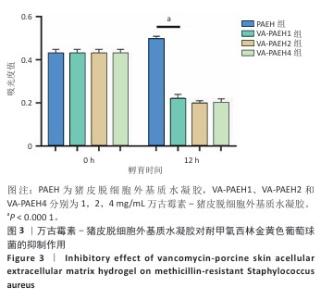
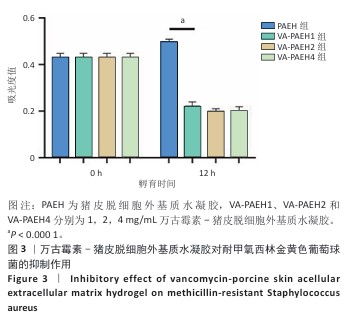
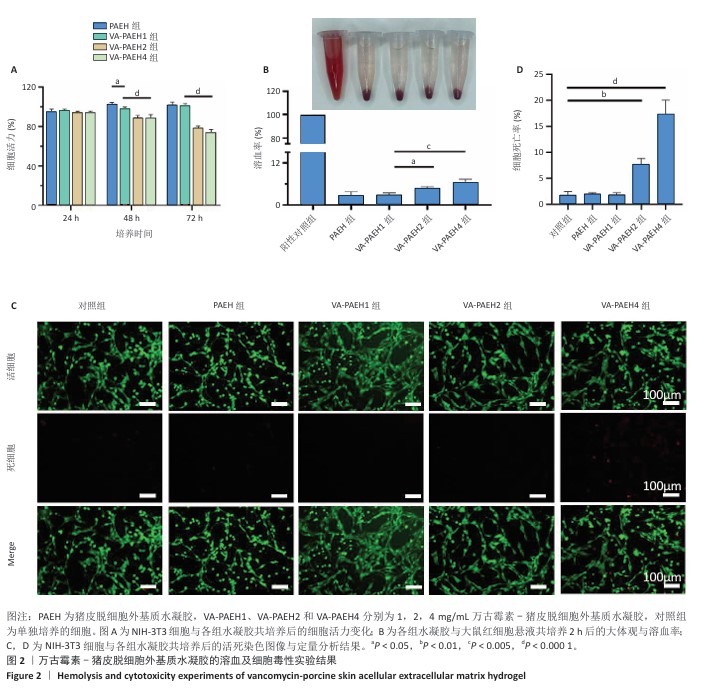
2.1.2 水凝胶的安全性 采用CCK-8法检测PAEH、VA-PAEH1、VA-PAEH2和VA-PAEH4水凝胶对NIH-3T3细胞活力的影响,结果见图2A。培养24 h,各组细胞活力没有明显差异;培养48,72 h,VA-PAEH2和VA-PAEH4组细胞活力明显低于VA-PAEH组、VA-PAEH1组(P < 0.001),说明VA-PAEH2和VA-PAEH4水凝胶抑制细胞活力,这可能与高浓度万古霉素的毒性作用有关。 红细胞溶血实验结果显示,阳性对照组发生溶血,而其他组别无此现象,取各组上清液测量吸光度值后,结果显示VA-PAEH2组和VA-PAEH4组溶血率高于VA-PAEH组、VA-PAEH1组(P < 0.05,P < 0.005),并且VA-PAEH2组和VA-PAEH4组溶血率都超过了生物材料溶血安全临界标准 (< 5%),见图2B。 活死染色结果显示,VA-PAEH2组和VA-PAEH4组可见较多的死细胞,对照组、PAEH组和VA-PAEH1组死细胞较少,见图2C,D。 同时测试了VA-PAEH1、VA-PAEH2和VA-PAEH4水凝胶的抗菌性能,结果显示VA-PAEH1、VA-PAEH2和VA-PAEH4水凝胶均有明显抗菌效果,并且该3组水凝胶间的抗菌效果无明显差异,见图3。 综合以上实验结果,为了兼顾抗菌效果和安全性能,选择VA-PAEH1水凝胶进行后续实验。"
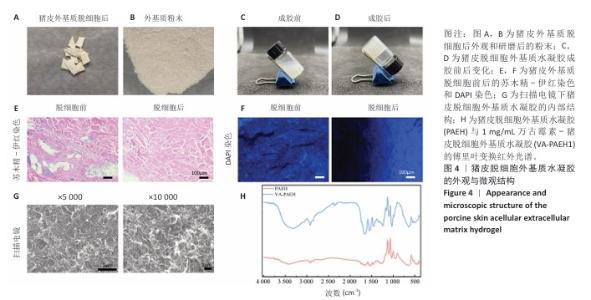
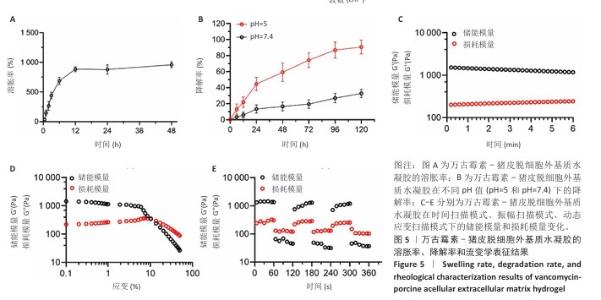
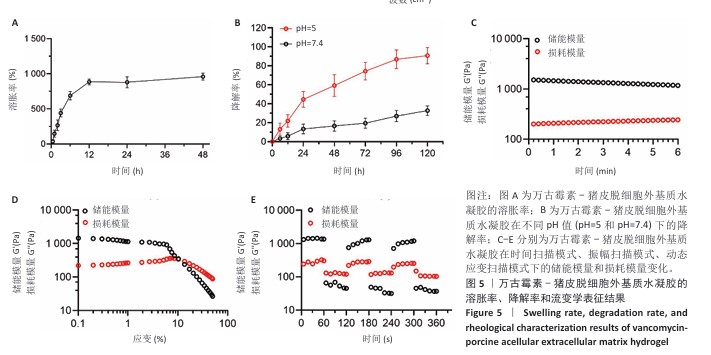
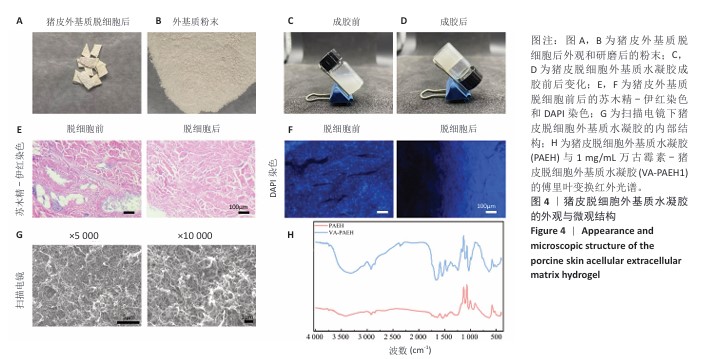
2.2 VA-PAEH1水凝胶的表征结果 新鲜猪皮经过脱细胞冷冻干燥后变成白色多孔结构(图4A),研磨得到外基质粉末(图4B)。在调节pH值、调整渗透压之后,猪皮凝胶溶液外观颜色从浅白色乳液变为半透明白色固体(图4C,D)。苏木精-伊红染色和DAPI染色显示,猪皮外基质中细胞被完全脱去,外基质结构保持完整,见图4E,F。扫描电镜下PAEH水凝胶由互相交织的纤维组成(图4G)。傅里叶变换红外光谱结果显示(图4H),PAEH水凝胶和VA-PAEH1水凝胶在3 300 cm-1和1 650 cm-1特征峰值没有明显差异,其中万古霉素以非共价键形式装载,装载万古霉素后PAEH水凝胶的主要成分并未发生较大的改变,不影响PAEH水凝胶的胶原结构和物理性能。 在前6 h,VA-PAEH1水凝胶快速吸水,达到溶胀平衡时的溶胀率约为958%,见图5A。VA-PAEH1水凝胶在模拟感染的酸性环境下降解较快,第3天的降解率约为73%,见图5B。 VA-PAEH1水凝胶的流变学特征检测结果,如图5C-E所示。由时间扫描曲线可知,VA-PAEH1水凝胶的储存模量(G’)大于损耗模量(G’’),表现出黏性固体行为,在测量时间内,VA-PAEH1水凝胶的储存模量和损耗模量基本保持稳定。在振幅扫描模式下,VA-PAEH1水凝胶的储存模量随着应变的增加逐渐减小,损耗模量逐渐增大,储存模量与损耗模量在应变约为10%时相交,超过这个临界点后损耗模量大于储存模量,说明VA-PAEH1水凝胶结构受到了破坏。由动态应变扫描曲线可知,在应变由25%转变成0.1%过程中,VA-PAEH1水凝胶的储存模量逐渐增大,说明VA-PAEH1水凝胶在结构遭到外力破坏后,外力消失后水凝胶结构可以自行恢复。 "
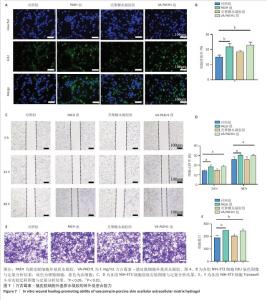
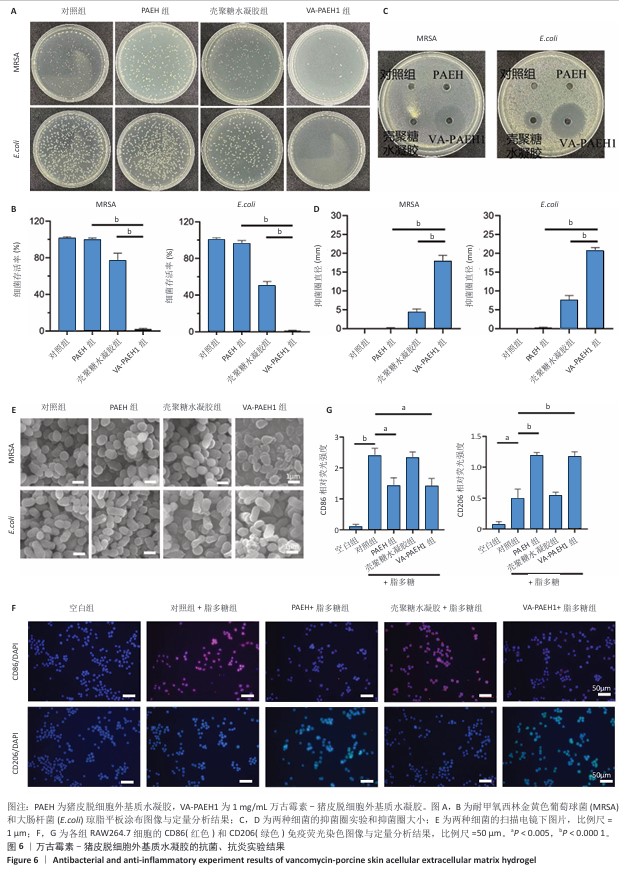
2.3 VA-PAEH1水凝胶的抗菌性能和抗炎活性检测结果 琼脂平板涂布实验结果见图6A,B。结果显示,经PAEH组、壳聚糖水凝胶组和VA-PAEH1组耐甲氧西林金黄色葡萄球菌存活率分别为99.3%,76.67%和2.28%,大肠杆菌存活率分别为96.3%,50.23%和1.17%,表明壳聚糖水凝胶对大肠杆菌具有较强的杀菌作用,而VA-PAEH1水凝胶对耐甲氧西林金黄色葡萄球菌和大肠杆菌有很强的杀菌作用。 抑菌圈实验结果见图6C,D。结果显示,相较于PAEH水凝胶、壳聚糖水凝胶,VA-PAEH1水凝胶可明显抑制耐甲氧西林金黄色葡萄球菌和大肠杆菌的生长繁殖。扫描电镜观察结果显示,VA-PAEH1组耐甲氧西林金黄色葡萄球菌和大肠杆菌形态发生了变化,细菌表现出形态塌陷和中心凹陷,见图6E。结果表明VA-PAEH1水凝胶具有良好的抗菌特性,是由于万古霉素可以破坏细菌细胞壁[32]。 良好的抗炎能力也是促进皮肤创面愈合的关键[33]。 首先,M1和M2型巨噬细胞分别促进炎症和产生抗炎因子[34],此次研究用脂多糖处理RAW264.7细胞模拟炎症反应,通过免疫荧光鉴定了CD86(一种M1型巨噬细胞标记物)和CD206(M2型巨噬细胞标记物)表达。免疫荧光染色结果显示,PAEH组、VA-PAEH1组CD86表达量低于对照组、壳聚糖水凝胶组,CD206表达高于壳聚糖水凝胶组和对照组,差异均有显著性意义,见图6F,G。以上结果表明,PAEH、VA-PAEH1水凝胶促进了巨噬细胞从M1型向M2型转化。 "
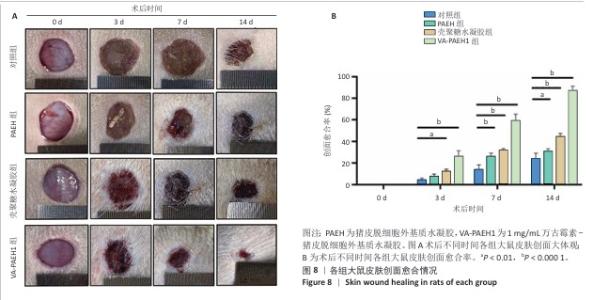
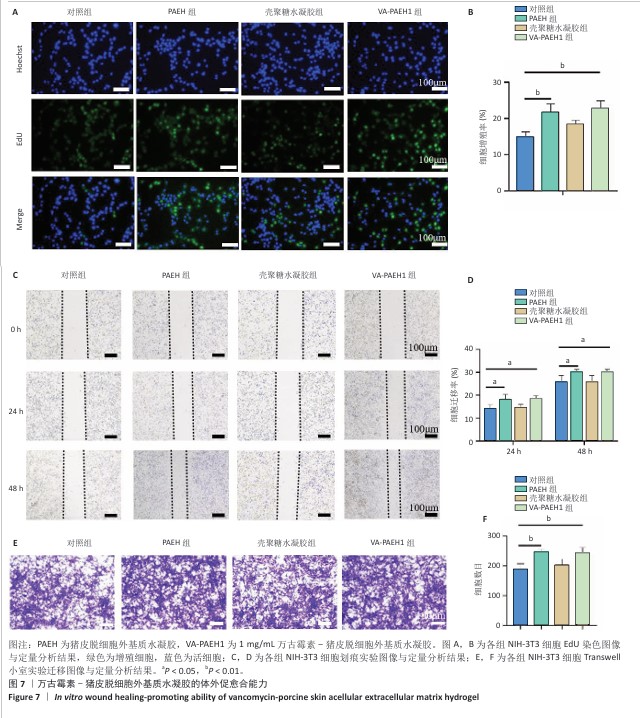
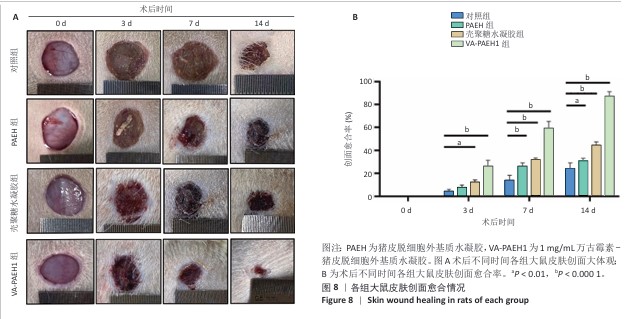
2.5 VA-PAEH1水凝胶修复大鼠感染皮肤创面实验结果 2.5.1 实验动物数量分析 28只SD大鼠全部进入结果分析。 2.5.2 创面愈合率 随着术后时间的延长,各组创面逐渐闭合,见图8A。术后第3天,对照组、PAEH组、壳聚糖水凝胶组和VA-PAEH1组创面愈合率分别为6.26%,8.21%,12.73%和28.13%;术后第7天,VA-PAEH组创面面积明显缩小,创面愈合率约为60.86%,至术后第14天创面几乎完全愈合,对照组、PAEH组和壳聚糖水凝胶组术后第7天的创面愈合率分别为15.21%,26.48%,24.47%,术后第14天的创面愈合率分别为21.41%,30.07%和44.76%,见图8B。 "
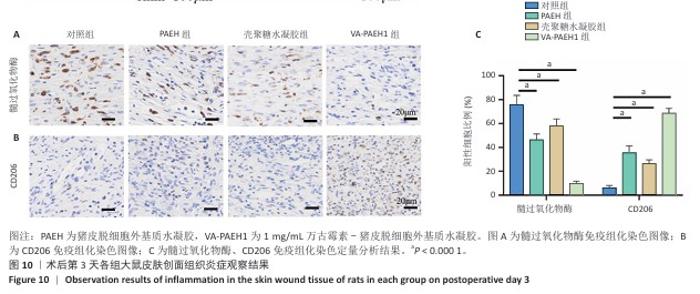
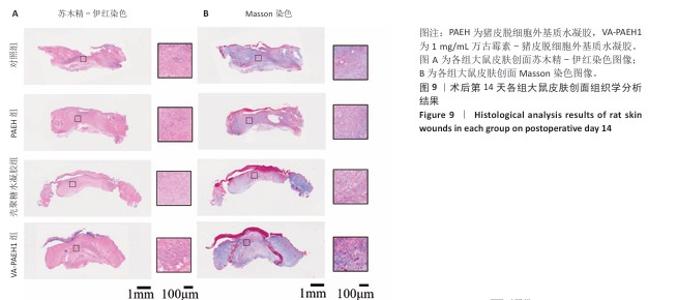
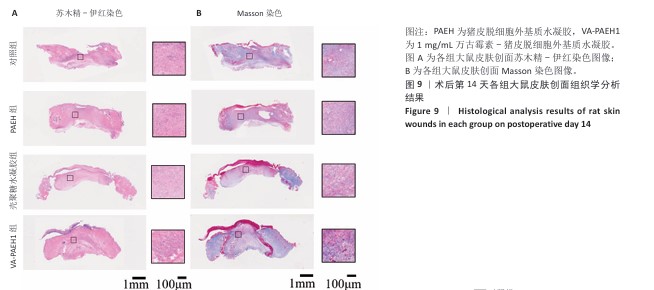
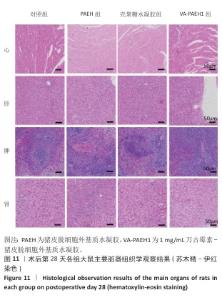
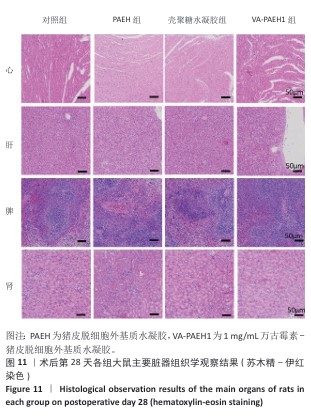
2.5.4 创面组织巨噬细胞表型极化分析 各组大鼠皮肤创面髓过氧化物酶与CD206免疫组化染色结果,见图10。 与对照组相比,PAEH组、壳聚糖水凝胶组和VA-PAEH1组髓过氧化物酶阳性细胞比例都降低,其中VA-PAEH1组降低更明显,这一结果提示VA-PAEH释放的万古霉素通过强效清除病原体消除感染对中性粒细胞的趋化刺激,从而抑制中性粒细胞向感染部位的募集[36]。在皮肤创面愈合早期阶段,M1型巨噬细胞转变为M2型巨噬细胞对组织肉芽过渡到增殖期至关重要[37]。与对照组相比,PAEH组、壳聚糖水凝胶组和VA-PAEH1组CD206阳性细胞比例均升高,其中VA-PAEH1组升高更明显。结果表明,VA-PAEH水凝胶可以实现感染控制与组织再生双重效果。 "
| [1] LI Q, SONG H, LI S, et al. Macrophage metabolism reprogramming EGCG-Cu coordination capsules delivered in polyzwitterionic hydrogel for burn wound healing and regeneration. Bioact Mater. 2023;29:251-264. [2] WANG Y, BEEKMAN J, HEW J, et al. Burn injury: Challenges and advances in burn wound healing, infection, pain and scarring. Adv Drug Deliv Rev. 2018;123:3-17. [3] SINGER AJ, CLARK RA. Cutaneous wound healing. N Engl J Med. 1999; 341(10):738-746. [4] QIAN Y, ZHENG Y, JIN J, et al. Immunoregulation in Diabetic Wound Repair with a Photoenhanced Glycyrrhizic Acid Hydrogel Scaffold. Adv Mater. 2022;34(29):e2200521. [5] 李小鸾.10%聚维酮碘乳膏治疗皮肤感染性创面的疗效观察[J]. 中国实用医药,2020,15(13):143-145. [6] 王廷宇,叶子青,郭晓雨,等.夫西地酸治疗浅II度烧伤的疗效及对烧伤常见菌的体外抗菌活性探讨[J].中国美容医学,2023, 32(4):34-37. [7] BLACKLOW SO, LI J, FREEDMAN BR, et al. Bioinspired mechanically active adhesive dressings to accelerate wound closure. Sci Adv. 2019; 5(7):eaaw3963. [8] ZHAO X, WU H, GUO B, et al. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials. 2017;122:34-47. [9] YANG Z, HUANG R, ZHENG B, et al. Highly Stretchable, Adhesive, Biocompatible, and Antibacterial Hydrogel Dressings for Wound Healing. Adv Sci (Weinh). 2021;8(8):2003627. [10] UBEROI A, MCCREADY-VANGI A, GRICE EA. The wound microbiota: microbial mechanisms of impaired wound healing and infection. Nat Rev Microbiol. 2024;22(8):507-521. [11] NOVIELLO S, COREY GR, HOLLAND TL, et al. A pooled analysis of patients with wound infections in the Phase 3 REVIVE trials: randomized, double-blind studies to evaluate the safety and efficacy of iclaprim versus vancomycin for treatment of acute bacterial skin and skin structure infections. J Med Microbiol. 2020;69(4):625-630. [12] 常欣悦,徐丽,吴晓旭.急性细菌性皮肤感染病原菌耐药性及万古霉素治疗效果[J].中华医院感染学杂志,2022,32(5):745-749. [13] CHEN Y, WANG X, TAO S, et al. Research advances in smart responsive-hydrogel dressings with potential clinical diabetic wound healing properties. Mil Med Res. 2023;10(1):37. [14] ZHANG W, WU W, WANG T, et al. Novel Supramolecular Hydrogel for Infected Diabetic Foot Ulcer Treatment. Adv Healthc Mater. 2024; 13(31):e2402092. [15] KOWALEWSKI M, PASIERSKI M, MAKHOUL M, et al. Topical vancomycin for sternal wound infection prophylaxis. A systematic review and updated meta-analysis of over 40,000 cardiac surgery patients. Surgery. 2023;174(5):1102-1112. [16] 刘旭良,李伯庭,郑介柏,等.负压封闭引流技术联合外用万古霉素治疗骶尾部难愈性压疮的效果[J].医学信息,2022,35(17): 37-40. [17] 何家伟,王菁,汪洋,等.局部应用万古霉素粉末预防膝和髋关节置换术后手术部位感染有效性和安全性的meta分析[J].中国现代应用药学,2024,41(6):812-822. [18] SALEH A, THABET A, BELKHAIR S. Topical Vancomycin for Prevention of Surgical Site Infection after Craniotomy: Meta-analysis and Systematic Literature Review. World Neurosurg. 2022;158:e605-e611. [19] MA H, SIU WS, LEUNG PC. The Potential of MSC-Based Cell-Free Therapy in Wound Healing-A Thorough Literature Review. Int J Mol Sci. 2023;24(11):9356. [20] 石晶,楚瑨,孙涛,等.脱细胞基质-甲基丙烯酰化明胶复合水凝胶的制备及其对肝细胞增殖的作用[J].国际生物医学工程杂志, 2025,48(1):47-55. [21] SRICHAIYAPOL O, MADDOCKS SE, THAMMAWITHAN S, et al. TA-AgNPs/Alginate Hydrogel and Its Potential Application as a Promising Antibiofilm Material against Polymicrobial Wound Biofilms Using a Unique Biofilm Flow Model. Microorganisms. 2022;10(11):2279. [22] JIN F, LI X, CHEN J, et al. Clinical study on the role of platelet-rich plasma in human acellular dermal matrix with razor autologous skin graft repair of giant congenital pigmented nevus in children. J Plast Reconstr Aesthet Surg. 2024;90:305-314. [23] TAMAYO L, SANTANA P, FORERO JC, et al. Coaxial fibers of poly(styrene-co-maleic anhydride)@poly(vinyl alcohol) for wound dressing applications: Dual and sustained delivery of bioactive agents promoting fibroblast proliferation with reduced cell adherence. Int J Pharm. 2022;611:121292. [24] DONG Q, ZU D, KONG L, et al. Construction of antibacterial nano-silver embedded bioactive hydrogel to repair infectious skin defects. Biomater Res. 2022;26(1):36. [25] KONG P, DONG J, LI W, et al. Extracellular Matrix/Glycopeptide Hybrid Hydrogel as an Immunomodulatory Niche for Endogenous Cardiac Repair after Myocardial Infarction. Adv Sci (Weinh). 2023; 10(23):e2301244. [26] IYER V, RAUT J, DASGUPTA A. Impact of pH on growth of Staphylococcus epidermidis and Staphylococcus aureus in vitro. J Med Microbiol. 2021;70(9).doi:10.1099/jmm.0.001421. [27] MO GY, ZHANG SC, LI YX, et al. [Establish mouse osteoblast -osteoclast cell co-culture system in a Transwell chamber]. Zhongguo Gu Shang. 2018;31(3):241-247. [28] LI Y, WANG Y, DING Y, et al. A Double Network Composite Hydrogel with Self-Regulating Cu2+/Luteolin Release and Mechanical Modulation for Enhanced Wound Healing [published correction appears in ACS Nano. 2024 Dec 17;18(50):34415-34418. [29] SIM HW, LEE WY, LEE R, et al. The Anti-Inflammatory Effects of Broccoli (Brassica oleracea L. var. italica) Sprout Extract in RAW 264.7 Macrophages and a Lipopolysaccharide-Induced Liver Injury Model. Curr Issues Mol Biol. 2023;45(11):9117-9131. [30] CAI D, CHEN S, WU B, et al. Construction of multifunctional porcine acellular dermal matrix hydrogel blended with vancomycin for hemorrhage control, antibacterial action, and tissue repair in infected trauma wounds. Mater Today Bio. 2021;12:100127. [31] FANG B, QIU P, XIA C, et al. Extracellular matrix scaffold crosslinked with vancomycin for multifunctional antibacterial bone infection therapy. Biomaterials. 2021;268:120603. [32] THITIANANPAKORN K, AIBA Y, TAN XE, et al. Association of mprF mutations with cross-resistance to daptomycin and vancomycin in methicillin-resistant Staphylococcus aureus (MRSA). Sci Rep. 2020; 10(1):16107. [33] LI F, LIU T, LIU X, et al. Ganoderma lucidum polysaccharide hydrogel accelerates diabetic wound healing by regulating macrophage polarization. Int J Biol Macromol. 2024;260(Pt 2):129682. [34] LEE J, BYUN H, MADHURAKKAT PERIKAMANA SK, et al. Current Advances in Immunomodulatory Biomaterials for Bone Regeneration. Adv Healthc Mater. 2019;8(4):e1801106. [35] WANG Y, GAO H, WANG X, et al. Dual cross-linked self-healing hydrogel enhanced by dopamine nanoparticles and raffinose for wound healing. Int J Biol Macromol. 2024;271(Pt 1):132615. [36] YAO J, XIAO L, LIN MJ, et al. Bifunctional copper ion/melittin incorporated hydrogel with antimicrobial and antioxidant capabilities for infected skin wound healing. Mater Design. 2025;250:113631. [37] XU Y, HU Q, WEI Z, et al. Advanced polymer hydrogels that promote diabetic ulcer healing: mechanisms, classifications, and medical applications. Biomater Res. 2023;27(1):36. [38] GE Y, RONG F, LU Y, et al. Glucose Oxidase Driven Hydrogen Sulfide-Releasing Nanocascade for Diabetic Infection Treatment. Nano Lett. 2023;23(14):6610-6618. [39] LIU S, HUANG Y, JENSEN S, et al. Molecular physiological characterization of the dynamics of persister formation in Staphylococcus aureus. Antimicrob Agents Chemother. 2024;68(1):e0085023. [40] SUN S, WANG Q, ZHANG B, et al. Vancomycin-Loaded in situ Gelled Hydrogel as an Antibacterial System for Enhancing Repair of Infected Bone Defects. Int J Nanomedicine. 2024;19:10227-10245. [41] ANDRIANOPOULOU A, SOKOLOWSKI K, WENZLER E, et al. Assessment of antibiotic release and antibacterial efficacy from pendant glutathione hydrogels using ex vivo porcine skin. J Control Release. 2024;365:936-949. [42] LIU W, GAO R, YANG C, et al. ECM-mimetic immunomodulatory hydrogel for methicillin-resistant Staphylococcus aureus-infected chronic skin wound healing. Sci Adv. 2022;8(27):eabn7006. [43] DEURENBERG RH, STOBBERINGH EE. The evolution of Staphylococcus aureus. Infect Genet Evol. 2008;8(6):747-763. [44] WALTERS MS, EGGERS P, ALBRECHT V, et al. Vancomycin-Resistant Staphylococcus aureus - Delaware, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(37):1056. [45] LI D, LI J, XU Y, et al. Topical vancomycin powder for the prevention of surgical site infections in spinal deformity surgery: a systematic review and meta-analysis. Eur Spine J. 2024;33(12):4653-4663. [46] GHOSH S, MUKHERJEE S, PATRA D, et al. Polymeric Biomaterials for Prevention and Therapeutic Intervention of Microbial Infections. Biomacromolecules. 2022;23(3):592-608. [47] Gao X, Chai X, Lou Y, et al. Fabrication of porcine acellular dermal matrix and oxidized hyaluronic acid conductive composite hydrogels for accelerating skin wound healing under electrical stimulation. Int J Biol Macromol. 2024;282(Pt 4):137179. [48] CANNON JGD, HO AL, MOHOLE J, et al. Topical vancomycin for surgical prophylaxis in non-instrumented pediatric spinal surgeries. Childs Nerv Syst. 2019;35(1):107-111. [49] FURNARY AP, WU Y. Clinical effects of hyperglycemia in the cardiac surgery population: the Portland Diabetic Project. Endocr Pract. 2006;12 Suppl 3:22-26. [50] LAZAR HL, KETCHEDJIAN A, HAIME M, et al. Topical vancomycin in combination with perioperative antibiotics and tight glycemic control helps to eliminate sternal wound infections. J Thorac Cardiovasc Surg. 2014;148:1035-1038;1038-1040. |
| [1] | Guo Yuchao, Ni Qianwei, Yin Chen, Jigeer·Saiyilihan, Gao Zhan . Quaternized chitosan hemostatic materials: synthesis, mechanism, and application [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2091-2100. |
| [2] | Diao Youlu, Gao Jia, Pan Guoqing. Recruitable tissue repair biomaterials: advantages of regulating cell and factor migration and improving tissue integration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5270-5281. |
| [3] | Wang Jieyan, Yao Jiayi, Xin Yingtong, Zhang Xinwen, Li Riwang, Liu Dahai. Chitosan hydrogel drug delivery system is a safer and more effective solution for treating oral ulcers [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5331-5340. |
| [4] | Liu Xiaohong, Zhao Tian, Mu Yunping, Feng Wenjin, Lyu Cunsheng, Zhang Zhiyong, Zhao Zijian, Li Fanghong. Acellular dermal matrix hydrogel promotes skin wound healing in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 395-403. |
| [5] | Kong Xiaojuan, Tan Zhenyu, Lei Lei. Repair of rat endometrial injury by using miR-424-5p modified exosome/Poloxam 407 hydrogel [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3626-3635. |
| [6] | Yang Biao, , Wu Zhonghuan, , Jiang Fugui, , He Chenglong, , Li Tingdong, . Conductive hydrogel with cell-free fat extract repairs spinal cord injuries in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3652-3662. |
| [7] | Gong Yukang, Ye Gaoqi, Wang Chenhao, Chen Dejin, Gao Wenshan. Effects and mechanisms of natural polyphenol-based hydrogels in promoting bone repair [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3675-3686. |
| [8] | Li Qingshan, Li Runmeng, Gao Yuyang, Han Gang, Chen Jiying, Guo Quanyi. Magnetocaloric antitumor and osteogenic properties of magnetic bioactive glass scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3494-3503. |
| [9] | Wang Hao, He Qin, Wang Pingxi, Zhang Jun, Wu Zhilin. Deferoxamine-loaded strontium alginate hydrogel promotes the repair of skull injury in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3609-3617. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||