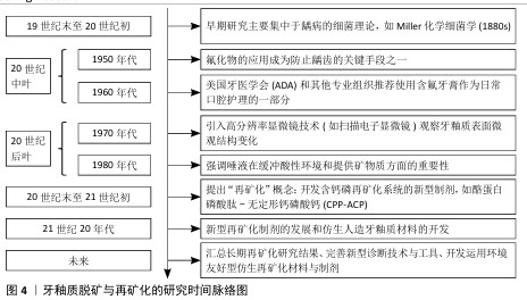
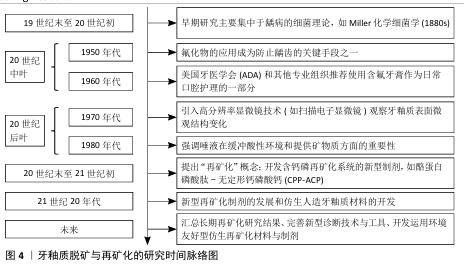
Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (14): 3726-3735.doi: 10.12307/2025.590
Previous Articles Next Articles
Role of enamel demineralization and remineralization in orthodontic treatment
Ma Xinyu, Li Mengying, Li Huang, Han Lei
- Department of Orthodontics, Nanjing Stomatological Hospital · Affiliated Stomatological Hospital of Medical School of Nanjing University, Institute of Stomatology of Nanjing University, Nanjing 210008, Jiangsu Province, China
-
Received:2025-02-20Accepted:2025-04-17Online:2026-05-18Published:2025-09-15 -
Contact:Han Lei, MD, Associate chief physician, Department of Orthodontics, Nanjing Stomatological Hospital · Affiliated Stomatological Hospital of Medical School of Nanjing University, Institute of Stomatology of Nanjing University, Nanjing 210008, Jiangsu Province, China -
About author:Ma Xinyu, Master candidate, Department of Orthodontics, Nanjing Stomatological Hospital · Affiliated Stomatological Hospital of Medical School of Nanjing University, Institute of Stomatology of Nanjing University, Nanjing 210008, Jiangsu Province, China -
Supported by:Nanjing Medical Science and Technology Development Fund Project, No. ZKX23055 (to HL); Lhasa Key Science and Technology Project (Jiangsu Aid Tibet), No. LSKJ202434 (to HL); 2024 Annual Research Program for Regulatory Science in Pharmaceuticals, Jiangsu Province No. 202430 (to HL); High-Level Hospital Construction Project of Nanjing Stomatological Hospital, No. 0224C046 (to HL)
CLC Number:
Cite this article
Ma Xinyu, Li Mengying, Li Huang, Han Lei. Role of enamel demineralization and remineralization in orthodontic treatment[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3726-3735.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
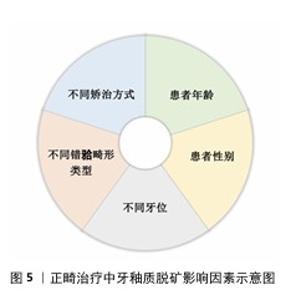
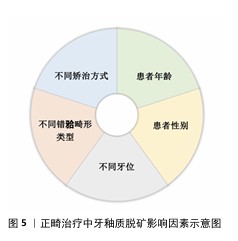
2.2 正畸治疗中牙釉质脱矿的影响因素 在未进行任何干预措施的情况下,正畸治疗患者牙釉质表面脱矿的发生率高达50%-60%[6]。年龄、性别、牙位、不同错牙合畸形类型、不同矫治方式的使用等因素均与正畸治疗中釉质脱矿发生率存在一定相关性[7],见图5。 2.2.1 牙釉质脱矿与年龄因素 正畸过程中牙釉质脱矿的发生趋势随年龄增加逐渐降低[8]。由于青少年年轻恒牙牙体硬组织矿化程度较低[9]、青春期激素水平影响[10]、缺乏口腔健康保健意识和良好依从性,青少年患者成为牙釉质脱矿的易感人群。鉴于早期矫治疗程较短,目前尚未见早期矫治过程中牙釉质脱矿发生率的研究报道。 2.2.2 牙釉质脱矿与性别因素 性别差异不是影响正畸患者牙釉质脱矿发生发展的主要因素[11],但有研究认为由于女性通常具有更高的口腔卫生标准与更强的牙齿健康行为意识,使正畸过程中女性患者釉质脱矿发生率明显低于男性患者[12]。 2.2.3 牙釉质脱矿与不同牙位 上下颌前牙是牙釉质脱矿的易感牙位[6],且由于唾液腺导管开口位置、食物优先接触牙面部位使上颌前牙更易感。在上牙弓中,上颌侧切牙是釉质脱矿的好发部位[13],由于上颌侧切牙临床牙冠较小,常伴随过小牙发生,粘接托槽或附件后釉质暴露面更小,口腔卫生清洁难度增加;在下牙弓中,前磨牙和第一磨牙是釉质脱矿的好发部位,由于下颌后牙牙冠舌倾,龈方区域刷牙时易遗漏,该位置粘接附件后菌斑聚集量随之增加,釉质脱矿现象更加明显[14]。 2.2.4 牙釉质脱矿与不同错牙合畸形类型 不同安氏分类或骨性分类的正畸患者牙釉质脱矿发生率的研究尚未见报道。目前,仅发现针对早期下颌后缩的骨性Ⅱ类患者,正畸治疗中若使用包裹上颌第一磨牙的活动矫治器(与无此装置的活动矫治器相比)更易于营造出脱矿环境[15]。 "
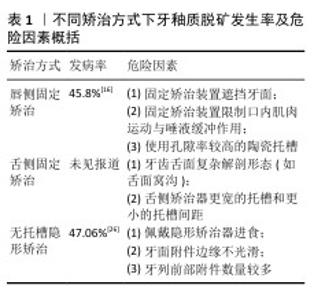
2.2.5 牙釉质脱矿与不同矫治方式 (1)唇侧固定矫治:唇侧固定托槽矫治期间,牙釉质脱矿的发病率和患病率分别为45.8%和68.4%[16]。固定矫治装置的存在促进菌斑滞留、限制口腔内肌肉运动和唾液冲刷自洁功能[17], 使固定矫治器周围成为釉质脱矿的好发部位[10]。通过分析使用唇侧传统托槽配合结扎丝或弹性橡皮圈结扎、唇侧自锁托槽正畸治疗前后,矫治器周围菌斑累积情况及菌斑唾液中致龋菌水平[18-20],有研究认为唇侧自锁托槽更易于口腔卫生清洁,可有效减少细菌滞留[20]。但多数研究则认为不同结扎方式对菌斑保留率和釉质表面白垩色斑产生无显著影响[18],釉质脱矿的发生更大程度上取决于患者口腔卫生状况[21]。针对结果的差异性,未来需要更大样本量、长期随访研究来全面了解不同结扎方式对釉质脱矿的影响。 此外,不同材质托槽与正畸过程中釉质脱矿也存在相关性:与金属托槽相比,陶瓷托槽粘接的牙面表现出更高脱矿率[22],这是由于陶瓷托槽材料表面孔隙率高,随着口腔内食物摩擦,其表面粗糙度随之增加,促进细菌生物膜附着[23]。 (2)舌侧固定矫治:舌侧固定矫治期间,牙釉质脱矿的发病率和患病率数据目前尚未见报道。多数研究认为与传统唇侧固定矫治器相比,舌侧矫治器美观舒适度提高的同时,可有效降低托槽基底下方牙釉质脱矿的发生率[24],这一方面是由于有效粘接条件下,三维个性化定制的舌侧矫治器可将牙齿舌面几乎完全封闭;另一方面是由于舌侧矫治器周围唾液冲刷带来良好自洁作用。但相反观点认为:①牙齿舌面复杂的解剖形态(如舌面窝沟等)是菌斑滞留的危险因素;②舌侧矫治器更宽的托槽和更小的托槽间距不利于口腔卫生清洁措施的实施,导致佩戴舌侧矫治装置的正畸患者表现出更高的釉质脱矿率[25]。未来仍需更大样本量、长时间随访的临床研究来明确舌侧矫治器对釉质脱矿的影响。 (3)无托槽隐形矫治:无托槽隐形矫治期间,牙釉质脱矿在成年患者中发病率为1.2%-47.06%[13,26],青少年患者中发病率为2.85%-35.5%[9,27],结果差异性大可能与受试者牙体硬组织矿化程度、口腔健康保健意识、研究地区医疗水平参差不齐相关[28]。 无托槽隐形矫治期间,患者每日需长时间戴用矫治器,限制了唾液冲刷对牙面的缓冲作用和唇颊舌肌运动对牙面的自洁作用,粘接附件的牙釉质表面成为脱矿的好发部位[26]。此过程中釉质脱矿发生发展的危险因素主要有以下3点:①佩戴矫治器进食者往往忽视对隐形矫治器的清洁,导致前牙切缘、后牙腭侧、附件周围易于聚集菌斑;②牙面附件边缘不光滑,通常是粘接附件后树脂溢出或多余树脂未去净所致[29];③牙列前部附件数量较多,研究发现隐形矫治中釉质脱矿者牙列前部附件数量明显多于未出现釉质脱矿者,使用优化附件或传统附件对釉质脱矿的影响则未见显著差异[9]。 正畸治疗中使用不同矫治方式,其牙釉质脱矿发生率及相关危险因素也存在差异,文章分析比较目前临床上3种常用矫治技术正畸治疗过程中牙釉质脱矿的发生情况并加以汇总,见表1。 "

2.3 正畸治疗中牙釉质脱矿的病因学研究 2.3.1 正畸治疗中牙釉质脱矿过程的口腔菌群变化 正畸治疗中牙釉质脱矿的发生发展类似牙釉质早期龋损的形成。口腔内环境变化、常驻菌群平衡失调的不利转变[30],使釉质中无机物脱矿、有机物分解。口腔微生物菌群组成改变除了受到机体免疫反应、激素水平影响,与正畸治疗也存在相关性[31]。正畸矫治器会诱导菌斑快速生长,影响细菌代谢,改变菌群坏境[32];与非正畸治疗者相比,正畸患者菌斑唾液中致龋菌和产酸菌比例显著增加[33],矫治器周围菌斑中pH值及钙磷离子含量下降,釉质脱矿进程加快。 正畸矫治器与口腔微生物群组成变化存在中高度关联性[32]。固定矫治过程中,包括牙龈卟啉单胞菌、福赛坦氏菌在内的部分牙周致病菌呈下降趋势[34],且固定矫治器会吸附丰度更高的纤毛菌[35],纤毛菌作为人体正常菌群,通常定植于口腔和泌尿生殖道,并与龋病的发生发展密切相关[36]。无托槽隐形矫治过程中,矫治器内表面菌群多样性显著减少,链球菌属比例大幅上升,龈下菌群结构相对稳定[37];且由于口腔卫生措施实施的便利性[38],隐形矫治器的戴用可获得更好的口腔健康指数[35]。 综上所述,正畸治疗中釉质脱矿引起的口腔菌群失调主要是矫治器促使龋病等相关细菌在菌斑唾液中大量繁殖、潜在革兰阴性致病菌比例上升所致[39],但这种变化尚不足以带来显著、严重的影响,牙釉质表面脱矿导致的白垩色斑可在合理应用再矿化制剂后有所改善。 2.3.2 牙釉质脱矿-再矿化平衡 (1)正畸治疗中的牙釉质脱矿:正畸患者戴入矫治器后,口腔卫生维护难度增加,加之不良饮食习惯,牙面有糖类存留且龈上菌斑难以完全清除,以蔗糖为主的多糖在口内水解为单糖后迅速扩散至致龋菌内,菌斑深部呈现缺氧状态[40],便于链球菌等致龋菌进行无氧糖酵解代谢,不断以糖类为底物并将其转化为以乳酸为主的大量有机酸,这些酸在致密、凝胶状的菌斑中难以清除,导致唾液缓冲作用无法完全发挥。酸的持续产生使口腔内局部pH值显著下降,当pH值下降至临界值5.5甚至以下时,釉质的脱矿-再矿化平衡被打破,脱矿过程占优势地位:微观上表现为羟基磷灰石晶体中钙离子溶解,磷酸根离子浓度降低,晶体结构逐渐丧失,釉质中无机物脱矿,有机物坏死分解;宏观上表现为釉质表面出现形状不规则、颜色不透明的白垩色斑[39],严重脱矿时,釉质表面进一步剥离,形成龋损。 此外,正畸过程中医源性因素对龈上菌斑的生存环境也会产生影响。釉质表面粘接托槽前处理时,酸蚀面积过大、酸蚀时间过长、粘接托槽或附件后未及时清除周围多余粘接剂均会促进菌斑附着,是釉质脱矿的不利因素[41]。随着正畸治疗持续时间的延长,釉质脱矿导致白垩色斑的发生率及严重程度也会随之增加[8]。 (2)正畸治疗中的牙釉质再矿化:由于糖的扩散作用、酸游离出菌斑、唾液缓冲作用[1],口腔内局部pH值可逐渐回升至中性附近。牙釉质羟基磷灰石晶体中游离出大量钙磷离子,在菌斑中达到过饱和状态,使钙磷离子在脱矿釉质晶体表面和孔隙中重新沉积,产生矿物质净增益的效果,即牙釉质再矿化[42]。 正畸治疗结束后,部分刚拆除矫治器的患者牙釉质表面脱矿明显,白垩色病损边缘清晰;拆除矫治器数月后,随着口腔卫生清洁措施更易实施,唾液缓冲作用更加充分,菌斑内酸性环境改变,釉质脱矿不再占主导地位,表现出一定程度的釉质再矿化:白垩色病损面积缩小、颜色变浅、边缘逐渐模糊。然而,在不进行任何再矿化制剂干预的情况下,唾液中钙磷离子浓度较低,正畸治疗后牙釉质表面的自然再矿化过程漫长且困难,多数白垩色病损短期内并不会有所好转[41]。但有研究探讨仿生再矿化疗法对正畸治疗中牙釉质早期脱矿的作用机制,已明确釉原蛋白、非釉原蛋白、釉原蛋白肽等成分对脱矿釉质表面再矿化的积极作用[43]。此外,有团队设计合成具备多尺度分层结构的纳米级类牙釉质材料,其强度、硬度、黏弹性等理化性质表现均不劣于天然牙釉质[2],新型人造仿生牙釉质材料的合成为促进正畸治疗中脱矿釉质再矿化提供新的思路。 2.3.3 口腔环境中的氧化应激 唾液中的氧化应激状态也是牙釉质脱矿发生发展的促进因素[44],氧化应激本质上是自由基的产生与机体通过抗氧化剂中和自由基来抵消或最小化其有害影响之间的不平衡。过量活性氧的产生会造成氧化应激状态,活性氧包括氧自由基和非自由基氧衍生物[45],这些自由基可在有氧条件下通过多种细胞代谢途径产生:线粒体呼吸链、微粒体细胞色素 P450酶、黄素蛋白氧化酶和真核细胞中的过氧化物酶体脂肪酸代谢均是活性氧的重要细胞内来源[46]。 由于口腔黏膜允许物质快速交换吸收,口腔内细胞对氧化应激损伤非常敏感,测定唾液中氧化应激状态可为监测和诊断龋病等口腔疾病提供有效工具[47]。临床研究表明唾液的总抗氧化能力与龋齿之间存在相关性,较高的龋病严重程度可增加唾液抗氧化活性,进而减轻氧化损伤[48];实验室研究证明口腔主要致龋菌变形链球菌具有Spx等调节因子,可激活氧化应激反应,在氧化应激水平较高的口腔环境中,对病原菌的生长繁殖起到促进作用[49-50]。 此外,有研究认为正畸治疗也可短期内改变受试者唾液中的氧化-抗氧化平衡[51],这是由于戴入矫治器营造了更加复杂的口腔内环境,被移动牙齿周围局部炎症反应产生的大量炎症因子参与正畸治疗中的机械力作用,导致自由基合成增加,氧化应激随之而来。但相反观点通过分析牙齿移动过程中唾液内炎症因子和氧化应激标志物水平的变化,认为正畸牙齿移动和矫治过程中使用的材料器械不会导致口腔氧化水平超过生理限度,唾液和龈沟液中均不存在氧化损伤[52]。 "

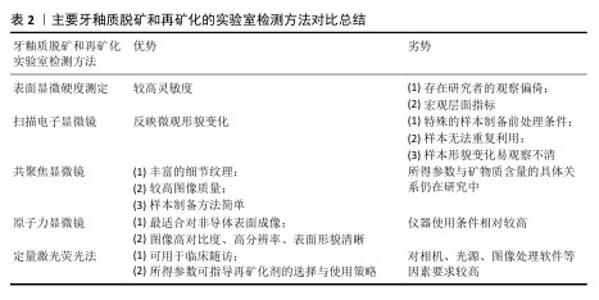
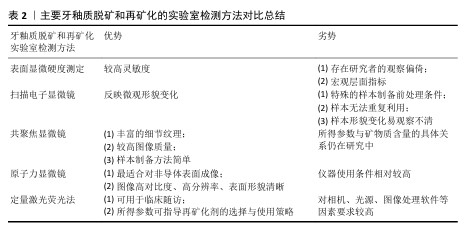
2.4 正畸治疗中牙釉质再矿化的方法与策略 2.4.1 牙釉质脱矿和再矿化检测方法 牙釉质表层相对完整而表层下脱矿是正畸治疗中牙釉质脱矿的典型病理特征。临床上传统的龋病检测方法如视诊、探诊、X射线片等难以发现这种早期病损,且口内检查时运用的国际龋病检测评估系统视觉评分受研究者主观影响较大[53],缺乏可重复性与再现性。目前已有研究团队采用体外实验室检测手段与体内模拟临床实际环境相结合的方法,将制备完成的脱矿釉质薄片置于患者口腔中进行再矿化制剂的疗效监测,尽管此种方法对受试者依从性要求较高,临床施行相对困难,但为牙釉质脱矿和再矿化研究提供了新的思路[54]。鉴于各类定性、定量、高敏感度的实验室检查方法对釉质脱矿早期监测与诊断的适用性和灵敏性,文章着重于探讨牙釉质脱矿和再矿化的各类实验室检测手段及方法,见表2。 (1)表面显微硬度测定:显微硬度压痕测定已成为目前研究牙体硬组织机械性能变化广泛使用的手段。硬度作为牙釉质的重要力学性能之一,可反映矿物质含量;硬度变化也可反映釉质中矿物质的获得和丢失[55]。该方法对釉质脱矿与再矿化的检测具有较高灵敏度,但仍属于宏观层面的定量指标分析,且测定时多存在研究者的观察偏倚[56]。 (2)扫描电子显微镜:作为一种用于观测牙釉质表层脱矿、再矿化程度的常用手段,可通过观察脱矿与再矿化前后釉质表面微观结构改变来评估牙体硬组织变化情况[55]。但测试前需对样本进行喷金处理,以满足适当的样本制备和检测条件[57],且放大倍数有限,样本无法重复利用,形貌变化指标易观察不清等不利因素也使扫描电镜的应用场景受到一定限制。 (3)共聚焦显微镜:相较于传统光学显微镜,共聚焦显微镜所得图像包含传统图像无法具备的细节纹理及更高图像质量;相较于扫描电子显微镜,共聚焦显微镜的样本制备更加简单,不会因样本前处理干燥脱水后造成微观结构的塌陷变形,可用于完整牙齿样本的表层下检测,并以三维形式呈现结果,但是所得参数与矿物质含量的具体关系仍在研究中[58]。 (4)原子力显微镜:是扫描探针显微镜家族中最适合对非导体表面进行成像的仪器,可提供高对比度、高分辨率、表面形貌清晰的图像[59]。现已应用于牙釉质脱矿和再矿化的微观形貌观测及相关量化分析[60],可直观监测随脱矿时间增加,牙釉质表面结构的疏松程度;经再矿化处理,釉质表面结构逐渐致密,粗糙度相应降低。相较于扫描电子显微镜,原子力显微镜可提供放大倍数更高的微观形貌,样本无需前处理,测试后的样本仍可用于后续实验研究,为评估釉质脱矿及再矿化效果提供了一种直接有效的方法。 (5)其他:除上述方法外,另有定量激光荧光法在监测牙釉质初期脱矿前后矿物质含量微小变化方面表现出高效性[61],且已有研究将此检测手段运用于临床随访中,根据体外实验结果和临床测定的定量激光荧光参数指导正畸治疗过程中再矿化剂的最佳选择和使用策略[62]。此外,分光光度计可用于宏观层面评估釉质颜色改变情况;显微拉曼光谱仪可用于微观层面评估釉质表面脱矿及再矿化程度;X射线衍射仪可用于评估脱矿与再矿化前后釉质表面沉积物的物相组成和晶体结构;X射线能谱分析仪可用于评估脱矿与再矿化前后釉质表面沉积物的钙磷元素组成、含量及比例[63-64]。单一的检测方法缺乏足够灵敏性和特异性,多种检测方法联合应用可一定程度上提高检测结果的准确性,达到更加明确的诊断。 "
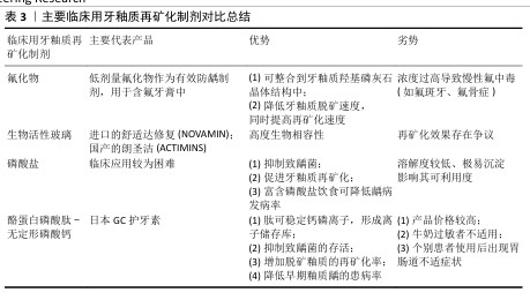
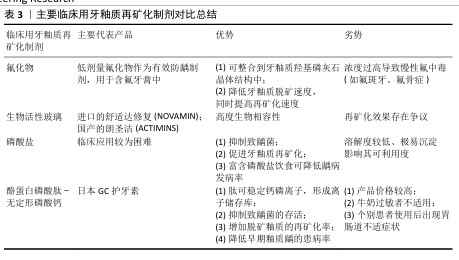
2.4.2 促进牙釉质再矿化的临床应用与进展 (1)氟化物:氟是与牙釉质脱矿和再矿化关系最密切的微量元素,也是能够整合到牙釉质羟基磷灰石晶体结构中最重要的一种离子[63,65]。氟化物主要包括氟化钠 、氟化亚锡等,目前临床上将低剂量的氟化物作为有效防龋制剂,主要运用于含氟牙膏中;局部高浓度氟化物(> 2 500 mg/L)可在牙齿表面和牙菌斑中形成氟库(类似氟化钙的沉积物),提供穿透菌斑的驱动力,将氟离子输送至釉质表面[30]。此外,氟离子还可作为抗菌剂和细菌酶烯醇化酶的抑制剂,有效减少细菌产酸。 氟化物已被证明可降低牙釉质脱矿速度,同时可提高牙釉质再矿化速度。在对抗釉质脱矿的过程中,氟离子主要表现在改善釉质羟基磷灰石晶体结构、降低溶解性、促进脱矿区域再矿化;抑制致龋菌生长及相关酶的活性;降低牙釉质表面自由能、减少对蛋白质及细菌的黏附作用3个方面[66]。在促进牙釉质再矿化过程中,氟离子通过置换羟基磷灰石中的羟基,与相邻的OH-和Ca2+形成OH-F氢键和Ca-F共价键[17],使磷灰石晶胞体积减小,晶格结构更稳定,形成的氟基磷灰石比羟基磷灰石结晶度更好,不易受酸溶解和釉质脱矿的影响,从而提高釉质的硬度和耐酸性(化学耐久性)。但摄入氟化物浓度过高也会导致慢性氟中毒,出现氟斑牙、氟骨症等不良并发症。 (2)生物活性玻璃:是以钙、磷、硅、钠为主要组成成分的生物活性材料[67],具有高度生物相容性,最初被开发运用于骨再生领域,近年来作为抗敏感和牙本质矿化材料,用于牙膏和抛光膏,以进口的舒适达修复(NOVAMIN)和国产的朗圣洁(ACTIMINS)为主要代表产品。模拟口腔条件下,生物活性玻璃在唾液等水性环境中可释放大量钙离子、钠离子及磷酸根离子,钠离子又迅速与氢离子(以H3O+的形式)置换出钙磷离子,形成离子储存库。钙磷离子以富硅层为成核位点,在生物活性玻璃表面沉积生长。由于钠离子的初期释放,使得局部pH值瞬时升高,有助于钙离子和磷酸根离子的沉淀,在釉质表面形成一层耐磨性佳的磷酸钙层[68],伴随反应持续发生,该层逐渐结晶为羟基碳酸磷灰石,羟基碳酸磷灰石具有与牙齿中天然存在的磷灰石相似的化学组成和结构[69]。目前针对生物活性玻璃再矿化效果的研究仍存在争议,体外研究表明其具有促进釉质再矿化的作用[55,70],但尚无足够证据证明对牙本质再矿化的影响[71],未来仍需深入研究证明生物活性玻璃的再矿化效果及临床运用的有效性。 (3)磷酸盐制剂:有研究认为局部使用磷酸盐制剂有抑制致龋菌、促进牙釉质再矿化的作用[72],富含磷酸盐的饮食可一定程度上降低大鼠和人类龋病的发病率和患病率[73],这是由于当牙体硬组织受损时,口腔内游离的钙磷离子可作为促进其再矿化的重要成分,丰富的磷酸钙可使菌斑、唾液中钙磷离子浓度达到饱和,磷酸盐缓冲体系也可有效缓冲菌斑内的有机酸,减少羟基磷灰石的溶解,抑制釉质脱矿;对于已经脱矿的釉质表面,磷酸钙的重新沉积可促进釉质再矿化进程。但是由于磷酸盐溶解度较低,高浓度的钙磷离子在溶液中极易沉淀,影响可利用度[74],导致磷酸盐再矿化系统在临床应用时较为困难。 (4)酪蛋白磷酸肽-无定形磷酸钙(CPP-ACP):是近年来新研制的一种非含氟再矿化制剂,它是由酪蛋白磷酸肽和无定形磷酸钙结合形成的复合体,其内部储存大量钙磷离子,起到离子储存库的作用。同时,作为一种纳米级防龋材料,其结构中的肽可稳定钙、磷等离子,防止离子间结合,保持游离[75]。钙磷离子持续释放形成的过饱和状态,促使磷酸钙沉积于釉质表面,达到较好的促进釉质再矿化的效果[64,76]。此外,酪蛋白磷酸肽-无定形磷酸钙还可抑制部分致龋菌,降低其存活率[77]。目前以日本GC护牙素为主要代表产品,已有研究证明此产品可降低早期釉质龋患病率、增加脱矿釉质的再矿化率[78]。另有团队对无定形磷酸钙材料进行改形与改性[63,79],发现该材料对口腔致龋菌的抑制和釉质再矿化均具有一定促进作用。 但相较于其他再矿化制剂,酪蛋白磷酸肽-无定形磷酸钙产品价格较高,且由于其所含酪蛋白从牛奶中提取,故对牛奶过敏者不适用[80];另有研究报道个别患者使用酪蛋白磷酸肽-无定形磷酸钙产品后出现胃肠道不适症状[78]。 主要临床用牙釉质再矿化制剂对比总结,见表3。 (5)抗菌光动力疗法:近年来,光动力疗法在口腔正畸学领域的应用越来越广泛,光敏剂、特定波长的光源、氧气存在是抗菌光动力疗法不可或缺的3个要素[81-82]。 在特定波长光源(如卤素灯、激光等)、氧气存在条件下激活光敏剂,可产生活性氧,促进细菌成分中不可逆的氧化过程,导致致龋菌死亡[81,83],故正畸治疗中可运用抗菌光动力疗法,去除釉质表面及矫治器周围菌斑,降低菌斑生物膜的细菌活力和代谢活性,达到抑制釉质早期脱矿的效果[84]。激光处理后的釉质表面抗酸性增强,抗龋性提高,这可能与釉质中的有机成分被分解、釉柱排列方向发生改变有关[85]。此外,活性氧可促进牙釉质和牙本质中色素分子的降解,减少光吸收,对非破坏性牙齿美白有效[86-87]。 "
| [1] 于世凤.口腔组织病理学[M].5版.北京:人民卫生出版社,2004. [2] ZHAO H, LIU S, WEI Y, et al. Multiscale engineered artificial tooth enamel. Science. 2022;375(6580):551-556. [3] FLYNN LN, JULIEN K, NOURELDIN A, et al. The efficacy of fluoride varnish vs a filled resin sealant for preventing white spot lesions during orthodontic treatment. Angle Orthod. 2022;92(2):204-212. [4] MALCANGI G, PATANO A, MOROLLA R, et al. Analysis of Dental Enamel Remineralization: A Systematic Review of Technique Comparisons. Bioengineering (Basel). 2023; 10(4):472. [5] WANG N, YU J, YAN J, et al. Recent advances in antibacterial coatings for orthodontic appliances. Front Bioeng Biotechnol. 2023; 11:1093926. [6] GORELICK L, GEIGER AM, GWINNETT AJ. Incidence of white spot formation after bonding and banding. Am J Orthod. 1982;81(2):93-98. [7] 张婧琦,杨一,高嫣子,等.隐形与固定矫治器正畸后白垩斑发病率的比较[J].口腔医学研究,2024,40(2):152-155. [8] RICHTER AE, ARRUDA AO, PETERS MC, et al. Incidence of caries lesions among patients treated with comprehensive orthodontics. Am J Orthod Dentofacial Orthop. 2011; 139(5):657-664. [9] LIU Q, SONG Z. Incidence, severity, and risk factors for white spot lesions in adolescent patients treated with clear aligners. Orthod Craniofac Res. 2024; 27(5):704-713. [10] ABBATE GM, CARIA MP, MONTANARI P, et al. Periodontal health in teenagers treated with removable aligners and fixed orthodontic appliances. J Orofac Orthop. 2015;76(3):240-250. [11] SAGARIKA N, SUCHINDRAN S, LOGANATHAN S, et al. Prevalence of white spot lesion in a section of Indian population undergoing fixed orthodontic treatment: An in vivo assessment using the visual International Caries Detection and Assessment System II criteria. J Conserv Dent. 2012;15(2):104-108. [12] JULIEN KC, BUSCHANG PH, CAMPBELL PM. Prevalence of white spot lesion formation during orthodontic treatment. Angle Orthod. 2013;83(4): 641-647. [13] BUSCHANG PH, CHASTAIN D, KEYLOR CL, et al. Incidence of white spot lesions among patients treated with clear aligners and traditional braces. Angle Orthod. 2019; 89(3):359-364. [14] KHALAF K. Factors Affecting the Formation, Severity and Location of White Spot Lesions during Orthodontic Treatment with Fixed Appliances. J Oral Maxillofac Res. 2014;5(1):e4. [15] ALEXANDER SA. The effect of fixed and functional appliances on enamel decalcifications in early Class II treatment. Am J Orthod Dentofacial Orthop. 1993; 103(1):45-47. [16] SUNDARARAJ D, VENKATACHALAPATHY S, TANDON A, et al. Critical evaluation of incidence and prevalence of white spot lesions during fixed orthodontic appliance treatment: A meta-analysis. J Int Soc Prev Community Dent. 2015;5(6):433-439. [17] O’REILLY MM, FEATHERSTONE JD. Demineralization and remineralization around orthodontic appliances: an in vivo study. Am J Orthod Dentofacial Orthop. 1987;92(1):33-40. [18] LAZAR L, VLASA A, BERESESCU L, et al. White Spot Lesions (WSLs)-Post-Orthodontic Occurrence, Management and Treatment Alternatives: A Narrative Review. J Clin Med. 2023;12(5):1908. [19] CHHIBBER A, AGARWAL S, YADAV S, et al. Which orthodontic appliance is best for oral hygiene? A randomized clinical trial. Am J Orthod Dentofacial Orthop. 2018; 153(2):175-183. [20] PELLEGRINI P, SAUERWEIN R, FINLAYSON T, et al. Plaque retention by self-ligating vs elastomeric orthodontic brackets: quantitative comparison of oral bacteria and detection with adenosine triphosphate-driven bioluminescence. Am J Orthod Dentofacial Orthop. 2009;135(4): 426.e1-9. [21] POLAT Ö, GÖKÇELIK A, ARMAN A, et al. A comparison of white spot lesion formation between a self-ligating bracket and a conventional preadjusted straight wire bracket. World J Orthod. 2008;9(2):e46-50. [22] TOZ ERTOP M, CICEK O, ERENER H, et al. Evaluation of the Demineralization Development around Different Types of Orthodontic Brackets. Materials (Basel). 2023;16(3):984. [23] ALMOSA NA, SIBAI BS, REJJAL OA, et al. Enamel demineralization around metal and ceramic brackets: an in vitro study. Clin Cosmet Investig Dent. 2019;11:37-43. [24] KNÖSEL M, KLANG E, HELMS HJ, et al. Occurrence and severity of enamel decalcification adjacent to bracket bases and sub-bracket lesions during orthodontic treatment with two different lingual appliances. Eur J Orthod. 2016; 38(5):485-492. [25] LOMBARDO L, ORTAN YÖ, GORGUN Ö, et al. Changes in the oral environment after placement of lingual and labial orthodontic appliances. Prog Orthod. 2013;14:28. [26] 胡丹艳,陈慧芬,吴峻青,等.无托槽隐形矫治中牙釉质脱矿情况的临床调查[J].口腔医学,2024,44(10):742-746. [27] AZEEM M, UL HAMID W. Incidence of white spot lesions during orthodontic clear aligner therapy. J World Fed Orthod. 2017;6(3): 127-130. [28] ALBHAISI Z, AL-KHATEEB SN, ABU ALHAIJA ES. Enamel demineralization during clear aligner orthodontic treatment compared with fixed appliance therapy, evaluated with quantitative light-induced fluorescence: A randomized clinical trial. Am J Orthod Dentofacial Orthop. 2020;157(5):594-601. [29] LOW B, LEE W, SENEVIRATNE CJ, et al. Ultrastructure and morphology of biofilms on thermoplastic orthodontic appliances in ‘fast’ and ‘slow’ plaque formers. Eur J Orthod. 2011;33(5):577-583. [30] PITTS NB, ZERO DT, MARSH PD, et al. Dental caries. Nat Rev Dis Primers. 2017; 3:17030. [31] SANTONOCITO S, POLIZZI A. Oral Microbiota Changes during Orthodontic Treatment. Front Biosci (Elite Ed). 2022; 14(3):19. [32] LUCCHESE A, BONDEMARK L, MARCOLINA M, et al. Changes in oral microbiota due to orthodontic appliances: a systematic review. J Oral Microbiol. 2018; 10(1):1476645. [33] MUMMOLO S, NOTA A, ALBANI F, et al. Salivary levels of Streptococcus mutans and Lactobacilli and other salivary indices in patients wearing clear aligners versus fixed orthodontic appliances: An observational study. PLoS One. 2020; 15(4):e0228798. [34] SANDIĆ MZ, POPOVIĆ B, CARKIĆ J, et al. Changes in subgingival microflora after placement and removal of fixed orthodontic appliances. Srp Arh Celok Lek. 2014; 142(5-6):301-305. [35] SHOKEEN B, VILORIA E, DUONG E, et al. The impact of fixed orthodontic appliances and clear aligners on the oral microbiome and the association with clinical parameters: A longitudinal comparative study. Am J Orthod Dentofacial Orthop. 2022;161(5): e475-e485. [36] ERIKSSON L, LIF HOLGERSON P, JOHANSSON I. Saliva and tooth biofilm bacterial microbiota in adolescents in a low caries community. Sci Rep. 2017;7(1):5861. [37] ROUZI M, JIANG Q, ZHANG H, et al. Characteristics of oral microbiota and oral health in the patients treated with clear aligners: a prospective study. Clin Oral Investig. 2023;27(11):6725-6734. [38] WANG Q, MA JB, WANG B, et al. Alterations of the oral microbiome in patients treated with the Invisalign system or with fixed appliances. Am J Orthod Dentofacial Orthop. 2019;156(5):633-640. [39] ZHOU XD. Dental Caries: Principles and Management. Berlin, Heidelberg: Springer Berlin Heidelberg, 2016. [40] 周学东.龋病学[M].北京:人民卫生出版社,2011. [41] 崔晶.多乐氟、护牙素及含氟牙膏促进釉质再矿化的实验研究[D].西安:第四军医大学,2016. [42] PHILIP N. State of the Art Enamel Remineralization Systems: The Next Frontier in Caries Management. Caries Res. 2019;53(3):284-295. [43] 蔺孝慧,杨梦源,李春年.仿生再矿化治疗在牙釉质早期脱矿的作用及机制[J].中国组织工程研究,2025,29(4):856-865. [44] BUCZKO P, ZALEWSKA A, SZARMACH I. Saliva and oxidative stress in oral cavity and in some systemic disorders. J Physiol Pharmacol. 2015;66(1):3-9. [45] BETTERIDGE DJ. What is oxidative stress? Metabolism. 2000;49(2 Suppl 1):3-8. [46] RAHMAN MT, HOSSAIN A, PIN CH, et al. Zinc and Metallothionein in the Development and Progression of Dental Caries. Biol Trace Elem Res. 2019;187(1):51-58. [47] SKUTNIK-RADZISZEWSKA A, ZALEWSKA A. Salivary Redox Biomarkers in the Course of Caries and Periodontal Disease. Appl Sci. 2020;10(18):6240. [48] ARAUJO HC, NAKAMUNE ACMS, GARCIA WG, et al. Carious Lesion Severity Induces Higher Antioxidant System Activity and Consequently Reduces Oxidative Damage in Children’s Saliva. Oxid Med Cell Longev. 2020;2020:3695683. [49] CHENG X, XU X, ZHOU X, et al. Oxidative stress response: a critical factor affecting the ecological competitiveness of Streptococcus mutans. J Oral Microbiol. 2023;16(1):2292539. [50] YU S, MA Q, LI Y, et al. Molecular and regulatory mechanisms of oxidative stress adaptation in Streptococcus mutans. Mol Oral Microbiol. 2023;38(1):1-8. [51] BUCZKO P, KNAŚ M, GRYCZ M, et al. Orthodontic treatment modifies the oxidant-antioxidant balance in saliva of clinically healthy subjects. Adv Med Sci. 2017;62(1):129-135. [52] ATUĞ ÖZCAN SS, CEYLAN I, OZCAN E, et al. Evaluation of oxidative stress biomarkers in patients with fixed orthodontic appliances. Dis Markers. 2014;2014:597892. [53] 胡轶,苏丽萍,王胜朝.国际龋病检测与评估系统(ICDAS)的介绍[J].牙体牙髓牙周病学杂志,2016,26(9):554-558. [54] OLIVEIRA PRA, BARBOZA CM, BARRETO LSDC, et al. Effect of CPP-ACP on remineralization of artificial caries-like lesion: an in situ study. Braz Oral Res. 2020;34:e061. [55] GANGWAR A, JHA KK, THAKUR J, et al. In Vitro evaluation of remineralization potential of novamin on artificially induced carious lesions in primary teeth using scanning electron microscope and vickers hardness. Indian J Dent Res. 2019;30(4):590-594. [56] 郝丽英,刘宇峰,文莺惠,等.原子力显微镜对牙釉质脱矿再矿化的分析[J].实验室研究与探索,2024,43(1):27-31+49. [57] RISNES S, SAEED M, SEHIC A. Scanning Electron Microscopy (SEM) Methods for Dental Enamel. Methods Mol Biol. 2019;1922:293-308. [58] CHOKSHI K, CHOKSHI A, KONDE S, et al. An in vitro Comparative Evaluation of Three Remineralizing Agents using Confocal Microscopy. J Clin Diagn Res. 2016;10(6):ZC39-42. [59] FARINA M, SCHEMMEL A, WEISSMÜLLER G, et al. Atomic force microscopy study of tooth surfaces. J Struct Biol. 1999;125(1):39-49. [60] SUBRAMANI K, KWOK C, NAPOLES JA, et al. Enamel remineralization potential of bioactive glass air abrasion studied via elemental and surface morphology analysis. J Clin Exp Dent. 2023;15(10):e835-e841. [61] PARK SW, KANG SM, LEE HS, et al. Lesion activity assessment of early caries using dye-enhanced quantitative light-induced fluorescence. Sci Rep. 2022;12(1): 11848. [62] DING L, HE D, ZHENG S, et al. In-vitro and in-vivo comparative studies of treatment effects on enamel demineralization during orthodontic therapy: implications for clinical early-intervention strategy. Clin Oral Investig. 2024;28(10):545. [63] YAN J, CAO L, LUO T, et al. In vitro evaluation of a novel fluoride-coated clear aligner with antibacterial and enamel remineralization abilities. Clin Oral Investig. 2023;27(10):6027-6042. [64] ABUFARWA M, NOURELDIN A, DZIAK R, et al. Efficacy of CPP-ACP fluoride varnish applied with and without acid etching in preventing enamel demineralization compared to light-curable fluoride varnish. Angle Orthod. 2022;92(2):213-219. [65] HU H, FENG C, JIANG Z, et al. Effectiveness of remineralizing agents in the prevention and reversal of orthodontically induced white spot lesions: a systematic review and network meta-analysis. Clin Oral Investig. 2020;24(12):4153-4167. [66] MARGOLIS HC, MORENO EC, MURPHY BJ. Effect of low levels of fluoride in solution on enamel demineralization in vitro. J Dent Res. 1986;65(1):23-29. [67] EL-WASSEFY NA. Remineralizing effect of cold plasma and/or bioglass on demineralized enamel. Dent Mater J. 2017;36(2):157-167. [68] ZHONG JP, GREENSPAN DC, FENG JW. A microstructural examination of apatite induced by Bioglass in vitro. J Mater Sci Mater Med. 2002;13(3):321-326. [69] HAGHGOO R, AHMADVAND M, MOSHAVERINIA S. Remineralizing Effect of Topical NovaMin and Nano-hydroxyapatite on caries-like Lesions in Primary teeth. J Contemp Dent Pract. 2016;17(8):645-649. [70] SHIHABI S, ALNESSER S, COMISI JC. Comparative Remineralization Efficacy of Topical NovaMin and Fluoride on Incipient Enamel Lesions in Primary Teeth: Scanning Electron Microscope and Vickers Microhardness Evaluation. Eur J Dent. 2021;15(3):420-424. [71] FERNANDO D, ATTIK N, PRADELLE-PLASSE N, et al. Bioactive glass for dentin remineralization: A systematic review. Mater Sci Eng C Mater Biol Appl. 2017;76:1369-1377. [72] MÜLLER WEG, NEUFURTH M, ACKERMANN M, et al. Bifunctional dentifrice: Amorphous polyphosphate a regeneratively active sealant with potent anti-Streptococcus mutans activity. Dent Mater. 2017;33(7): 753-764. [73] GILMORE ND. The effect on dental caries-activity of supplementing diets with phosphates; a review. J Public Health Dent. 1969;29(3):188-207. [74] COCHRANE NJ, CAI F, HUQ NL, et al. New approaches to enhanced remineralization of tooth enamel. J Dent Res. 2010;89(11): 1187-1197. [75] CROSS KJ, HUQ NL, STANTON DP, et al. NMR studies of a novel calcium, phosphate and fluoride delivery vehicle-alpha(S1)-casein(59-79) by stabilized amorphous calcium fluoride phosphate nanocomplexes. Biomaterials. 2004;25(20):5061-5069. [76] BAKRY AS, ABBASSY MA. Increasing the efficiency of CPP-ACP to remineralize enamel white spot lesions. J Dent. 2018; 76:52-57. [77] DASHPER SG, SHEN P, SIM CPC, et al. CPP-ACP Promotes SnF2 Efficacy in a Polymicrobial Caries Model. J Dent Res. 2019;98(2):218-224. [78] HANI TB, O’CONNELL AC, DUANE B. Casein phosphopeptide-amorphous calcium phosphate products in caries prevention. Evid Based Dent. 2016;17(2):46-47. [79] HUA F, YAN J, ZHAO S, et al. In vitro remineralization of enamel white spot lesions with a carrier-based amorphous calcium phosphate delivery system. Clin Oral Investig. 2020;24(6):2079-2089. [80] GUPTA R, PRAKASH V. CPP-ACP complex as a new adjunctive agent for remineralisation: a review. Oral Health Prev Dent. 2011;9(2): 151-165. [81] HOSSEINPOUR-NADER A, KARIMI N, GHAFARI HA. Ex-vivo effects of propolis quantum dots-nisin-nanoquercetin-mediated photodynamic therapy on Streptococcus mutans biofilms and white spot lesions. Photodiagnosis Photodyn Ther. 2023;41:103255. [82] OLEK M, MACHOROWSKA-PIENIĄŻEK A, STÓS W, et al. Photodynamic Therapy in Orthodontics: A Literature Review. Pharmaceutics. 2021;13(5):720. [83] ALQERBAN A. Effectiveness of Riboflavin and Rose Bengal Photosensitizer Modified Adhesive Resin for Orthodontic Bonding. Pharmaceuticals (Basel). 2021; 14(1):48. [84] HUANG Y, LIU Y, PANDEY NK, et al. Iron oxide nanozymes stabilize stannous fluoride for targeted biofilm killing and synergistic oral disease prevention. Nat Commun. 2023;14(1):6087. [85] 许颖华,刘婧,尤权,等.掺钕钇铝钙钛晶体激光结合两种再矿化制剂与早期釉质龋的再矿化[J].中国组织工程研究, 2024,28(3):360-365. [86] PAN G, WANG H, LI Z, et al. Photodynamic therapy based on bismuth oxyiodide nanoparticles for nondestructive tooth whitening. Colloids Surf B Biointerfaces. 2024;243:114133. [87] TORRES C, MOECKE SE, MAFETANO A, et al. Influence of Viscosity and Thickener on the Effects of Bleaching Gels. Oper Dent. 2022;47(3):E119-E130. [88] LUO T, YAN J, CAO L, et al. Novel orthodontic adhesives with antibacterial, mineralization and fluorescence properties for enamel demineralization prevention. Chem Eng J. 2024;500:156737. [89] RAJENDRAN R, HUSSAIN MS, SANDHYA R, et al. Effect of Remineralization Agents on White Spot Lesions: A Systematic Review. J Pharm Bioallied Sci. 2022;14(Suppl 1): S7-S12. |
| [1] | Shao Ziyu, Li Qian, Qumanguli·Abudukelimu, Han Youjun, Hu Yang. Preparation and characterization properties of three different ratios of biphasic calcium phosphate [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1952-1961. |
| [2] | Li Congcong, Wufanbieke·Baheti, Zhao Li, Chen Xiaotao, Kong Chuifan, Yu Min. Physicochemical properties and biocompatibility of hydroxyapatite/graphene oxide/interleukin-4 composite coating materials [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 404-413. |
| [3] | Wang Zhuo, Sun Panpan, Cheng Huanzhi, Cao Tingting. Application of chitosan in repair and regeneration of oral hard and soft tissues [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 459-468. |
| [4] | Ma Lisha, He Huiyu, Wufanbieke·Baheti, Lyu Shangyi, Han Xiangzhen. effect of graphene oxide/hydroxyapatite composite coating on immune activity of RAW264.7 macrophages [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(10): 2023-2029. |
| [5] | Dumanbieke·Amantai, He Huiyu, Han Xiangzhen. Hydroxyapatite-graphene oxide composite coating promotes bone defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(10): 2030-2037. |
| [6] |
Wang Dexin, Xu Zhanwu, Pei Guoxian.
Osteogenesis of bone marrow mesenchymal stem cells on hydroxyapatite/icariin/poly(lactic-co-glycolic acid) scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(25): 3974-3980. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||