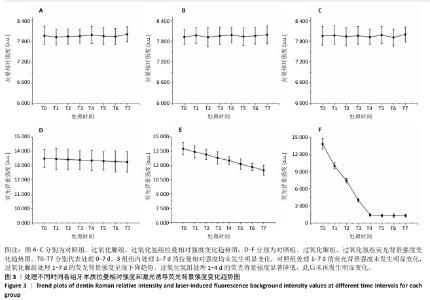
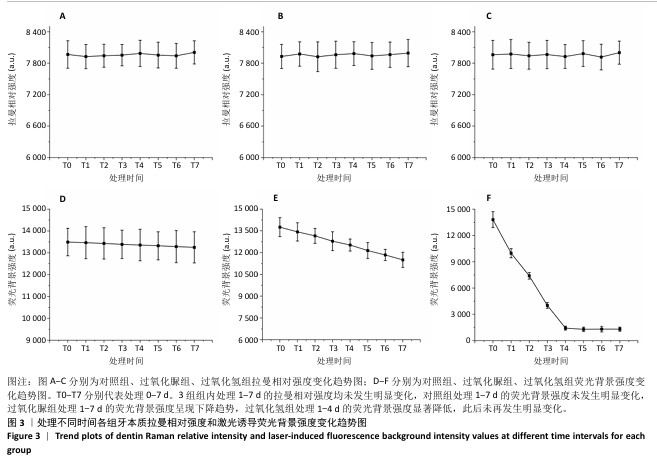
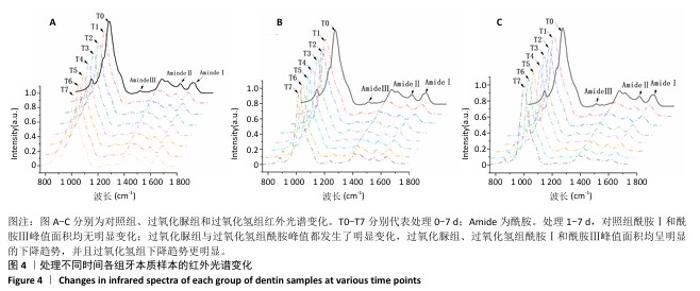
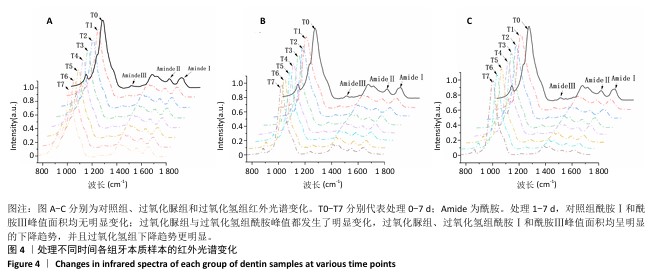
[1] HAYWOOD VB. Nightguard vital bleaching: current concepts and research. J Am Dent Assoc. 1997;128 Suppl:19S-25S.
[2] DAHL JE, PALLESEN U. Tooth bleaching--a critical review of the biological aspects. Crit Rev Oral Biol Med. 2003;14(4):292-304.
[3] KAHLER B. Present status and future directions - Managing discoloured teeth. Int Endod J. 2022;55 Suppl 4(Suppl 4):922-950.
[4] EPPLE M, MEYER F, ENAX J. A Critical Review of Modern Concepts for Teeth Whitening. Dent J (Basel). 2019;7(3):79.
[5] ALKAHTANI R, STONE S, GERMAN M, et al. A review on dental whitening. J Dent. 2020;100:103423.
[6] UTHAPPA R, SUPRITH ML, BHANDARY S, et al. A comparative study of different bleaching agents on the morphology of human enamel: an in vitro SEM study. J Contemp Dent Pract. 2012;13(6):756-759.
[7] MELO M, FIORESTA R, SANZ JL, et al. Effect of highly concentrated bleaching gels on enamel microhardness and superficial morphology, and the recovery action of four remineralizing agents. BMC Oral Health. 2022;22(1):645.
[8] XAVIER GMB, GIL GS, PAES YFO, et al. Assessment of the effect of experimental gel of pregabalin associated with 35% hydrogen peroxide bleaching on bovine dental enamel: an in vitro study. Odontology. 2024. doi: 10.1007/s10266-024-00978-2.
[9] TORRES CR, ZANATTA RF, GODOY MM, et al. Influence of Bleaching Gel Peroxide Concentration on Color and Penetration through the Tooth Structure. J Contemp Dent Pract. 2021;22(5):479-483.
[10] WIJETUNGA CL, OTSUKI M, ABDOU A, et al. The effect of in-office bleaching materials with different pH on the surface topography of bovine enamel. Dent Mater J. 2021;40(6):1345-1351.
[11] ACUÑA ED, PARREIRAS SO, FAVORETO MW, et al. In-office bleaching with a commercial 40% hydrogen peroxide gel modified to have different pHs: Color change, surface morphology, and penetration of hydrogen peroxide into the pulp chamber. J Esthet Restor Dent. 2022;34(2):322-327.
[12] DA SILVA KL, FAVORETO MW, CENTENARO GG, et al. Can all highly concentrated in-office bleaching gels be used as a single-application? Clin Oral Investig. 2023;27(7):3663-3671.
[13] TORRES C, MOECKE SE, MAFETANO A, et al. Influence of Viscosity and Thickener on the Effects of Bleaching Gels. Oper Dent. 2022;47(3): E119-E130.
[14] BARROS JÚNIOR ES, RIBEIRO MES, LIMA RR, et al. Excessive Dental Bleaching with 22% Carbamide Peroxide Combined with Erosive and Abrasive Challenges: New Insights into the Morphology and Surface Properties of Enamel. Materials (Basel). 2022;15(21):7496.
[15] HARDAN L, BOURGI R, FLORES-LEDESMA A, et al. Is a White Diet Necessary for Tooth Bleaching Procedures? A Systematic Review and Meta-Analysis. Dent J (Basel). 2024;12(4):118.
[16] CARLOS NR, PINTO A, DO AMARAL F, et al. Influence of Staining Solutions on Color Change and Enamel Surface Properties During At-home and In-office Dental Bleaching: An In Situ Study. Oper Dent. 2019;44(6):595-608.
[17] CARVALHO RF, DA MATA GALVÃO A, CAMPOLINA MG, et al. Does polishing of bleached enamel affect roughness and tooth color stability after exposure to coffee? J Esthet Restor Dent. 2022;34(2):351-359.
[18] SOUZA JM, AGUIAR JP, NEVES WJ, et al. Influence of diet and red wine exposure on the velocity of at home bleaching: A randomized controlled clinical trial. Am J Dent. 2022;35(4):191-196.
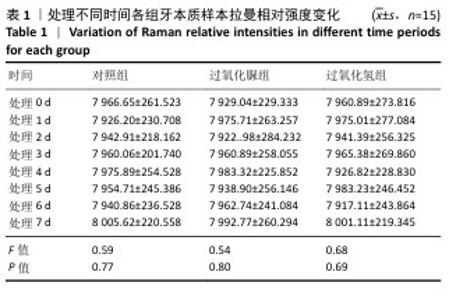
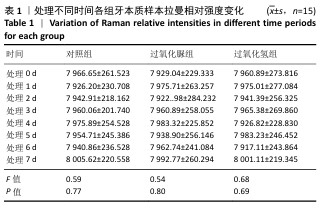
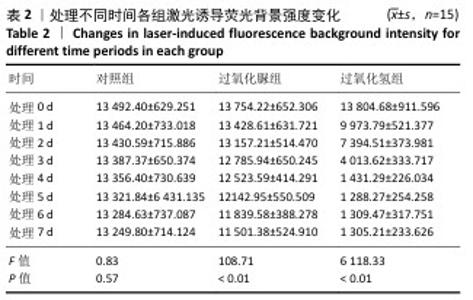
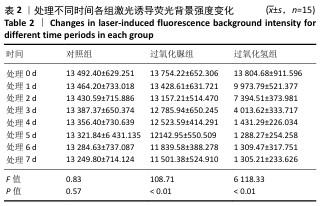
[19] LIANG S, JIANG T, WANG Y. Effect of hydrogen peroxide with different pH values on the color, translucency and laser-induced fluorescence of human dentin. Chinese J Stomatol. 2013;48(8):453-457.
[20] OTEL I. Overall Review on Recent Applications of Raman Spectroscopy Technique in Dentistry. Quantum Beam Sci. 2023;7:5.
[21] JIANG T, MA X, WANG Y, et al. Investigation of the effects of 30% hydrogen peroxide on human tooth enamel by Raman scattering and laser-induced fluorescence. J Biomed Opt. 2008;13(1):014019.
[22] PASCHALIS EP, MENDELSOHN R, BOSKEY AL. Infrared assessment of bonequality: a review. Clin Orthop Relat Res. 2011;469(8):2170-2178.
[23] BOSKEY A, MENDELSOHN R. Infrared analysis of bone in health and disease. Biomed Opt. 2005;10(3):31102.
[24] TSUDA H, RUBEN J, ARENDS J. Raman spectra of human dentin mineral. Eur J Oral Sci. 1996;104(2 (Pt 1)):123-131.
[25] JIANG T, MA X, WANG Y, et al. Effects of hydrogen peroxide on human dentin structure.J Dent Res. 2007;86(11):1040-1045.
[26] PLOTINO G, BUONO L, GRANDE NM, et al. Nonvital tooth bleaching: a review of the literature and clinical procedures. J Endod. 2008;34(4): 394-407.
[27] LIANG S, WANG M, WANG Y, et al. A Pilot Study About the Effect of Laser-Induced Fluorescence on Color and Translucency of Human Enamel During Tooth Bleaching. Photobiomodul Photomed Laser Surg. 2020;38(3):151-159.
[28] HANNIG C,HAMKENS A, BECKER K, et al. Erosive effects of different acids on bovine enamel: release of calcium and phosphate in vitro. Arch Oral Biol. 2005;50(6):541-552.
[29] GILCHRIST F, SANTINI A, HARLEY K, et al. The use of micro Raman spectroscopy to differentiate between sound and eroded primary enamel. Int Jpaediatr Dent. 2007;17(4):274-280.
[30] SUN L, LIANG S, SA Y, et al. Surface alteration of human tooth enamel subjected to acidic and neutral 30% hydrogen peroxide. J Dent. 2011; 39(10):686-692.
[31] GODINHO M, DE ATAIDE IN, LAMBOR R, et al. Influence of two remineralizing agents on bleached enamel surface morphology and mineral composition-An In Vitro study. Indian J Dent Res. 2022;33(2): 188-192.
[32] ARAGÃO WAB, CHEMELO VS, ALENCAR CM, et al. Biological action of bleaching agents on tooth structure: A review. Histol Histopathol. 2024;39(10):1229-1243.
[33] MANSO AP, DE MORAIS DC, YAMAMOTO K, et al. Effects of prolonged use of over-the-counter bleaching agents on enamel: An in vitro study. Microsc Res Tech. 2022;85(3):1016-1027.
[34] TSUDA M, ONO T, OGAWA T, et al. Pyridinoline is a real moiety of collagen. Biochem Biophys Res Commun. 1982;104(4):1407-1412.
[35] AMER M. Intracoronal tooth bleaching - A review and treatment guidelines. Aust Dent J. 2023;68 Suppl 1:S141-S152.
[36] BRIK A, HASKELL E, BRIK V, et al. Anisotropy effects of EPR signals and mechanisms of mass transfer in tooth enamel and bones. Appl Radiat Isot. 2000;52(5):1077-1083.
[37] JUNQUERA LB, CARLOS NR, OTSUKI M, et al. Effect of Bleaching Treatments on the Mechanical Properties of the Dentin Matrix and on Collagen Biodegradation by Endogenous Protease. Oper Dent. 2024;49(5):564-573. |