Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (32): 6963-6970.doi: 10.12307/2025.997
Previous Articles Next Articles
Blood flow restriction training intervention in the elderly with sarcopenic obesity
Liu Chenchen1, Liu Ruize2, Bao Mengmeng1, Fang Li1, Cao Liquan1, 3, Wu Jiangbo1, 3
- 1School of Sports and Health, 2School of Physical Education, 3Tianjin Key Laboratory of Integration of Sports and Hygiene and Health Promotion, Tianjin University of Sport, Tianjin 301617, China
-
Received:2024-11-18Accepted:2025-01-20Online:2025-11-18Published:2025-04-29 -
Contact:Wu Jiangbo, MD, Lecturer, Master’s supervisor, School of Sports and Health, Tianjin University of Sport, Tianjin 301617, China; Tianjin Key Laboratory of Integration of Sports and Hygiene and Health Promotion, Tianjin University of Sport, Tianjin 301617, China -
About author:Liu Chenchen, Master candidate, School of Sports and Health, Tianjin University of Sport, Tianjin 301617, China -
Supported by:Tianjin Municipal Education Commission Scientific Research Project, No. 2020KJ102 (to WJB)
CLC Number:
Cite this article
Liu Chenchen, Liu Ruize, Bao Mengmeng, Fang Li, Cao Liquan, Wu Jiangbo. Blood flow restriction training intervention in the elderly with sarcopenic obesity[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6963-6970.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
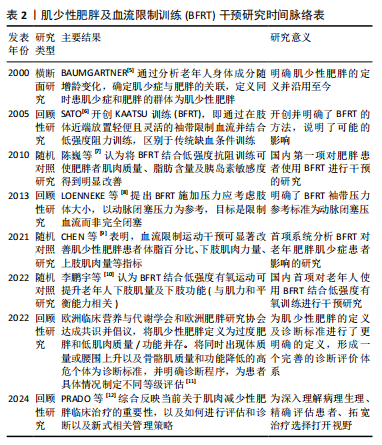
肌少性肥胖已经成为危害老年人健康的公共卫生问题。早在2000年,BAUMGARTNER[5]通过对新墨西哥州老龄化进程研究和新墨西哥州老年人健康调查,正式开启了对肌少性肥胖的研究。BFRT作为一个新兴的运动模式,近年来也备受关注。2005年,SATO[6]开创了KAATSU训练(BFRT),为肌肉训练引入了新的方法。 2010年,陈巍等[7]在国内率先将BFRT应用于肥胖人群,他们发现BFRT对肥胖患者体成分及胰岛素敏感度有积极影响。2013年,LOENNEKE等[8]通过深入探索,将个人动脉闭塞压百分比作为训练压力,为后续训练统一标准。2021年,CHEN等[9]首次揭示了BFRT在老年肌少性肥胖患者中的作用。2022年,李鹏宇等[10]证实BFRT与低强度有氧运动结合对老年人下肢肌肉质量和功能也有提升作用。同年,欧洲临床营养与代谢学会和欧洲肥胖研究协会达成的共识从宏观层面明确了肌少性肥胖的定义和诊断标准[11]。直至 2024 年,PRADO等[12]通过综合整理相关内容,深入和全面地探究了肌少性肥胖的病理生理,从基础概念定义到干预手段创新,再到诊断标准的完善,逐步构建肌少性肥胖研究的知识体系。见表2。"
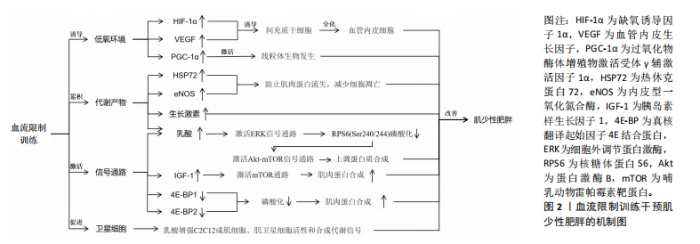
2.1 BFRT的作用机制 BFRT的作用机制主要包括加压导致的缺血低氧、代谢物质的积累、潜在的信号通路激活以及卫星细胞的增殖等,见图2。 2.1.1 诱导低氧环境 BFRT通过使用袖带施加压力限制血液流向肌肉,减少肌肉的氧气供应,从而在肌肉内部创造一个相对缺氧的环境。BFRT提高了肌细胞的氧气利用率,并激活一系列促进肌肉生长和增加力量的生理途径。 缺氧诱导因子由α亚基和β亚基组成[13]。缺氧诱导因子1α是一种对细胞内氧气水平发生变化做出反应的关键蛋白[14],它在低氧条件下会积累并进入细胞核,激活一系列基因的表达[15],包括促进血管生成的因子以及参与能量代谢的基因,帮助细胞适应低氧环境。研究发现,在限制血流后步行运动3 h,缺氧诱导因子1α、过氧化物酶体增殖物激活受体γ辅激活因子1α和血管内皮生长因子蛋白含量显著增加,这表明血流限制结合低强度有氧运动可以通过激活线粒体生物发生、血管生成和骨骼肌蛋白表达的细胞信号传导途径,引起肌肉肥大[16],这可能与缺氧诱导因子1α的过度表达能够显著促进血管生成因子的激活,使得血管生成增加有关[17],血管生成对肌肉生长和修复至关重要。一篇Meta分析也报道了类似结果,BFRT后机体缺氧程度增高,导致缺氧诱导因子1α水平显著升高,进而促进了血管内皮生长因子的激活[18]。缺血会引起血管内皮生长因子表达升高,促进间充质干细胞进一步分化为血管内皮细胞,提高毛细血管密度并改善肌肉功能[19]。上述研究表明BFRT能够通过制造缺氧缺血的环境,诱导血管内皮生长因子表达升高,进而产生肌肉增长和功能增强的效果。 2.1.2 代谢产物的累积 肌少性肥胖的发生与合成代谢功能失调密切相关。老年人具有较高的合成代谢阈值,与年轻人相比,他们需要更多可利用的营养摄入,以维持代谢产物的累积与消耗[20],因此诱导代谢产物的累积有助于改善老年肌少性肥胖的发展。 BFRT通过外部加压限制肢体血液流动,减少静脉血液回流和阻塞部分动脉血流,增加肌肉代谢产物(乳酸等)的累积。乳酸可以增加C2C12成肌细胞、肌卫星细胞活性和合成代谢信号[21]。在运动恢复阶段,受试者将加压带松解,使得外周血流量增加,补充能源物质的同时将代谢废物排除,刺激与肌肉增长相关激素及蛋白的释放,"
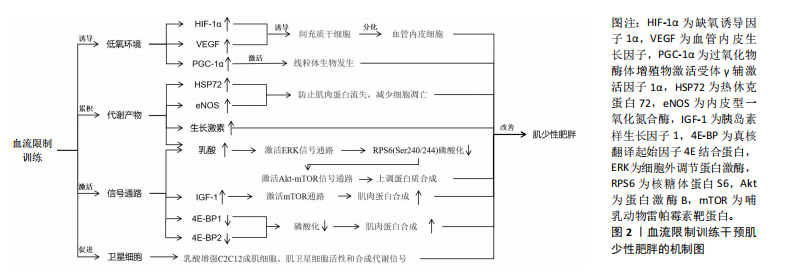

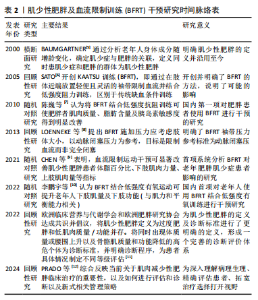
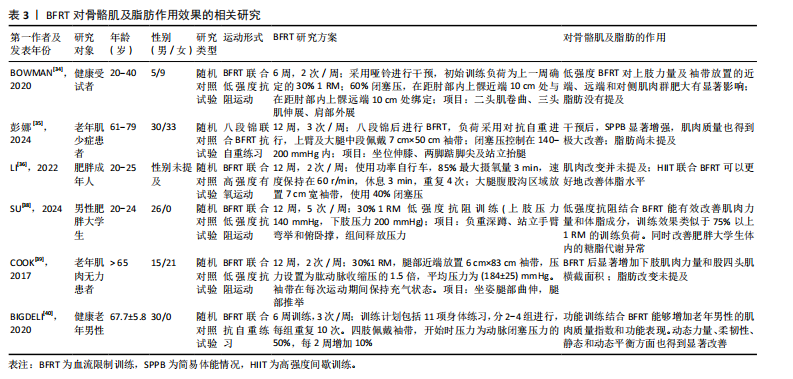
促进肌肉增长和力量提升。研究表明,BFRT能够增加肌肉一氧化氮合成酶和热休克蛋白72的分泌,这些蛋白可以缓解失用性肌萎缩导致的蛋白质合成效率下降,防止肌肉蛋白流失,减少细胞凋亡[22]。GOTO等[23]通过在运动过程中缩短休息时间来增加代谢压力,这种运动策略能够显著激发乳酸、生长激素、肾上腺素和去甲肾上腺素的反应,这表明通过增加代谢物质的累积能够促进肌肉力量和质量的显著增长,但这些变化并非特定于BFRT,可能还涉及到其他未被明确识别的信号传导途径。 2.1.3 潜在的信号通路 老年人的肌肉蛋白合成能力随着年龄的增加而减弱,而研究证实在BFRT干预后这一现象有显著改善[24]。BFRT通过促进释放胰岛素样生长因子1激活哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)通路,促进肌肉蛋白合成[25]。BFRT通过增加血乳酸浓度激活细胞外调节蛋白激酶(extracellular regulated protein kinases,ERK)信号通路增强核糖体蛋白S6(ribosomal protein S6,RPS6)在Ser240/244位点的磷酸化。而磷酸化RPS6(Ser240/244)水平的增加可能进一步激活蛋白激酶B(protein kinase B,AKT)/mTOR信号通路,是促进蛋白质合成的关键因素。然而,低强度抗阻和有氧运动不能增加磷酸化RPS6(Ser240/244)的表达,因此不能通过这一途径促进肌肉蛋白质的合成[26]。此外,MAY等[27]认为运动通过减少真核翻译起始因子4E结合蛋白(eIF4E-binding protein,4E-BP)1在Thr37/46位点的磷酸化,促进机体的肌肉修复和生长,并证实低负荷BFRT在有足够机械张力的情况下,会导致4E-BP1(Thr37/46)磷酸化水平下降。NGUYEN等[28]发现敲除小鼠4E-BP1和4E-BP2相关基因后,肌肉蛋白合成增加,证明了4E-BP1和4E-BP2的磷酸化增加极大地影响了蛋白质合成,不利于维持衰老的肌肉质量和功能。敲除4E-BP1和4E-BP2基因的老年野生型小鼠的肌肉损失比正常老年野生型小鼠低了6%,也从侧面说明了BFRT对延缓老年肌少性肥胖的发展有积极作用。 2.1.4 卫星细胞增殖 肌少性肥胖是肌少症与肥胖症的综合性疾病,其发生与衰老密不可分,特征是骨骼肌质量下降、肌肉功能损失及脂肪的增加[29]。骨骼肌质量增加依赖于卫星细胞的激活与增殖,而这一过程会随着年龄增长而效率降低。BJ?RNSEN等[30]发现进行一次性BFRT干预后肌卫星细胞的数量增加了约70%,在干预10 d后增加了144%,Ⅰ型肌纤维面积也增加了约19%。另一篇文章也证实,即便是急性BFRT后,与测试前相比,测试腿Ⅰ型纤维中卫星细胞数量增加90%-100%,Ⅱ型纤维中卫星细胞数量增加45%-55%以及肌核数量增加20%。但肌肉大小以及力量的增加并不迅速,这可能与训练强度有关[31]。成肌分化因子和生肌因子5作为肌肉特异性转录因子,在调节骨骼肌发育和生长中起重要作用[32],BFRT可以通过诱导卫星细胞增殖,上调成肌分化因子和生肌因子5的表达促进肌肉生长,提高基础代谢率,增加能量消耗,进一步抑制肌少性肥胖的发生与发展。尽管BFRT能够有效促进卫星细胞的增殖,但抗阻运动对卫星细胞的增殖也具有相同影响,因此并不能作为改善肌少症的特定途径。 2.2 BFRT对肌少性肥胖的影响 2.2.1 BFRT对增肌及减脂的作用效果 BFRT能够促进肌肉质量和功能的增加,并减少脂肪。在一篇Meta分析报告中,低强度BFRT和传统阻力训练都有一定改善老年人肌肉减少症的潜力,在肌肉质量方面也有类似的改善,但BFRT在肌肉力量方面的改善更大[33]。BOWMAN等[34]与彭娜等[35]实验结果显示BFRT后肌肉质量和横截面积都显著增加,身体活动能力也得到有效提高。而肌肉质量越高,新陈代谢就越快,消耗的能量就越多,这也有助于脂肪的消耗。LI等[36]发现高强度间歇训练联合BFRT比单独高强度间歇训练更有利于改善血糖代谢和减少体脂含量,他们认为这一运动模式主要通过增加乳酸、脂肪分解相关激素水平及运动后过量耗氧等改善内脏脂肪。同时,BFRT结合阻力训练通过诱导肌因子(如白细胞介素6、白细胞介素10、白细胞介素15、肌肉生长抑制素、鸢尾素、成纤维细胞生长因子21等)和脂肪因子(如脂联素和爱帕琳肽等)通路控制骨骼肌肌肉蛋白合成与脂肪分解,进而减少了蛋白质降解和肌肉萎缩[37]。有研究证实使用低强度抗阻结合BFRT能有效改善肌肉力量和体脂成分,训练效果与75% 1 RM的负荷抗阻训练类似,它还可以改善肥胖人群体内的糖脂代谢异常[38]。 与年轻人相比,老年患者的肌肉质量、强度及骨密度更低,抗阻运动可能会引起过度训练、增加骨骼肌损伤及骨折的发生率。因此,BFRT可能更适合老年肌少性肥胖患者。COOK等[39]及BIGDELI等[40]发现BFRT后的老人股四头肌力量与横截面积都有显著增加,同时力量、柔韧性、静态和动态平衡能力也得到极大改善。此外,老人体内血清集聚蛋白羧基片段水平也有所下降,血清集聚蛋白羧基片段水平被认为是肌少症发生的标志物之一,这也侧面反映BFRT能够有效延缓肌少性肥胖的发生与发展。BFRT对骨骼肌及脂肪作用效果的相关研究见表3。 2.2.2 BFRT的优势与安全性 人在40岁左右时骨骼肌质量和力量损失加快,肌肉蛋白加速分解。与年龄相关的肌肉质量和力量损失会导致身体功能下降,从而增加老年人虚弱、残疾、跌倒和死亡的发生概率,同时较少的运动会增加脂肪组织的合成[41]。多项研究证实老年肌肉减少性肥胖往往会伴随着心血管疾病[42]、慢性肝病[43]、2型糖尿病[44]、骨质疏松等疾病的发生[45],而这些慢性疾病的存在将很大程度影响到运动干预方式的选择。 在医学领域,BFRT常用于术后康复,如前交叉韧带断裂后的恢复过程。JACK等[46]研究发现,患者在前交叉韧带重建后立即开始BFRT,并结合常规物理治疗方案,减少了患者术后肌肉质量和骨密度的损失,这说明BFRT是一种相对安全有效的方案。心血管疾病在老年人群中非常常见,BFRT在增加肌肉质量、功能的同时,也能通过激活血管内皮生长因子和内皮型一氧化氮合酶等[47],对心血管功能产生有利影响。NASCIMENTO等[48]也提出假设“BFRT对于健康的成年人、患有缺血性心脏病的中年人和健康的老年人来说都是相对安全的”。研究发现,对20名老年人进行BFRT干预,在运动期间心率和血压始终保持的正常范围内[49]。对肥胖人群来说,低强度抗阻训练联合BFRT可安全有效地改善由高肥胖和低肌肉质量引起的代谢功能障碍[38]。在一项针对老年慢性阻塞性肺疾病患者的研究中,BFRT干预2周后肌肉力量和质量增加的同时,没有其他不良反应,并且在生化方面没有明显差异,这表明BFRT所具备的安全性可以满足在其他疾病康复过程中的应用[50]。TEIXEIRA等[51]和BARBALHO等[52]认为主动和被动使用BFRT都不会对肌肉产生不利影响,能够很大程度上对肌肉力量增加及肌肥大产生作用,也是减少重症监护病房患者肌肉萎缩率的有效策略。迄今为止,研究或文献中尚未报道在进行BFRT期间发生心血管事件,这可能与试验设计的运动强度较小、实验干预周期较短有关,但有报道过肌肉损伤,因此,在这些特殊人群更广泛地进行BFRT之前,了解BFRT潜在风险与注意事项是有必要的。 从上述的多项研究成果来看,BFRT在多种应用中都表现出一定的优势与安全性[53],无论是术后康复、老年人群生活质量提高还是肥胖人群的代谢改善等方面都有显著改善。然而,和其他治疗方法一样,虽已对BFRT安全性有了一定程度的认知,但仍需持续对潜在风险保持高度警惕。BFRT的优势与安全性的相关研究见表4。"
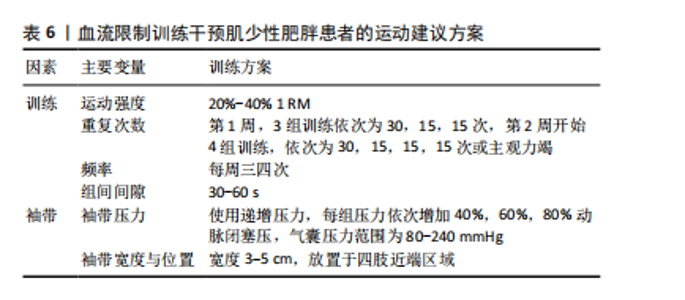
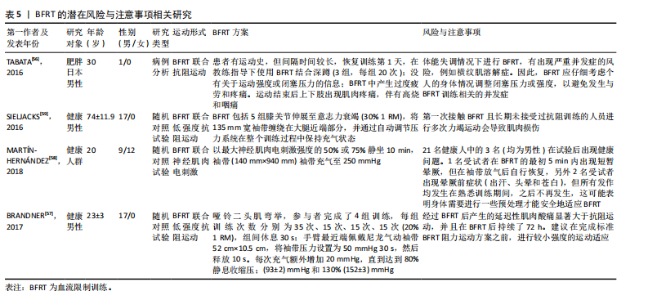
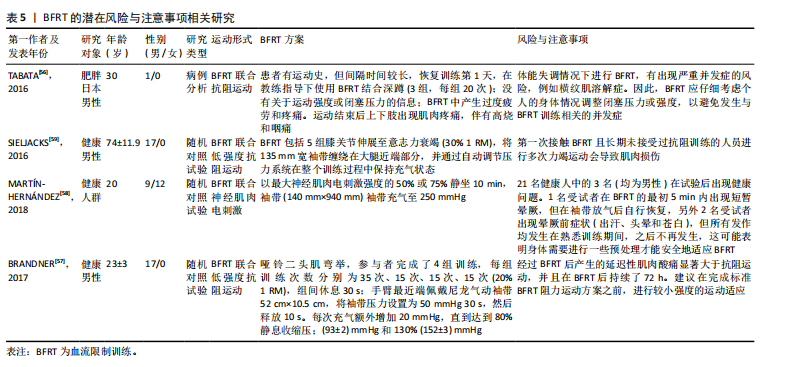
2.2.3 BFRT的潜在风险与注意事项 长期BFRT可能会增加各种风险,虽然定期的身体活动有利于纤维蛋白溶解,但高强度运动可能会诱发血栓形成,因为高强度的BFRT会导致细小的微血管损伤(加压训练后观察到白细胞介素6略微升高),从而可能引发局部血栓形成[54]。此外,长期BFRT会引起静脉过度充血,并可能对静脉瓣膜造成损害。TAKARADA等[54]发现,阻力训练(20% 1 RM)与加压(214 mmHg)结合不会引起较大的组织损伤,但很多研究人员使用并建议在运动期间将绑带压力设置为个人动脉闭塞压百分比,而不是一个固定值。 虽然在试验过程中几乎没有出现因血栓导致的并发症,但老年患者依旧应定期进行凝血和血压监测。肌少性肥胖往往会伴随多种慢性疾病,如2型糖尿病等,患者进行BFRT应避免运动后低血糖,及时进行血糖检查、评估身体状况以及调整运动前后的碳水化合物摄入量[55]。TABATA等[56]表明,采用不适应的BFRT强度与压力,尤其是在体能失调的情况下还会出现其他风险,例如横纹肌溶解症。BRANDNER等[57]发现,BFRT后产生的延迟性肌肉酸痛显著大于抗阻运动,并且在BFRT后持续了72 h。MARTíN-HERNáNDEZ等[58]对21位年轻人进行观察,有3人在进行BFRT时发生健康问题,1人出现晕厥前症状。在对老年人群的观察中,第一次接触BFRT且未接受过阻力训练的受试者,在进行多次力竭运动中出现一定的肌肉损伤。当然,这一现象出现在多种运动模式中,但这也是在进行BFRT中值得注意的一点[59]。 上述研究表明,无论是从微观的血管、组织层面,还是宏观的身体整体机能反应,都有可能因BFRT的不当应用而受到损害,尤其将BFRT应用于老年人群。因此,选择合适的运动强度与加压压力十分重要。只有对BFRT的潜在风险有足够的重视,并将注意事项严格落实到每一次的训练中,才能确保BFRT在未来的应用中实现效益最大化与风险最小化的理想平衡。BFRT的潜在风险与注意事项相关研究见表5。 2.3 运动建议 为避免并发症带来的不良后果,老年肌少性肥胖患者应谨慎制定运动方案。仅了解BFRT对肌少性肥胖的影响机制、作用、安全性与潜在风险还不够,针对肌少性肥胖患者制定BFRT的运动处方也同样重要。BFRT主要受到训练方式与袖带选择两个方面影响。在训练方式中,确定运动强度、运动负荷、运动频率、间歇时间十分重要;袖带主要涉及由袖带宽窄与施加压力两个方面。文章基于已有的证据提出BFRT干预肌少性肥胖患者的运动建议方案,见表6。"

2.3.1 训练方式 对于老年肌少性肥胖患者,选取合适的运动强度非常重要。过大的运动强度不仅达不到预期效果,可能还会带来严重的肌肉损伤。苏艳红等[60]发现BFRT伴随低强度抗阻训练对肌肉激活的效果较好,同时小强度运动也能减少受伤风险。在一篇针对老年人BFRT的Meta分析中,大部分文章采用20%-30% 1 RM范围的运动强度,只有少数用到10%或50% 1 RM强度,其中20% 1 RM强度结合BFRT显著增加了生长激素、乳酸、血管内皮生长因子等血液标志物并增加了肌肉质量与力量。也有文章提出30% 1 RM能够有效改善老年人身体功能[2]。LIXANDR?O等[61]认为以非常低的运动强度(20% 1 RM)的BFRT方案可能受益于更高水平的闭塞压力,相反,在使用更高强度(例如40% 1 RM)的BFRT方案中,BFRT的影响可能不如单纯抗阻运动,因为将较高的运动"

强度与更高的闭塞压力相结合似乎没有带来任何额外的好处[61]。同时SIELJACKS等[59]与MARTíN-HERNáNDEZ等[58]的试验中也出现第一次接触和运动开始阶段发生身体不适与肌肉损伤事件。因此,作者认为刚接触BFRT的老年肌少性肥胖患者第1周以20% 1 RM结合BFRT进行运动,从第2周开始逐渐增加至40%效果更好也更安全。 PATTERSON等[62]认为BFRT的运动组数应设置4组,其中第1组重复30次,后3组均重复15次或主观力竭,这一训练方式被多篇文献采用且并未发生任何不适反应。BFRT结合抗阻训练期间的组间休息通常较短。例如,LOENNEKE等[63]发现30-60 s的组间休息能够有效增加肌肉力量。同时30-60 s的休息时间也常见于BFRT的研究中。例如,JACK等[46]使用30 s休息时间进行BFRT,肌肉质量增加的同时,还减少了患者重返运动的时间。SU等[38]的研究也通过35-15-15-15的运动次数及30 s的休息时间进行BFRT,显著减少了受试者的身体脂肪。但上述研究大多数是年轻受试者,考虑到老年人群身体功能下降,建议休息时间应为 30-60 s。对于训练频率,CAHALIN等[64]发现老年人采用了每周4次BFRT,训练过程中没有发生受伤事件的同时身体功能也得到了极大改善。 2.3.2 袖带的选择与压力的大小 袖带规格的选择与袖带压力的大小都是影响BFRT效果的重要因素。LIXANDR?O等[61]发现当运动负荷较低(20% 1 RM)时,较高的闭塞压力(80%的闭塞压力)对肌肉力量的改善更明显。LETIERI等[65]发现低强度BFRT可增加老年女性肌肉力量。此外,在CHANG等[66]发表的文章中,被纳入的5篇文章选择递增压力,压力依次增加为40%,60%和80%动脉闭塞压,气囊压力范围为80-240 mmHg,平均压力约为158 mmHg,气囊宽度范围为3-5 cm;5篇文章施加了固定压力,气囊压力范围为50-187.5 mmHg,气囊宽度为6-14 cm,结果显示,施加递增压力的BFRT比固定压力的BFRT干预后肌肉力量增加更明显。"

| [1] HSU KJ, LIAO CD, TSAI MW, et al. Effects of Exercise and Nutritional Intervention on Body Composition, Metabolic Health, and Physical Performance in Adults with Sarcopenic Obesity: A Meta-Analysis. Nutrients. 2019;11(9):2163. [2] LIM ZX, GOH J. Effects of blood flow restriction (BFR) with resistance exercise on musculoskeletal health in older adults: a narrative review. Eur Rev Aging Phys Act. 2022;19(1):15. [3] 唐小蝶,袁淑娟.血流限制训练在康复医学中的应用进展[J].中国体育科技, 2023,59(1):81-85. [4] 魏佳,李博,杨威,等.血流限制训练的应用效果与作用机制[J].体育科学, 2019,39(4):71-80. [5] BAUMGARTNER RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000; 904:437-448. [6] SATO Y. The history and future of KAATSU Training. Int J Kaatsu Train Res. 2005; 1(1):1-5. [7] 陈巍,李娟,陈庆合,等.抗阻训练中运动肌血流限制对肥胖者体成分及胰岛素敏感度的影响[J].中国运动医学杂志,2010,29(6):646-649. [8] LOENNEKE JP, FAHS CA, ROSSOW LM, et al. Blood flow restriction pressure recommendations: a tale of two cuffs. Front Physiol. 2013;4:249. [9] CHEN N, HE X, ZHAO G, et al. Efficacy of low-load resistance training combined with blood flow restriction vs. high-load resistance training on sarcopenia among community-dwelling older Chinese people: study protocol for a 3-arm randomized controlled trial. Trials. 2021;22(1):518. [10] 李鹏宇,何穗,韦兆颜,等.血流限制结合低强度有氧运动对老年人下肢肌量和功能的影响[J].循证护理,2022,8(16):2223-2227. [11] DONINI LM, BUSETTO L, BISCHOFF SC, et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Clin Nutr. 2022; 41(4):990-1000. [12] PRADO CM, BATSIS JA, DONINI LM, et al. Sarcopenic obesity in older adults: a clinical overview. Nat Rev Endocrinol. 2024;20(5):261-277. [13] KANG JS, KIM D, RHEE J, et al. Baf155 regulates skeletal muscle metabolism via HIF-1a signaling. PLoS Biol. 2023;21(7):e3002192. [14] ZHANG H, HE H, CUI Y, et al. Regulatory effects of HIF-1α and HO-1 in hypoxia-induced proliferation of pulmonary arterial smooth muscle cells in yak. Cell Signal. 2021;87:110140. [15] 赵丽,李杰.生命的低氧适应:2019年度诺贝尔生理学奖或医学奖成果解析[J].科技导报,2020,38(2):79-85. [16] BARJASTE A, MIRZAEI B, RAHMANI-NIA F, et al. Concomitant aerobic- and hypertrophy-related skeletal muscle cell signaling following blood flow-restricted walking. Science & Sports. 2021;36(2):e51-e58. [17] DENG H, TIAN X, SUN H, et al. Calpain-1 mediates vascular remodelling and fibrosis via HIF-1α in hypoxia-induced pulmonary hypertension. J Cell Mol Med. 2022;26(10):2819-2830. [18] LI S, LI S, WANG L, et al. The Effect of Blood Flow Restriction Exercise on Angiogenesis-Related Factors in Skeletal Muscle Among Healthy Adults: A Systematic Review and Meta-Analysis. Front Physiol. 2022;13:814965. [19] CHEN T, YE B, TAN J, et al. CD146+Mesenchymal stem cells treatment improves vascularization, muscle contraction and VEGF expression, and reduces apoptosis in rat ischemic hind limb. Biochem Pharmacol. 2021;190:114530. [20] NASCIMENTO CM, INGLES M, SALVADOR-PASCUAL A, et al. Sarcopenia, frailty and their prevention by exercise. Free Radic Biol Med. 2019;132:42-49. [21] OISHI Y, TSUKAMOTO H, YOKOKAWA T, et al. Mixed lactate and caffeine compound increases satellite cell activity and anabolic signals for muscle hypertrophy. J Appl Physiol (1985). 2015;118(6):742-749. [22] GUNDERMANN DM, WALKER DK, REIDY PT, et al. Activation of mTORC1 signaling and protein synthesis in human muscle following blood flow restriction exercise is inhibited by rapamycin. Am J Physiol Endocrinol Metab. 2014;306(10): E1198-E1204. [23] GOTO K, ISHII N, KIZUKA T, et al. The impact of metabolic stress on hormonal responses and muscular adaptations. Med Sci Sports Exerc. 2005;37(6):955-963. [24] GUNDERMANN DM, FRY CS, DICKINSON JM, et al. Reactive hyperemia is not responsible for stimulating muscle protein synthesis following blood flow restriction exercise. J Appl Physiol (1985). 2012;112(9):1520-1528. [25] 何穗,汪菊培,吴礼,等.血流限制训练的生理机制与应用进展[J].医学新知,2023,33(2):143-148. [26] YOSHIKAWA M, MORIFUJI T, MATSUMOTO T, et al. Effects of combined treatment with blood flow restriction and low-current electrical stimulation on muscle hypertrophy in rats. J Appl Physiol (1985). 2019;127(5):1288-1296. [27] MAY AK, RUSSELL AP, DELLA GATTA PA, et al. Muscle Adaptations to Heavy-Load and Blood Flow Restriction Resistance Training Methods. Front Physiol. 2022; 13:837697. [28] NGUYEN LH, XU Y, MAHADEO T, et al. Expression of 4E-BP1 in juvenile mice alleviates mTOR-induced neuronal dysfunction and epilepsy. Brain. 2022;145(4): 1310-1325. [29] PAPADOPOULOU SK. Sarcopenia: A Contemporary Health Problem among Older Adult Populations. Nutrients. 2020;12(5):1293. [30] BJØRNSEN T, WERNBOM M, LØVSTAD A, et al. Delayed myonuclear addition, myofiber hypertrophy, and increases in strength with high-frequency low-load blood flow restricted training to volitional failure. J Appl Physiol (1985). 2019; 126(3):578-592. [31] BJØRNSEN T, WERNBOM M, PAULSEN G, et al. Frequent blood flow restricted training not to failure and to failure induces similar gains in myonuclei and muscle mass. Scand J Med Sci Sports. 2021;31(7):1420-1439. [32] SHI LL, ZHU KC, WANG HL. Characterization of myogenic regulatory factors, myod and myf5 from Megalobrama amblycephala and the effect of lipopolysaccharide on satellite cells in skeletal muscle. Gene. 2022;834:146608. [33] KONG J, LI Z, ZHU L, et al. Comparison of blood flow restriction training and conventional resistance training for the improvement of sarcopenia in the older adults: A systematic review and meta-analysis. Sports Med Health Sci. 2022; 5(4):269-276. [34] BOWMAN EN, ELSHAAR R, MILLIGAN H, et al. Upper-extremity blood flow restriction: the proximal, distal, and contralateral effects-a randomized controlled trial. J Shoulder Elbow Surg. 2020;29(6):1267-1274. [35] 彭娜,苗笛,王红,等.八段锦联合血流限制训练对老年肌少症的应用效果研究[J].中华保健医学杂志,2024,26(3):332-335. [36] LI S, GUO R, YU T, et al. Effect of High-Intensity Interval Training Combined with Blood Flow Restriction at Different Phases on Abdominal Visceral Fat among Obese Adults: A Randomized Controlled Trial. Int J Environ Res Public Health. 2022;19(19):11936. [37] ALIZADEH PAHLAVANI H. Exercise Therapy for People With Sarcopenic Obesity: Myokines and Adipokines as Effective Actors. Front Endocrinol (Lausanne). 2022; 13:811751. [38] SU Y, WANG F, WANG M, et al. Effects of blood flow restriction training on muscle fitness and cardiovascular risk of obese college students. Front Physiol. 2024;14:1252052. [39] COOK SB, LAROCHE DP, VILLA MR, et al. Blood flow restricted resistance training in older adults at risk of mobility limitations. Exp Gerontol. 2017;99:138-145. [40] BIGDELI S, DEHGHANIYAN MH, AMANI-SHALAMZARI S, et al. Functional training with blood occlusion influences muscle quality indices in older adults. Arch Gerontol Geriatr. 2020;90:104110. [41] MARQUES A, QUEIRÓS C. Frailty, Sarcopenia and Falls. In: Hertz K, Santy-Tomlinson J, editors. Fragility Fracture Nursing: Holistic Care and Management of the Orthogeriatric Patient [Internet]. Cham (CH): Springer; 2018. Chapter 2. [42] ZUO X, LI X, TANG K, et al. Sarcopenia and cardiovascular diseases: A systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2023;14(3):1183-1198. [43] BUNCHORNTAVAKUL C. Sarcopenia and Frailty in Cirrhosis: Assessment and Management. Med Clin North Am. 2023;107(3):589-604. [44] SANZ-CÁNOVAS J, LÓPEZ-SAMPALO A, COBOS-PALACIOS L, et al. Management of Type 2 Diabetes Mellitus in Elderly Patients with Frailty and/or Sarcopenia. Int J Environ Res Public Health. 2022;19(14):8677. [45] LIU C, LIU N, XIA Y, et al. Osteoporosis and sarcopenia-related traits: A bi-directional Mendelian randomization study. Front Endocrinol (Lausanne). 2022; 13:975647. [46] JACK RA 2ND, LAMBERT BS, HEDT CA, et al. Blood Flow Restriction Therapy Preserves Lower Extremity Bone and Muscle Mass After ACL Reconstruction. Sports Health. 2023;15(3):361-371. [47] VOLGA FERNANDES R, TRICOLI V, GARCIA SOARES A, et al. Low-Load Resistance Exercise with Blood Flow Restriction Increases Hypoxia-Induced Angiogenic Genes Expression. J Hum Kinet. 2022;84:82-91. [48] NASCIMENTO DDC, PETRIZ B, OLIVEIRA SDC, et al. Effects of blood flow restriction exercise on hemostasis: a systematic review of randomized and non-randomized trials. Int J Gen Med. 2019;12:91-100. [49] PARKINGTON T, MADEN-WILKINSON T, KLONIZAKIS M, et al. Comparative Perceptual, Affective, and Cardiovascular Responses between Resistance Exercise with and without Blood Flow Restriction in Older Adults. Int J Environ Res Public Health. 2022;19(23):16000. [50] LAU CW, LEUNG SY, WAH SH, et al. Effect on muscle strength after blood flow restriction resistance exercise in early in-patient rehabilitation of post-chronic obstructive pulmonary disease acute exacerbation, a single blinded, randomized controlled study. Chron Respir Dis. 2023;20:14799731231211845. [51] TEIXEIRA EL, DE SALLES PAINELLI V, SILVA-BATISTA C, et al. Blood Flow Restriction Does Not Attenuate Short-Term Detraining-Induced Muscle Size and Strength Losses After Resistance Training With Blood Flow Restriction. J Strength Cond Res. 2021;35(8):2082-2088. [52] BARBALHO M, ROCHA AC, SEUS TL, et al. Addition of blood flow restriction to passive mobilization reduces the rate of muscle wasting in elderly patients in the intensive care unit: a within-patient randomized trial. Clin Rehabil. 2019; 33(2):233-240. [53] SCARPELLI MC, BERGAMASCO JGA, ARRUDA EAB, et al. Resistance Training With Partial Blood Flow Restriction in a 99-Year-Old Individual: A Case Report. Front Sports Act Living. 2021;3:671764. [54] TAKARADA Y, NAKAMURA Y, ARUGA S, et al. Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. J Appl Physiol (1985). 2000;88(1):61-65. [55] NASCIMENTO DDC, ROLNICK N, NETO IVS, et al. A Useful Blood Flow Restriction Training Risk Stratification for Exercise and Rehabilitation. Front Physiol. 2022;13: 808622. [56] TABATA S, SUZUKI Y, AZUMA K, et al. Rhabdomyolysis After Performing Blood Flow Restriction Training: A Case Report. J Strength Cond Res. 2016;30(7):2064-2068. [57] BRANDNER CR, WARMINGTON SA. Delayed Onset Muscle Soreness and Perceived Exertion After Blood Flow Restriction Exercise. J Strength Cond Res. 2017;31(11):3101-3108. [58] MARTÍN-HERNÁNDEZ J, SANTOS-LOZANO A, FOSTER C, et al. Syncope Episodes and Blood Flow Restriction Training. Clin J Sport Med. 2018;28(6):e89-e91. [59] SIELJACKS P, MATZON A, WERNBOM M, et al. Muscle damage and repeated bout effect following blood flow restricted exercise. Eur J Appl Physiol. 2016; 116(3):513-525. [60] 苏艳红,杨孝磊,于海强.血流限制伴不同强度抗阻热身训练对肌肉激活及下肢肌力影响的差异[J].中国体育科技,2023,59(12):10-17. [61] LIXANDRÃO ME, UGRINOWITSCH C, LAURENTINO G, et al. Effects of exercise intensity and occlusion pressure after 12 weeks of resistance training with blood-flow restriction. Eur J Appl Physiol. 2015;115(12):2471-2480. [62] PATTERSON SD, HUGHES L, WARMINGTON S, et al. Blood Flow Restriction Exercise: Considerations of Methodology, Application, and Safety. Front Physiol. 2019;10:533. [63] LOENNEKE JP, WILSON JM, MARÍN PJ, et al. Low intensity blood flow restriction training: a meta-analysis. Eur J Appl Physiol. 2012;112(5):1849-1859. [64] CAHALIN LP, FORMIGA MF, ANDERSON B, et al. A call to action for blood flow restriction training in older adults with or susceptible to sarcopenia: A systematic review and meta-analysis. Front Physiol. 2022;13:924614. [65] LETIERI RV, TEIXEIRA AM, FURTADO GE, et al. Effect of 16 weeks of resistance exercise and detraining comparing two methods of blood flow restriction in muscle strength of healthy older women: A randomized controlled trial. Exp Gerontol. 2018;114:78-86. [66] CHANG H, YAN J, LU G, et al. Muscle strength adaptation between high-load resistance training versus low-load blood flow restriction training with different cuff pressure characteristics: a systematic review and meta-analysis. Front Physiol. 2023;14:1244292. |
| [1] | Li Kaiying, Wei Xiaoge, Song Fei, Yang Nan, Zhao Zhenning, Wang Yan, Mu Jing, Ma Huisheng. Mechanism of Lijin manipulation regulating scar formation in skeletal muscle injury repair in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1600-1608. |
| [2] | Li Huayuan, Li Chun, Liu Junwei, Wang Ting, Li Long, Wu Yongli. Effect of warm acupuncture on PINK1/Parkin pathway in the skeletal muscle of rats with chronic fatigue syndrome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1618-1625. |
| [3] | Wang Xuanqiang, Zhang Wenyang, Li Yang, Kong Weiqian, Li Wei, Wang Le, Li Zhongshan, Bai Shi. Effects of chronic exposure to low-frequency pulsed magnetic fields on contractility and morphology of the quadriceps muscle in healthy adults [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1634-1642. |
| [4] | Zhang Zixian, Xu Youliang, Wu Shaokui, Wang Xiangying. Effects of blood flow restriction training combined with resistance training on muscle indicators in college athletes: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1705-1713. |
| [5] | Zhang Wenhua, Li Xun, Zhang Weichao, Li Xinying, Ma Guoao, Wang Xiaoqiang . Promoting myogenesis based on the SphK1/S1P/S1PR2 signaling pathway: a new perspective on improving skeletal muscle health through exercise [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1265-1275. |
| [6] | Wen Zixing, Xu Xin, Zhu Shengqun. Correlations between gastrocnemius morphology parameters and physical activity capacity in elderly females under high-frequency ultrasound [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1058-1063. |
| [7] | Wang Zilin, Mu Qiuju, Liu Hongjie, Shen Yuxue, Zhu Lili. Protective effects of platelet-rich plasma hydrogel on oxidative damage in L929 cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 771-779. |
| [8] | Geng Zhizhong, Wang Jinhao, Cao Guohuan, Tan Chenhao, Li Longji, Qiu Jun. Difference of energy metabolism and skeletal muscle oxygenation in athletes under high temperature, high humidity and low oxygen environment [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6866-6876. |
| [9] | Wang Qifei, Du Xingbin, Kong Jianda. Neural physiological basis and exercise-induced mechanism of central fatigue [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6979-6988. |
| [10] | Wang Jiaqian, , Jiang Changjun, Peng Yi, Ma Mi, Li Junhan. Study on the role of aerobic exercise in regulating the CNPY2-mediated AKT/GSK3β pathway for improving non-alcoholic fatty liver [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6441-6448. |
| [11] | Zhang Songjiang, Li Longyang, Zhou Chunguang, Gao Jianfeng. Central anti-inflammatory effect and mechanism of tea polyphenols in exercise fatigue model mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6474-6481. |
| [12] | Hu Shujuan, Liu Dang, Ding Yiting, Liu Xuan, Xia Ruohan, Wang Xianwang. Ameliorative effect of walnut oil and peanut oil on atherosclerosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6482-6488. |
| [13] | Zhang Jian, Cai Feng, Li Tingwen, Ren Pengbo. Fatigue gait recognition of athletes based on fish swarm algorithm [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6489-6498. |
| [14] | Zhang Zihan¹, Wang Jiaxin¹, Yang Wenyi², Zhu Lei¹. Regulatory mechanism of exercise promoting mitochondrial biogenesis in skeletal muscle [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6499-6508. |
| [15] | Chen Jun, Jia Shaohui, Guo Chenggen, Xue Xinxuan, Dong Kunwei. Role and mechanism of resveratrol in delaying exercise-induced fatigue [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(29): 6285-6294. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||