Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (36): 7848-7855.doi: 10.12307/2025.754
Previous Articles Next Articles
Epigenetic characteristics of hepatogenic differentiation of mesenchymal stem cells in three-dimensional culture
Huang Haina, Yu Yanrong, Bi Jian, Huang Miao, Peng Weijie
- Gannan Medical University, Ganzhou 341000, Jiangxi Province, China
-
Received:2024-09-23Accepted:2024-11-09Online:2025-12-28Published:2025-03-18 -
Contact:Peng Weijie, PhD, Professor, Doctoral supervisor, Gannan Medical University, Ganzhou 341000, Jiangxi Province, China -
About author:Huang Haina, Master candidate, Gannan Medical University, Ganzhou 341000, Jiangxi Province, China -
Supported by:National Natural Science Foundation of China, No. 32460236 (to PWJ); Jiangxi Province Graduate Innovation Special Project, No. YC2023-S935 (to HHN)
CLC Number:
Cite this article
Huang Haina, Yu Yanrong, Bi Jian, Huang Miao, Peng Weijie. Epigenetic characteristics of hepatogenic differentiation of mesenchymal stem cells in three-dimensional culture[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7848-7855.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
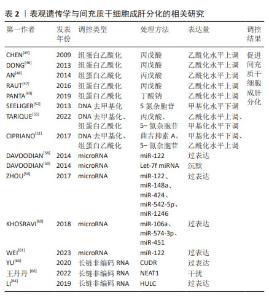
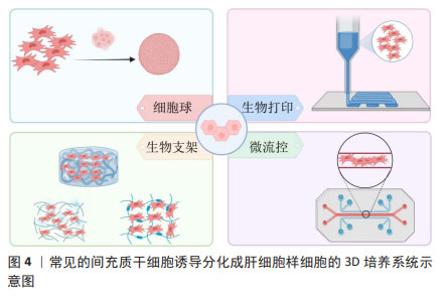
2.2.1 细胞球 主要通过限制外部环境或者利用物理力如重力聚集等方式将细胞局限在有限空间内,增加细胞之间的相互接触,使细胞依靠它们之间的黏附分子自发聚集成球,这些细胞聚集体被认为可以很好地模拟天然组织微环境结构和功能特征[13]。常见的细胞成球方式有悬滴法、旋转培养法或搅拌法以及低吸附自聚集等[14-15]。细胞球具有特殊的立体3D结构,从细胞球表面至细胞球中心,一定程度上模拟肝小叶从汇管区至中央静脉存在的氧气、营养物质浓度梯度,是一种良好的肝细胞3D培养模式[6,16-17]。OKURA等[18]在细胞球培养系统中,将脂肪来源间充质干细胞诱导成肝细胞球,这些细胞球展现出典型的成熟肝细胞功能特征,包括白蛋白的表达与分泌、尿素的合成、细胞色素P450酶活性,以及对低密度脂蛋白的摄取和糖原的储存能力。进一步将这些具备肝细胞特征的细胞球移植到肝损伤小鼠体内,可显著提升血清中白蛋白含量及总胆红素水平,显示出良好的治疗潜力。相对于2D单层诱导,细胞球培养具有更为成熟的肝功能和更高的肝特异性基因的表达[19-20]。总之,细胞球可以显著增强成肝效应且操作简单,成本较低且不需要考虑组织相容性。但是,这种微球模型也存在一定的局限性,如球尺寸的大小、存在缺氧坏死核心、缺乏营养物质和代谢物交换系统等。 2.2.2 生物支架 在类似脚手架结构的支架上进行细胞培养,这些支架具有的孔隙结构、表面活性、机械强度及生物相容性有利于干细胞的黏附、增殖与分化[21-22]。通过控制3D支架的材料特性,如机械性能、孔隙率、化学功能化及几何形态,可模拟肝脏组织的微环境[23-24]。目前,研究者已开发出多种基于不同支架材料的3D培养系统,以提供一个重现肝脏生理微环境的平台,有效促进了间充质干细胞的肝向分化。常用的支架材料包括水凝胶、胶原和纳米纤维等。①水凝胶支架:ASADI等[25]将脱细胞外基质水凝胶用于脂肪间充质干细胞的成肝分化,结果显示出高水平的白蛋白和尿素生成,且生成的肝细胞片可维持肝细胞功能。WANG等[26]利用功能化细胞外基质微孔水凝胶进行成肝诱导,其白蛋白分泌远远高于传统的2D培养。XU等[27]将间充质干细胞接种到丝素蛋白基质支架上生成类肝细胞,移植到急性肝衰竭小鼠体内展示出明显的治疗效果。②胶原支架:ALEAHMAD等[28]研究发现,在3D支架中分化的肝细胞具有更接近成熟肝细胞的功能。相较于2D培养系统,3D支架增强了成肝诱导的效果。例如,采用胶原蛋白涂层的聚乳酸-乙醇酸3D支架进行间充质干细胞成肝诱导,与2D单层诱导相比,表现出更高的肝细胞特异性标记物的mRNA和蛋白水平表达[29]。③纳米纤维支架:在3D纳米纤维支架上产生的间充质干细胞来源肝细胞样细胞高表达白蛋白和尿素,支架结构不仅提供了物理支撑,使细胞能够保持其天然形态并维持细胞间连接,而且其独特的结构有助于模拟Disse空间,为成肝分化提供了一个理想的微环境[30]。以上这些结果揭示了生物支架促进成肝分化效应的巨大潜能。然而,尽管各种各样的支架材料在成肝细胞培养中显示出了良好的结果,但其生物相容性要求高,细胞毒性等因素使得支架材料需要进一步改良,才能更好地应用于肝脏组织工程。 2.2.3 生物打印 生物打印是组织工程领域的一种增材制造技术,这项技术将负载细胞的生物材料作为生物墨水,在计算机辅助下按特定空间排列逐层沉积[31-32]。3D生物打印技术可以再现复杂的3D结构与微环境,较传统的2D培养模型可更好地维持细胞存活与功能[33]。LEE等[34]采用胶原蛋白/细胞外基质、藻酸盐作为生物墨水,负载人脂肪间充质干细胞通过3D生物打印技术构建功能性人源肝细胞,结果表明诱导产生的肝细胞样细胞可以发挥白蛋白分泌、尿素合成等成熟肝细胞功能。ZHANG等[35]通过模拟肝小叶结构,负载脂肪间充质干细胞制造了一个3D打印的血管化微型肝脏,植入急性肝损伤小鼠体内时展示出良好的治疗效果。通过3D生物打印技术构建的具有血管网络和细胞外基质的肝组织,能够更接近真实肝脏的功能,有助于模拟肝脏的生理和病理状态。然而,生物打印技术目前仍面临着许多技术挑战,如打印速度、分辨率、生物材料选择和细胞活力的保持等。用于3D生物打印的生物墨水需要具有良好的生物相容性和生物降解性,以确保打印出的组织能够与宿主组织整合。目前,开发理想的生物墨水仍然是一个难题。总之,3D生物打印在肝损伤修复中具有巨大的应用潜力,有望未来实现临床转化,这将为肝脏组织工程的发展带来了新希望。 2.2.4 微流控 微流控技术是以微流控芯片为载体,在连续灌注的微米级通道中培养细胞,进而模拟体内组织器官的生理代谢[36]。微流控系统使新鲜培养基连续流动,有助于不断清除代谢废物,从而提供一个稳定的微环境,在较长的培养时间内维持细胞活力和功能[37]。微流控芯片可以精确控制细胞培养的环境,提供稳定的营养和氧气供应[38]。芯片中的微通道可以模拟肝脏的微血管网络,促进向肝分化。微流控芯片中的流体剪切应力类似于血压效应,在肝组织发育中很重要[39]。YEN等[39]构建了一个5层微流控装置,可以保持恒定的流速和均匀的介质流动,有效应用于间充质干细胞的成肝分化。总之,微流控系统可以有效控制单个细胞的周围环境,使其更接近体内微环境,提高了成肝分化效应,然而也存在成本高、操作复杂等问题。微流控芯片的尺寸有限,可能限制了大规模的细胞培养且实验仍然受限于细胞本身的特性和分化潜能。 2.3 间充质干细胞成肝分化的表观遗传学 间充质干细胞向肝细胞分化的过程中,表观遗传学调控起到关键作用。表观遗传学是指基因的核苷酸序列不发生改变而基因表达发生了可遗传变化,这种变化是在染色质水平上的基因表达,取决于染色质的变化形式[12,40]。表观遗传学被认为是基因型和表型之间的桥梁,并为调节细胞分化、发育和组织再生等关键生物学特征提供了框架[41]。表观遗传学涉及许多复杂的调控机制,包括DNA甲基化、组蛋白修饰和非编码RNA等,这些机制相互作用共同参与调控干细胞分化的命运[42-43]。例如,组蛋白修饰酶会影响微小RNA的表达。组蛋白修饰引起的染色质结构的动态变化同时影响DNA甲基化,两者相互作用,共同决定特定基因的转录活性。组蛋白修饰会影响细胞内信号通路,这些通路中某些分子可能参与长链非编码RNA的转录调控。过去的几年中,大多数研究调查了表观遗传学对于间充质干细胞诱导成肝分化的影响。 2.3.1 组蛋白修饰 组蛋白修饰是间充质干细胞成肝分化过程中的一个关键表观遗传学机制,可通过影响组蛋白与DNA双链的亲和性来影响染色质的构象和稳定性,进一步改变染色质对参与转录蛋白质的可及性,这种调控作用在调节基因表达和细胞功能方面发挥着至关重要的作用[44]。组蛋白的修饰位点较多,其中研究最早最透彻的是组蛋白乙酰化修饰。组蛋白乙酰化是在乙酰转移酶和组蛋白去乙酰化酶共同动态调控下,使得DNA链变得松弛,从而改变染色质的构象,调控基因的激活与转录[45]。当前研究主要聚焦于探讨组蛋白去乙酰化酶抑制剂如丙戊酸、丁酸钠以及曲古抑素A在干细胞向肝细胞诱导分化过程中的作用机制。CHEN等[45]研究发现丙戊酸处理组肝脏特异性标志物的表达在mRNA和蛋白水平显著上调,增强了分化细胞的肝功能,包括糖原储存、细胞色素P450活性、分泌白蛋白以及产生尿素,这表明使用组蛋白去乙酰化酶可以显著促进人骨髓间充质干细胞的成肝分化。DONG等[46]研究发现丙戊酸在促进骨髓间充质干细胞向肝细胞分化的过程中,主要通过增强组蛋白H3和H4的乙酰化修饰,提高DNA对核酸酶的敏感性,以及促进染色质结构解聚等表观遗传学变化,使得相关肝脏特异性基因更易被转录因子所识别,从而增加了转录活性。进一步研究发现,丙戊酸在H3K14、H4K8乙酰化上调,促进了参与脐带间充质干细胞成肝分化基因的早期转录[47],同时也增强了部分miRNA 的表达,这些 miRNA 通常在胎儿肝脏发育过程中上调,例如miR-23b簇(miR-27b-3p、miR-24-1-5p和miR-23b-3p)、miR-30a-5p、miR-26a-5p、miR-148a-3p、miR-192-5p 和 miR-122-5p,提高了成肝分化效率[47],这表明不同表观遗传机制之间存在相互作用,它们共同影响基因表达。AN等[48]研究发现通过提升CXCR4基因启动子区域组蛋白乙酰化水平,可以激活AKT与ERK信号传导途径,从而促进了内胚层相关基因包括CXCR4、Sox17、FOXA1、Foxa2、GSC、c-met、EOMES及HNF-β的表达,有效推动了位于中胚层脐带间充质干细胞向内胚层肝细胞方向的分化。PANTA等[49]诱导脐带间充质干细胞成肝分化前用丁酸钠预处理显著提升了成熟肝细胞功能,包括白蛋白分泌、角蛋白18表达、糖原储备及尿素合成能力。有研究认为丁酸钠是通过增强H4K5乙酰化水平,提高SOX17和HNF3β等内胚层特异性基因,促进脐带间充质干细胞向内胚层分化。 综上所述,通过抑制组蛋白去乙酰化作用,可以增强染色质的乙酰化状态,进而改变染色质结构并调控成肝分化相关基因表达,有效促进间充质干细胞向肝细胞转化。然而,目前关于组蛋白修饰主要集中在组蛋白乙酰化方面,对于其他类型的组蛋白修饰及其特定修饰位点在间充质干细胞成肝分化中的作用了解仍然有限。因此,后续研究应着重探讨组蛋白乙酰化在间充质干细胞肝向分化过程中所涉及的信号传导途径、关键修饰位点,组蛋白乙酰化与成肝分化阶段诱导因子之间的相互作用以及不同组蛋白修饰间的相互关系,深入解析表观遗传学在调控间充质干细胞成肝分化中的机制。 2.3.2 DNA甲基化 在间充质干细胞成肝分化过程中,DNA甲基化发挥着重要作用。DNA甲基化是指在甲基转移酶家族的作用下将甲基转移至胞嘧啶C5位上形成5-甲基胞嘧啶[50]。一方面,甲基化状态的改变影响基因的直接表达;另一方面,它还可能通过改变染色质的开放程度间接影响基因表达。去甲基化可能激活肝细胞特异性基因的表达,而甲基化可能抑制与间充质干细胞分化特性相关的基因。DNA甲基转移酶在染色质重塑和基因表达调控起着至关重要的作用。研究表明DNA甲基转移酶抑制剂显著提高了细胞代谢Ⅰ/Ⅱ期酶活性以及肝脏富集转录因子在不同细胞系(如HeLa细胞、人肝癌细胞系或小鼠肝细胞)中的表达[51]。肝细胞样细胞的表观遗传变化是通过抑制DNA甲基转移酶并导致DNA序列去甲基化,从而使成肝分化过程中基因转录更为容易,促进间充质干细胞成肝分化。例如,SEELIGER等[52]通过使用5-氮杂胞苷(DNA甲基转移酶抑制剂)降低细胞全局甲基化状态来启动脂肪间充质干细胞成肝分化,显著提高了生成的肝样细胞色素酶活性,并具有与人原代肝细胞类似的尿素代谢能力。DNA甲基化受到表观遗传修饰网络调控,主要是在染色质水平通过影响转录因子与DNA的接触对基因转录活性进行调节。抑制基因表达通常与DNA甲基化和致密的染色质结构有关,而主动转录与未甲基化的DNA和高乙酰化染色质有关[53-54]。因此,DNA甲基化和组蛋白乙酰化之间存在紧密的相关性,DNA甲基转移酶和组蛋白去乙酰化酶在调节细胞稳态方面具有协同作用。最新的研究表明,表观遗传修饰剂(丙戊酸和5-氮杂胞苷)与小分子(丹酚酸B、地塞米松和胰岛素)联合使用,无需使用生长因子即可有效启动人脐带间充质干细胞的肝脏分化,进一步提高成肝分化效率[55]。 综上所述,DNA甲基转移酶抑制剂促进DNA去甲基化,从而增强基因表达。组蛋白修饰和DNA甲基化可共同调控间充质干细胞成肝分化过程,在此过程中多能干性基因逐渐被沉默,肝脏谱系特异性基因逐渐被激活。然而,目前关于DNA甲基化在间充质干细胞成肝分化中的作用相对较少,未来的研究需要更深入地探究甲基化变化与成肝分化之间的具体联系。此外,探索调节这些甲基化变化的潜在干预策略,可能为肝脏替代治疗提供新的途径。 2.3.3 非编码RNA 除了组蛋白修饰和DNA甲基化,非编码RNA也可以调控间充质干细胞的成肝分化。非编码RNA是一种功能性非蛋白编码的RNA分子,通过促进mRNA降解或减弱蛋白质翻译来调节基因表达。miRNA在肝脏的多个生理和病理过程中起关键作用。在转录后水平,miRNA是干细胞分化过程中控制细胞命运的关键参与者,可以直接将间充质干细胞转化为肝细胞样细胞。例如,ZHOU等[56]发现在不添加细胞因子的情况下脐带间充质干细胞中5种miRNA的组合可以在7 d内诱导成功能性肝细胞。miR-122作为肝脏特异性miRNA,在成人肝脏中的表达最高,约占所有miRNA的70%[57]。 miR-122通过靶向肝脏特异性基因转录因子,在肝功能和病理发展的调节中起着重要作用。研究表明,miR-122可以刺激肝细胞特异性基因和大多数肝细胞富集转录因子的表达,形成正反馈回路并在体外诱导肝细胞分化[58],同时在间充质干细胞的肝向诱导分化过程中miR-122 的含量是逐渐增加的,二者呈现相辅相成的作用。敲低Let-7f miRNA可上调HNF4α促进间充质干细胞成肝分化[59]。miR-122-5p 可下调干性基因SOX11和VIM的表达,促进内胚层分化[59]。与传统细胞因子诱导的细胞相比,在细胞中过表达miR-106a、miR-574-3p和miR-451可表达更高水平的白蛋白、角蛋白18和HNF4α[60]。转染miR-122的间充质干细胞在四面体DNA纳米平台上可被诱导成功能性肝细胞,体内移植可挽救急性肝衰竭小鼠生命[61]。长链非编码RNA是一类长度大于200个核苷酸且不能编码蛋白质的RNA,与细胞增殖、分化和凋亡等生物过程密切相关[62],通过干预某些长链非编码RNA的表达可以促进干细胞分化。长链非编码RNA参与 Wnt/β-catenin 信号转导,可以影响成肝分化的进程[63-64]。YU等[65]发现间充质干细胞成肝分化过程中长链非编码RNA UCA1呈高表达,过表达UCA1后显著促进成肝分化。王丹丹等[66]研究发现长链非编码RNA NEAT1抑制了脐带间充质干细胞肝向分化。 综上所述,miRNA和长链非编码RNA均可以介导间充质干细胞成肝分化。一种或几种特异性miRNA可用于将间充质干细胞诱导分化为功能性肝细胞。然而,miRNA在此诱导过程的分子机制未见报道,深入探索其分子机制有利于优化肝细胞诱导方案。长链非编码RNA在成肝分化过程中发挥重要作用,特定的长链非编码RNA可以调控肝细胞特异性基因的表达,进而影响成肝分化进程,深入研究长链非编码RNA在成肝分化中的具体作用机制,可有助于开发新策略促进成肝分化,也为肝脏疾病的治疗提供了新的视角和潜在的靶点。 表观遗传学与间充质干细胞成肝分化的相关研究,见表2。 "

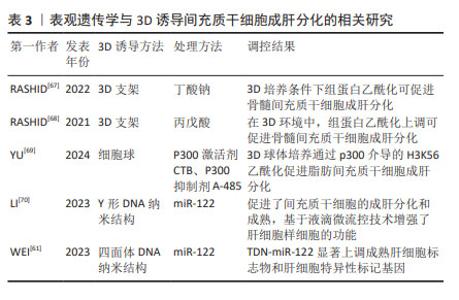
2.4 3D诱导间充质干细胞成肝分化过程中的表观遗传学变化 3D诱导能有效提升间充质干细胞向肝细胞方向的分化效率,使得诱导后的肝样细胞展现出更为成熟的肝功能特征及更高水平的肝脏特异性基因表达。当前,大量研究聚焦于深入探讨3D诱导环境下间充质干细胞向肝细胞分化的潜在机制,其中表观遗传学尤其是组蛋白乙酰化在分化过程中的调控作用受到关注。 在3D培养条件下诱导肝细胞分化的过程中,表观遗传学修饰发挥了重要作用,其中组蛋白乙酰化有助于转录因子与染色质的结合,从而促进肝细胞分化。RASHID等[67]利用3D水凝胶支架对人类骨髓间充质干细胞进行成肝分化诱导,结果表明在3D支架中分化的肝细胞展现出更成熟的特征;此外,只有在3D条件下观察到了成熟肝细胞特异性标志物——酪氨酸氨基转移酶(TAT)的表达上调,进一步证明了3D诱导在肝细胞分化成熟度方面的优势。在2D与3D诱导过程中加入组蛋白去乙酰化酶抑制剂,研究结果显示,相较于2D诱导,3D诱导通过组蛋白乙酰化更有效地提升了肝脏特异性基因的表达水平,并且在无需添加特定肝细胞生长因子的条件下,3D培养环境显著提高了肝细胞分化的效率[68],这表明培养环境能影响表观遗传学修饰,进而影响肝脏基因的表达。与2D培养相比,3D培养更接近体内的微环境,能激活类似于体内的细胞信号传导路径,使得表观遗传学修饰作用更为显著。细胞球培养诱导的肝样细胞表现出更为成熟的肝细胞功能,同时进行乙酰化修饰蛋白质组学检测发现,与2D相比,3D细胞球诱导的肝样细胞中H3K56乙酰化水平显著升高,且乙酰转移酶p300乙酰化水平也升高。3D细胞球诱导可通过p300 这一染色质调控组蛋白的H3K56乙酰化修饰,促进了白蛋白基因的转录活性,进而有效提升了间充质干细胞向肝细胞分化的诱导效率[69]。总之,以上研究结果表明不同培养维度可影响间充质干细胞的成肝分化,这一过程与组蛋白乙酰化修饰密切相关。 研究表明,miRNA参与调控间充质干细胞成肝分化,然而高效的miRNA递送仍然面临着不稳定、细胞摄取效率低、易生物降解、传递效率差等挑战,最近有研究表明将miRNA与3D培养相结合,可以提高递送效率,增强成肝诱导效果。例如,LI等[70]设计了Y形DNA纳米结构结合miRNA-122(Y-miR-122)促进了间充质干细胞的成肝分化和成熟,是一种很有前途的miR-122递送平台,基于微流控技术的微凝胶封装细胞进行细胞移植避免了炎症微环境和细胞免疫排斥的影响,而微凝胶的通透性允许营养和代谢物与外部环境交换,同时3D微环境促进了细胞内细胞-细胞和细胞-基质相互作用。微凝胶支架也为维持细胞活力和血管化提供了最佳的3D条件,在细胞治疗中提供更好的治疗效果。WEI等[61]研究发现,与miR-122相比,在四面体DNA纳米平台上结合miR-122(TDN-miR-122)显著上调成熟肝细胞标志物和肝特异性基因的表达,表现出成熟肝细胞形态和表型;四面体DNA纳米结构模拟了体内3D微环境,提高了miR-122的细胞摄取效率。总之,在3D培养条件下更有利于表观遗传学调控,miRNA和3D培养环境之间的相互作用是一个复杂的过程,它们相互影响,共同调控细胞的行为和基因表达。通过深入研究这些相互作用,可以更好地理解细胞在3D环境中的生物学行为,并为组织工程和疾病治疗提供新的策略。 3D培养条件下,细胞内部表观遗传学调控网络发生改变,这些变化进一步影响细胞分化的路径。通过深入剖析3D诱导间充质干细胞向肝细胞分化过程中的表观遗传学机制,可以进一步挖掘间充质干细胞分化的机制,并为优化干细胞向肝样细胞转化的效率和开发基于间充质干细胞的新型3D诱导策略提供全新的科研视角。 表观遗传学与3D诱导间充质干细胞成肝分化的相关研究,见表3。 "

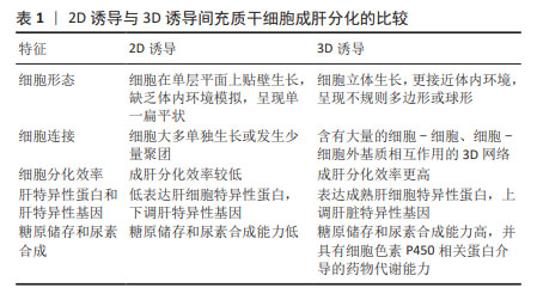
| [1] GADD VL, ALEKSIEVA N, FORBES SJ. Epithelial Plasticity during Liver Injury and Regeneration. Cell Stem Cell. 2020;27(4):557-573. [2] OLIVO R, GUARRERA JV, PYRSOPOULOS NT. Liver Transplantation for Acute Liver Failure. Clin Liver Dis. 2018;22(2):409-417. [3] DEVARBHAVI H, ASRANI SK, ARAB JP, et al. Global burden of liver disease: 2023 update. J Hepatol. 2023;79(2):516-537. [4] ZHONG Y, YU JS, WANG X, et al. Chemical-based primary human hepatocyte monolayer culture for the study of drug metabolism and hepatotoxicity: Comparison with the spheroid model. FASEB J. 2021;35(3):e21379. [5] NAJI A, EITOKU M, FAVIER B, et al. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci. 2019;76(17):3323-3348. [6] AFSHARI A, SHAMDANI S, UZAN G, et al. Different approaches for transformation of mesenchymal stem cells into hepatocyte-like cells. Stem Cell Res Ther. 2020; 11(1):54. [7] YE JS, SU XS, STOLTZ JF, et al. Signalling pathways involved in the process of mesenchymal stem cells differentiating into hepatocytes. Cell Prolif. 2015;48(2): 157-165. [8] BEHBAHAN IS, DUAN Y, LAM A, et al. New approaches in the differentiation of human embryonic stem cells and induced pluripotent stem cells toward hepatocytes. Stem Cell Rev Rep. 2011;7(3):748-759. [9] 彭蕾,杨骁,王敏君.获得诱导性肝细胞样细胞的策略研究[J].中国细胞生物学学报,2022,44(3):512-519. [10] SUN H, SHI C, YE Z, et al. The role of mesenchymal stem cells in liver injury. Cell Biol Int. 2022;46(4):501-511. [11] OZKUL Y, GALDERISI U. The Impact of Epigenetics on Mesenchymal Stem Cell Biology. J Cell Physiol. 2016;231(11):2393-2401. [12] MORTADA I, MORTADA R. Epigenetic changes in mesenchymal stem cells differentiation. Eur J Med Genet. 2018;61(2):114-118. [13] JAUKOVIĆ A, ABADJIEVA D, TRIVANOVIĆ D, et al. Specificity of 3D MSC Spheroids Microenvironment: Impact on MSC Behavior and Properties. Stem Cell Rev Rep. 2020;16(5):853-875. [14] RYU NE, LEE SH, PARK H. Spheroid Culture System Methods and Applications for Mesenchymal Stem Cells. Cells. 2019;8(12):1620. [15] WU X, SU J, WEI J, et al. Recent Advances in Three-Dimensional Stem Cell Culture Systems and Applications. Stem Cells Int. 2021;2021: 9477332. [16] KOUROUPIS D, CORREA D. Increased Mesenchymal Stem Cell Functionalization in Three-Dimensional Manufacturing Settings for Enhanced Therapeutic Applications. Front Bioeng Biotechnol. 2021;9: 621748. [17] XU Q. Human Three-Dimensional Hepatic Models: Cell Type Variety and Corresponding Applications. Front Bioeng Biotechnol. 2021;9:730008. [18] OKURA H, KOMODA H, SAGA A, et al. Properties of hepatocyte-like cell clusters from human adipose tissue-derived mesenchymal stem cells. Tissue Eng Part C Methods. 2010;16(4):761-770. [19] CIPRIANO M, FREYER N, KNÖSPEL F, et al. Self-assembled 3D spheroids and hollow-fibre bioreactors improve MSC-derived hepatocyte-like cell maturation in vitro. Arch Toxicol. 2017;91(4):1815-1832. [20] OCK SA, KIM SY, JU WS, et al. Adipose Tissue-Derived Mesenchymal Stem Cells Extend the Lifespan and Enhance Liver Function in Hepatocyte Organoids. Int J Mol Sci. 2023;24(20):15429. [21] MCKEE C, CHAUDHRY GR. Advances and challenges in stem cell culture. Colloids Surf B Biointerfaces. 2017;159:62-77. [22] RAGHAV PK, MANN Z, AHLAWAT S, et al. Mesenchymal stem cell-based nanoparticles and scaffolds in regenerative medicine. Eur J Pharmacol. 2022;918: 174657. [23] YAN XZ, VAN DEN BEUCKEN JJ, BOTH SK, et al. Biomaterial strategies for stem cell maintenance during in vitro expansion. Tissue Eng Part B Rev. 2014;20(4):340-354. [24] LI YS, HARN HJ, HSIEH DK, et al. Cells and materials for liver tissue engineering. Cell Transplant. 2013;22(4):685-700. [25] ASADI M, LOTFI H, SALEHI R, et al. Hepatic cell-sheet fabrication of differentiated mesenchymal stem cells using decellularized extracellular matrix and thermoresponsive polymer. Biomed Pharmacother. 2021;134:111096. [26] WANG Y, LEE JH, SHIRAHAMA H, et al. Extracellular Matrix Functionalization and Huh-7.5 Cell Coculture Promote the Hepatic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells in a 3D ICC Hydrogel Scaffold. ACS Biomater Sci Eng. 2016;2(12):2255-2265. [27] XU L, WANG S, SUI X, et al. Mesenchymal Stem Cell-Seeded Regenerated Silk Fibroin Complex Matrices for Liver Regeneration in an Animal Model of Acute Liver Failure. ACS Appl Mater Interfaces. 2017;9(17):14716-14723. [28] ALEAHMAD F, EBRAHIMI S, SALMANNEZHAD M, et al. Heparin/Collagen 3D Scaffold Accelerates Hepatocyte Differentiation of Wharton’s Jelly-Derived Mesenchymal Stem Cells. Tissue Eng Regen Med. 2017;14(4):443-452. [29] LI J, TAO R, WU W, et al. 3D PLGA scaffolds improve differentiation and function of bone marrow mesenchymal stem cell-derived hepatocytes. Stem Cells Dev. 2010;19(9):1427-1436. [30] PROCTOR WR, FOSTER AJ, VOGT J, et al. Utility of spherical human liver microtissues for prediction of clinical drug-induced liver injury. Arch Toxicol. 2017;91(8):2849-2863. [31] YANG Y, JIA Y, YANG Q, et al. REVIEW ARTICLE Engineering bio-inks for 3D bioprinting cell mechanical microenvironment. Int J Bioprint. 2022;9(1):632. [32] MURPHY SV, ATALA A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014; 32(8):773-785. [33] XIE R, PAL V, YU Y, et al. A comprehensive review on 3D tissue models: Biofabrication technologies and preclinical applications. Biomaterials. 2024;304: 122408. [34] LEE HJ, KIM YB, AHN SH, et al. A New Approach for Fabricating Collagen/ECM-Based Bioinks Using Preosteoblasts and Human Adipose Stem Cells. Adv Healthc Mater. 2015;4(9):1359-1368. [35] ZHANG J, CHEN X, CHAI Y, et al. 3D Printing of a Vascularized Mini-Liver Based on the Size-Dependent Functional Enhancements of Cell Spheroids for Rescue of Liver Failure. Adv Sci (Weinh). 2024;11(17):e2309899. [36] MASTRANGELI M, MILLET S, ORCHID PARTNERS T, et al. Organ-on-chip in development: Towards a roadmap for organs-on-chip. ALTEX. 2019;36(4):650-668. [37] EHRLICH A, DUCHE D, OUEDRAOGO G, et al. Challenges and Opportunities in the Design of Liver-on-Chip Microdevices. Annu Rev Biomed Eng. 2019;21:219-239. [38] UNDERHILL GH, KHETANI SR. Bioengineered Liver Models for Drug Testing and Cell Differentiation Studies. Cell Mol Gastroenterol Hepatol. 2017;5(3):426-439.e1. [39] YEN MH, WU YY, LIU YS, et al. Efficient generation of hepatic cells from mesenchymal stromal cells by an innovative bio-microfluidic cell culture device. Stem Cell Res Ther. 2016;7(1):120. [40] LI Z, SUN X. Epigenetic regulation in liver regeneration. Life Sci. 2024;353:122924. [41] WALEWSKA A, JANUCIK A, TYNECKA M, et al. Mesenchymal stem cells under epigenetic control - the role of epigenetic machinery in fate decision and functional properties. Cell Death Dis. 2023;14(11):720. [42] JANG S, HWANG J, JEONG HS. The Role of Histone Acetylation in Mesenchymal Stem Cell Differentiation. Chonnam Med J. 2022;58(1): 6-12. [43] SUI BD, ZHENG CX, LI M, et al. Epigenetic Regulation of Mesenchymal Stem Cell Homeostasis. Trends Cell Biol. 2020;30(2):97-116. [44] TSAI WL, YEH PH, TSAI CY, et al. Efficient programming of human mesenchymal stem cell-derived hepatocytes by epigenetic regulations. J Gastroenterol Hepatol. 2017;32(1):261-269. [45] CHEN Y, PAN RL, ZHANG XL, et al. Induction of hepatic differentiation of mouse bone marrow stromal stem cells by the histone deacetylase inhibitor VPA. J Cell Mol Med. 2009;13(8B):2582-2592. [46] DONG X, PAN R, ZHANG H, et al. Modification of histone acetylation facilitates hepatic differentiation of human bone marrow mesenchymal stem cells. PLoS One. 2013;8(5):e63405. [47] RAUT A, KHANNA A. Enhanced expression of hepatocyte-specific microRNAs in valproic acid mediated hepatic trans-differentiation of human umbilical cord derived mesenchymal stem cells. Exp Cell Res. 2016;343(2):237-247. [48] AN SY, HAN J, LIM HJ, et al. Valproic acid promotes differentiation of hepatocyte-like cells from whole human umbilical cord-derived mesenchymal stem cells. Tissue Cell. 2014;46(2):127-135. [49] PANTA W, IMSOONTHORNRUKSA S, YOISUNGNERN T, et al. Enhanced Hepatogenic Differentiation of Human Wharton’s Jelly-Derived Mesenchymal Stem Cells by Using Three-Step Protocol. Int J Mol Sci. 2019;20(12):3016. [50] JONES PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13(7):484-492. [51] CIPRIANO M, CORREIA JC, CAMÕES SP, et al. The role of epigenetic modifiers in extended cultures of functional hepatocyte-like cells derived from human neonatal mesenchymal stem cells. Arch Toxicol. 2017;91(6):2469-2489. [52] SEELIGER C, CULMES M, SCHYSCHKA L, et al. Decrease of global methylation improves significantly hepatic differentiation of Ad-MSCs: possible future application for urea detoxification. Cell Transplant. 2013;22(1):119-131. [53] SNYKERS S, VANHAECKE T, DE BECKER A, et al. Chromatin remodeling agent trichostatin A: a key-factor in the hepatic differentiation of human mesenchymal stem cells derived of adult bone marrow. BMC Dev Biol. 2007;7:24. [54] 何宇涛,冉江华.DNA甲基化在肝脏再生中的研究现状与展望[J].中国普外基础与临床杂志,2020,27(4):494-498. [55] TARIQUE S, NAEEM N, SALIM A, et al. The role of epigenetic modifiers in the hepatic differentiation of human umbilical cord derived mesenchymal stem cells. Biol Futur. 2022;73(4):495-502. [56] ZHOU X, CUI L, ZHOU X, et al. Induction of hepatocyte-like cells from human umbilical cord-derived mesenchymal stem cells by defined microRNAs. J Cell Mol Med. 2017;21(5):881-893. [57] LAUDADIO I, MANFROID I, ACHOURI Y, et al. A feedback loop between the liver-enriched transcription factor network and miR-122 controls hepatocyte differentiation. Gastroenterology. 2012;142(1):119-129. [58] DAVOODIAN N, LOTFI AS, SOLEIMANI M, et al. MicroRNA-122 overexpression promotes hepatic differentiation of human adipose tissue-derived stem cells. J Cell Biochem. 2014;115(9):1582-1593. [59] DAVOODIAN N, LOTFI AS, SOLEIMANI M, et al. Let-7f microRNA negatively regulates hepatic differentiation of human adipose tissue-derived stem cells. J Physiol Biochem. 2014;70(3):781-789. [60] KHOSRAVI M, AZARPIRA N, SHAMDANI S, et al. Differentiation of umbilical cord derived mesenchymal stem cells to hepatocyte cells by transfection of miR-106a, miR-574-3p, and miR-451. Gene. 2018;667: 1-9. [61] WEI H, LI F, XUE T, et al. MicroRNA-122-functionalized DNA tetrahedron stimulate hepatic differentiation of human mesenchymal stem cells for acute liver failure therapy. Bioact Mater. 2023;28:50-60. [62] ZHANG G, LAN Y, XIE A, et al. Comprehensive analysis of long noncoding RNA (lncRNA)-chromatin interactions reveals lncRNA functions dependent on binding diverse regulatory elements. J Biol Chem. 2019;294(43):15613-15622. [63] WANG Y, HE L, DU Y, et al. The long noncoding RNA lncTCF7 promotes self-renewal of human liver cancer stem cells through activation of Wnt signaling. Cell Stem Cell. 2015;16(4):413-425. [64] 李梦,周云,禹亚彬,等.长链非编码RNA肝癌高表达转录本促进人骨髓间充质干细胞向肝样细胞的分化[J].中国组织工程研究,2019,23(29):4656-4661. [65] YU Y, LI M, SONG Y, et al. Overexpression of long noncoding RNA CUDR promotes hepatic differentiation of human umbilical cord mesenchymal stem cells. Mol Med Rep. 2020;21(3):1051-1058. [66] 王丹丹,禹亚彬,刘世奇,等.人脐带间充质干细胞向肝细胞分化过程中长链非编码RNA核富集转录本1的作用[J].中国组织工程研究,2022,26(30): 4847-4851. [67] RASHID S, SALIM A, QAZI REM, et al. Sodium Butyrate Induces Hepatic Differentiation of Mesenchymal Stem Cells in 3D Collagen Scaffolds. Appl Biochem Biotechnol. 2022;194(8):3721-3732. [68] RASHID S, QAZI RE, MALICK TS, et al. Effect of valproic acid on the hepatic differentiation of mesenchymal stem cells in 2D and 3D microenvironments. Mol Cell Biochem. 2021;476(2):909-919. [69] YU Y, HUANG H, YE J, et al. 3D Spheroids Facilitate Differentiation of Human Adipose-Derived Mesenchymal Stem Cells into Hepatocyte-Like Cells via p300-Mediated H3K56 Acetylation. Stem Cells Transl Med. 2024;13(2):151-165. [70] LI F, WEI H, JIN Y, et al. Microfluidic Fabrication of MicroRNA-Induced Hepatocyte-Like Cells/Human Umbilical Vein Endothelial Cells-Laden Microgels for Acute Liver Failure Treatment. ACS Nano. 2023;17(24):25243-25256. |
| [1] | Lai Pengyu, Liang Ran, Shen Shan. Tissue engineering technology for repairing temporomandibular joint: problems and challenges [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [2] | Liu Lin, Liu Shixuan, Lu Xinyue, Wang Kan. Metabolomic analysis of urine in a rat model of chronic myofascial trigger points [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1585-1592. |
| [3] | Su Xiaoyang, Chen Wenting, Fu Yidan, Zhao Yan, Lan Danfeng, Yang Qiuping. Correlation between Mer receptor tyrosine kinase and diabetic peripheral neuropathy in Sprague-Dawley rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1593-1599. |
| [4] | Li Kaiying, Wei Xiaoge, Song Fei, Yang Nan, Zhao Zhenning, Wang Yan, Mu Jing, Ma Huisheng. Mechanism of Lijin manipulation regulating scar formation in skeletal muscle injury repair in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1600-1608. |
| [5] | Li Jun, Gong Jingjing, Sun Guobin, Guo Rui, Ding Yang, Qiang Lijuan, Zhang Xiaoli, Fang Zhanhai . miR-27a-3p promotes the proliferation of human hypertrophic scar fibroblasts by regulating mitogen-activated protein kinase signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1609-1617. |
| [6] | Li Huayuan, Li Chun, Liu Junwei, Wang Ting, Li Long, Wu Yongli. Effect of warm acupuncture on PINK1/Parkin pathway in the skeletal muscle of rats with chronic fatigue syndrome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1618-1625. |
| [7] | Jing Ruyi, Chen Yingxin, Cao Lei . Prognosis of deep lamellar keratoplasty versus penetrating keratoplasty in the treatment of stromal corneal dystrophy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1626-1633. |
| [8] | Wang Xuanqiang, Zhang Wenyang, Li Yang, Kong Weiqian, Li Wei, Wang Le, Li Zhongshan, Bai Shi. Effects of chronic exposure to low-frequency pulsed magnetic fields on contractility and morphology of the quadriceps muscle in healthy adults [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1634-1642. |
| [9] | Zhang Yuxin, Yu Cong, Zhang Cui, Ding Jianjun, Chen Yan. Differences in postural control ability between older adults with mild cognitive impairment and those with normal cognition under different single-task and dual-task conditions [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1643-1649. |
| [10] | Zhou Panpan, Cui Yinglin, Zhang Wentao, Wang Shurui, Chen Jiahui, Yang Tong . Role of cellular autophagy in cerebral ischemic injury and the regulatory mechanism of traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1650-1658. |
| [11] | Yu Jingbang, Wu Yayun. Regulatory effect of non-coding RNA in pulmonary fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1659-1666. |
| [12] | Wang Qiuyue, Jin Pan, Pu Rui . Exercise intervention and the role of pyroptosis in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1667-1675. |
| [13] | Zhu Hanmin, Wang Song, Xiao Wenlin, Zhang Wenjing, Zhou Xi, He Ye, Li Wei, . Mitophagy regulates bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1676-1683. |
| [14] | Yuan Weibo, Liu Chan, Yu Limei. Potential application of liver organoids in liver disease models and transplantation therapy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1684-1692. |
| [15] | Wang Yida, Liu Jun, Wang Xiaoling, Wang Liyan, Yang Chengru, Zhang Xuexiao. Effects of wearable electronic device-based interventions on physical activity and sedentary behavior in healthy adolescents: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1693-1704. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||