Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (26): 5688-5694.doi: 10.12307/2025.709
Previous Articles Next Articles
Cellular biomechanisms of Wallerian degeneration in peripheral nerve axon injury
Shalayiding·Aierxiding1, 2, Aikebaierjiang·Aisaiti1,2, Kutiluke·Shoukeer1, 2, Gulimire·Yilihamu3, Aikeremujiang·Muheremu1, 2
- 1Department of Orthopedics, The Sixth Affiliated Hospital of Xinjiang Medical University, Urumqi 830002, Xinjiang Uygur Autonomous Region, China; 2Key Laboratory of Orthopedic Regenerative Medicine, The Sixth Affiliated Hospital of Xinjiang Medical University, Urumqi 830002, Xinjiang Uygur Autonomous Region, China; 3The First Affiliated Hospital of Xinjiang Medical University, Urumqi 830054, Xinjiang Uygur Autonomous Region, China
-
Received:2024-07-12Accepted:2024-09-21Online:2025-09-18Published:2025-02-28 -
Contact:Aikeremujiang·Muheremu, MD, Chief physician, Associate professor, Master’s supervisor, Department of Orthopedics, The Sixth Affiliated Hospital of Xinjiang Medical University, Urumqi 830002, Xinjiang Uygur Autonomous Region, China; Key Laboratory of Orthopedic Regenerative Medicine, The Sixth Affiliated Hospital of Xinjiang Medical University, Urumqi 830002, Xinjiang Uygur Autonomous Region, China -
About author:Shalayiding·Aierxiding, Master’s candidate, Department of Orthopedics, The Sixth Affiliated Hospital of Xinjiang Medical University, Urumqi 830002, Xinjiang Uygur Autonomous Region, China; Key Laboratory of Orthopedic Regenerative Medicine, The Sixth Affiliated Hospital of Xinjiang Medical University, Urumqi 830002, Xinjiang Uygur Autonomous Region, China -
Supported by:Natural Science Foundation of Xinjiang Uygur Autonomous Region (General Program), No. 2022D01C330 (to AM); National Natural Science Foundation of China (General Program), No. 82374487 (to AM); National Natural Science Foundation of China (Regional Program), No. 82260252 (to AM); Natural Science Foundation of Xinjiang Uygur Autonomous Region (General Program), 2022D01C331 (to AM [project participant]); Provincial-Ministerial Jointly-Built State Key Laboratory of Causes and Prevention of High Morbidity in Central Asia Open Topic Faceted Project, No. SKL-HIDCA-2022-16 (to AM); Key Scientific Research Program of Xinjiang Medical University, No. XYD2024ZX09 (to AM); “Tianchi Talent” Young Doctoral Project (to AM)
CLC Number:
Cite this article
Shalayiding·Aierxiding, Aikebaierjiang·Aisaiti, Kutiluke·Shoukeer, Gulimire·Yilihamu, Aikeremujiang·Muheremu. Cellular biomechanisms of Wallerian degeneration in peripheral nerve axon injury[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5688-5694.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
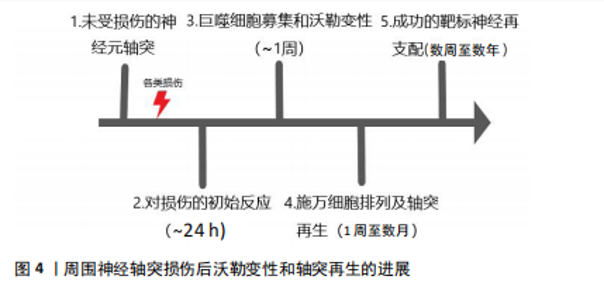
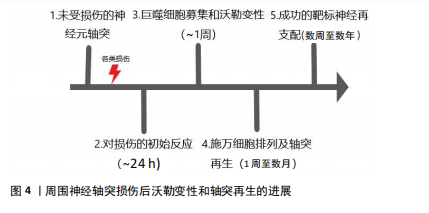
2.1 沃勒变性轴突退化与再生的细胞生物学机制 巨噬细胞、施万细胞和小胶质细胞是驻扎在脑和脊髓中的吞噬免疫细胞,一旦受损的轴突开始破坏,它们都参与轴突和髓鞘碎片的清除,在轴突损伤和沃勒变性中起积极作用[9]。在小鼠中,受损坐骨神经轴突碎片化的清除在损伤后1 d开始,整个过程可持续1周以上[10-14]。同时,这些细胞在病变部位形成胶质瘢痕,将受损区域封闭,并向细胞外基质释放促进轴突再生的硫酸软骨素蛋白聚糖[15]。研究发现,胶质瘢痕的完全发育可能需要相当长的时间,例如:在脊髓损伤患者中,反应性星形胶质细胞早在损伤后4 d就被发现,而晚期星形胶质细胞反应在损伤后4个月开始,形成致密、持久的胶质瘢痕[16],见图4。 轴突发生沃勒变性时表现出典型的形态改变,其过程可分为4个病理阶段:急性反应期、潜伏期、变性期和清除期。然而,病理阶段并不总与细胞和分子水平的变化相对应[17]。例如,虽然在潜伏期很少发生轴突破坏,但几个关键的遗传信号分子途径在这一阶段被激活,将损伤信号传递给下游级联反应和启动轴突退化的效应物[18-19]。"
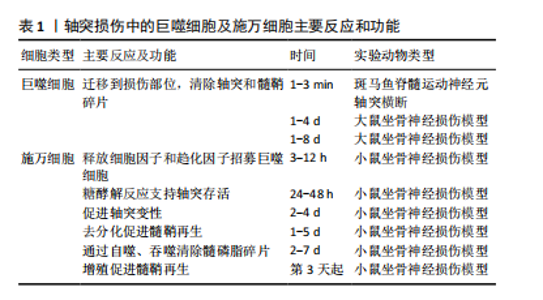
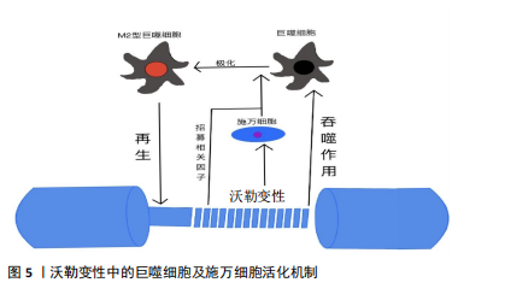
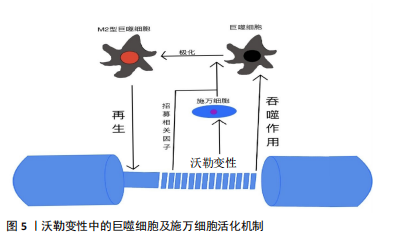
2.1.1 施万细胞对周围神经轴突损伤的反应 在周围神经轴突损伤后的三四周内,轴突残端几乎所有的神经生长锥都没有核和髓鞘。但从第4周开始,有时甚至更早,带有细长细胞核的梭形细胞就会在纤维周围生长,这些细胞是由施万细胞增殖产生的[33-34]。 施万细胞对正常神经功能和神经修复至关重要,占周围神经内有核细胞的90%[35],为发育、成熟和再生的轴突提供营养支持。此外,施万细胞产生的基膜包围轴突,支持轴突生长。成人周围神经中有2种类型的施万细胞:髓鞘施万细胞和无髓鞘施万细胞[36]。髓鞘施万细胞在大口径轴突(运动或感觉)周围形成多层膜性髓鞘,与单个轴突的一段相关联,并沿轴突的纵向均匀分布[37],绝缘的髓鞘结构使轴突能够比无髓鞘神经纤维轴突形成更快的动作电位。相反,无髓鞘施万细胞或Remak细胞松散地包裹多个小直径的无髓鞘轴突,其细胞质突起隔离并包围轴突。 (1)施万细胞是周围神经轴突损伤后前期最主要的吞噬细胞:外周神经损伤后不久,远端神经中的施万细胞开始去分化,这一过程依赖于泛素-蛋白酶体系统[38]。在轴突退化发生之前,与轴突相关的髓鞘施万细胞通过改变基因表达来对损伤做出反应[39-41]。在损伤的48 h内,这些施万细胞停止产生髓鞘蛋白[42-43],上调再生相关基因(生长相关蛋白43、神经营养因子及其受体、神经调节蛋白及其受体)并开始增殖[44-47]。髓鞘施万细胞和Remak细胞均有分裂,在损伤后4 d左右达到增殖高峰,增殖的施万细胞被限制在基板管中,排列成Büngner带,为再生轴突提供支持底物和生长因子[48-49]。 最近的研究揭示了施万细胞和免疫细胞最初感知附近轴突损伤的分子机制。其中,最重要的发现是Toll样受体,通过识别微生物病原体和激活天然免疫,在免疫稳态和抗病毒过程中发挥重要作用[50-53],通过结合通常不存在于细胞外环境中的内源性配体(例如热休克蛋白[54]、mRNA[55]、降解的细胞外基质成分[56])来识别组织损伤。施万细胞表达多种Toll样受体,如Toll样受体1、Toll样受体3、Toll样受体4和Toll样受体7。Toll样受体1在轴突损伤后上调[57]。Toll样受体的表达模式表明,施万细胞在周围神经系统中起着前哨作用。 髓鞘碎片是远端神经轴突再生的屏障,这些髓鞘碎片含有抑制轴突生长的分子,包括髓鞘相关糖蛋白和少突胶质细胞髓鞘磷脂糖蛋白[58-59]。施万细胞在早期清除髓鞘碎片方面发挥了重要作用,其中失神经施万细胞是损伤后前5 d的主要吞噬细胞[60]。轴突退化和消失后,施万细胞髓鞘纵向分裂,形成小的卵圆形结构[47]。施万细胞表达主要组织相容性复合体Ⅱ类分子,然而它们是否可以作为抗原提呈细胞尚不清楚[61-63]。 (2)施万细胞负责外周神经轴突损伤后的髓鞘初始降解:外周神经损伤后髓鞘的初始降解依赖于磷脂酶A2家族的激活[64]。施万细胞胞质内分泌形式的磷脂酶A2将磷脂酰胆碱水解为溶血磷脂酰胆碱(导致髓鞘分解)和花生四烯酸(可代谢为促炎二十烷类化合物)。炎症递质可能调控外周神经损伤后施万细胞中磷脂酶A2的激活[65-66]。值得注意的是,在损伤后几小时内,胞浆和分泌形式的磷脂酶A2在施万细胞和巨噬细胞中上调,并在2周内保持升高[66],这一时间进程与外周神经损伤后的沃勒变性相关。外周神经损伤后3周恢复到基础磷脂酶A2水平,与轴突再生和重新髓鞘形成有关。Stirling等[66]还发现,阻断坐骨神经损伤远端磷脂酶A2的表达显著延长了变性髓鞘和轴突的清除时间。因此,施万细胞表达磷脂酶A2水平是外周神经损伤后髓鞘降解和沃勒变性进展所必需的。 (3)激活的施万细胞启动了细胞因子/趋化素级联反应:除外周神经损伤后的增殖和吞噬碎片外,施万细胞还释放能使免疫细胞招募到受损神经中的细胞因子和化学因子。招募的各种免疫细胞,特别是巨噬细胞,在沃勒变性的下一阶段碎片清除和生长因子产生方面发挥主要作用[67-68]。在小鼠坐骨神经切断后,施万细胞表达的白细胞介素6和白血病抑制因子mRNA在神经损伤后3 h内增加[68],5 h内合成促炎细胞因子肿瘤坏死因子α和白细胞介素1α,而白细胞介素1β的产生延迟到24 h[69]。这些因子由施万细胞产生,是免疫细胞趋化所必需的[70]。肿瘤坏死因子α还诱导产生单核细胞趋化蛋白1[71]、基质金属蛋白酶9 [72],两者都是切断轴突诱导巨噬细胞聚集所必需的。总而言之,这些研究表明,激活的施万细胞启动了细胞因子/趋化素级联反应,从而放大和微调外周神经损伤后的炎症反应。 (4)施万细胞通过分泌细胞外基质分子和营养因子促进轴突再生:受损神经残端的施万细胞分泌促进轴突生长的细胞外基质和营养因子。层粘连蛋白是外周神经系统中第二常见的细胞外基质成分(仅次于胶原蛋白),支持轴突生长[73],它们的表达在外周神经损伤后增加[74]。研究发现,施万细胞中层粘连蛋白γ1亚单位的条件性敲除,改变了施万细胞生理学,抑制了外周神经损伤后的轴突生长[75]。因此,层粘连蛋白对于维护和修复受损的外周神经系统是不可或缺的。 施万细胞还分泌不同的营养因子,支持神经元的生存和生长。促炎症细胞因子白细胞介素6在外周神经损伤后的神经元和非神经元细胞中都升高[76-77],通过其受体发出信号,增加神经元中再生相关基因的表达,促进轴突生长[78-79]。外周神经损伤会增加神经营养因子的释放,如神经生长因子,并可能促进轴突再生[80-83]。 综上所述,外周神经系统轴突损伤后的施万细胞通过增殖、吞噬髓鞘碎片以及分泌营养因子和细胞因子来促进神经修复。 2.1.2 免疫细胞对周围神经损伤的反应 免疫细胞对组织损伤和感染的反应在病理性外周神经系统中很大程度上是保守的,中性粒细胞和巨噬细胞在损伤后几小时或几天内到达,而远端损伤神经节段的淋巴细胞聚集延迟了1周或更长时间。此外,VOET等[84]研究表明,背根神经节损伤后神经元细胞周围的巨噬细胞激活可以促进轴突再生,这表明在远离原发损伤的区域(但仍在受影响细胞附近)通过损伤诱导的巨噬细胞激活也可以促进神经修复。因此,目前普遍认为外周神经系统轴突的再生依赖于快速而有效的免疫细胞反应。见表1。"
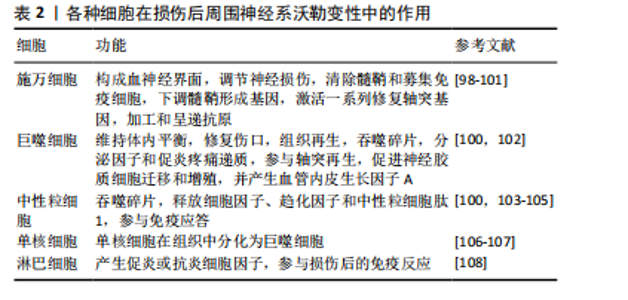
(1)中性粒细胞是第一个从循环中侵入受损组织的炎性白细胞:中性粒细胞作为髓系白细胞,是免疫系统的第一道防线,占体内所有循环白细胞的50%-70%[84]。中性粒细胞主要负责吞噬损伤细胞碎片,并在沃勒变性过程中调节其他白细胞(主要是单核细胞)的招募和激活[85]。虽然中性粒细胞在未损伤的大鼠神经中分布稀少,但其在损伤部位附近区域的密度在8 h内显著增加,并在24 h达到高峰(只有少数中性粒细胞渗透到远端残端的更远端区域)[86]。中性粒细胞在发生凋亡之前会短暂地吞噬损伤细胞碎片[87]。目前尚不清楚中性粒细胞如何影响外周神经损伤后轴突再生。 (2)外周神经轴突的再生依赖于快速而有效的巨噬细胞反应:巨噬细胞是另一个对外周神经损伤有反应的主要免疫细胞群。重要的是,它们在沃勒变性的后期阶段清除了髓鞘碎片。在未损伤的周围神经中,内膜巨噬细胞占有核细胞的29%[88]。巨噬细胞表达主要组织相容性复合体分子和补体受体3,这些表面分子分别赋予巨噬细胞抗原提呈和监视功能[89]。巨噬细胞通过增殖和吞噬髓鞘来对外周神经损伤做出反应[59]。血源性巨噬细胞对有效的髓鞘吞噬是必不可少的[90],并产生激活施万细胞的细胞因子(例如白细胞介素1)和帮助轴突再生的营养因子(例如神经生长因子)。巨噬细胞还重建远端神经的细胞外基质,为再生轴突做准备[91]。巨噬细胞在神经中停留几天到几个月,之后它们一部分通过循环迁移到淋巴器官,一部分因细胞凋亡而死亡[64,92]。DAVID团队已经详细研究了巨噬细胞的迁移情况,发现巨噬细胞为了吞噬退化的髓鞘,穿透施万细胞基板管,接收信号并迁移到附近的血管[64]。 (3)单核细胞在组织中分化为巨噬细胞:外周神经系统中增殖的巨噬细胞在沃勒变性的早期阶段有重要作用。单核细胞是由施万细胞和巨噬细胞衍生的细胞因子和趋化因子从血液中招募。损伤后4 d,大量循环单核细胞在损伤神经内积累[93-94],并且在组织中分化为巨噬细胞,局部趋化因子将巨噬细胞引导到不同的损伤区域。 (4)T淋巴细胞主要塑造沃勒变性后期的免疫反应:T淋巴细胞通过产生支持细胞免疫和体液免疫的促炎或抗炎细胞因子来调节免疫反应。1型辅助T(Th1)细胞分泌促炎细胞因子(例如肿瘤坏死因子α、γ-干扰素激活附近的巨噬细胞、中性粒细胞和自然杀伤细胞),2型辅助T (Th2)细胞释放抗炎细胞因子(例如白细胞介素4、白细胞介素10)。两种辅助T细胞通过调节各种巨噬细胞的功能,调节细胞级联反应[95]。BEAHRS等[96]通过研究转基因小鼠发现,辅助T细胞反应能够保护面部运动神经元,使一小部分神经元在轴突损伤后仍存活,因此得出Th1和Th2细胞都是促进轴突再生所必需的结论。 外周神经损伤后沃勒变性启动一种广泛的、涉及多种细胞类型的炎症反应,并持续数月。虽然施万细胞在沃勒变性的早期阶段调节髓鞘碎片的清除,但神经内膜和血源性巨噬细胞在外周神经损伤后1周内开始的碎片清除和神经修复中发挥关键作用[97]。见表2。 2.2 沃勒变性研究对周围神经损伤治疗研究的前景 许多脊髓损伤、创伤性脑损伤或创伤性轴突损伤患者只有小部分轴突被完全切断、压碎或拉伸,但在损伤后仍与躯体相连的轴突以及在最初损伤中未受损的神经束内的轴突,最终都会与其他受损的轴突一起退化。因此,了解控制轴突沃勒变性过程中的细胞生物学机制可以保护这些轴突,并尽可能地保持受伤神经的生理功能。"

| [1] 刘梦婷,沈霞,鲍关爱,等.运动干预对化疗诱导周围神经损伤影响的研究进展[J].中国康复医学杂志,2023,38(1):120-123. [2] YI S, ZHANG Y, GU X, et al. Application of stem cells in peripheral nerve regeneration. Burns Trauma. 2020;8:tkaa002. [3] JIANG L, JONES S, JIA X. Stem Cell Transplantation for Peripheral Nerve Regeneration: Current Options and Opportunities. Int J Mol Sci. 2017; 18(1):94. [4] LI X, GUAN Y, LI C, et al. Immunomodulatory effects of mesenchymal stem cells in peripheral nerve injury. Stem Cell Res Ther. 2022;13(1):18. [5] ZHENG C, YANG Z, CHEN S, et al. Nanofibrous nerve guidance conduits decorated with decellularized matrix hydrogel facilitate peripheral nerve injury repair. Theranostics. 2021;11(6):2917-2931. [6] ESTERA LA, WALSH SP, HEADEN JA, et al. Neuroinflammation: Breaking barriers and bridging gaps. Neurosci Res. 2023;197:9-17. [7] MAENG WY, TSENG WL, LI S, et al. Electroceuticals for peripheral nerve regeneration. Biofabrication. 2022;14(4):10. [8] YONG HYF, RAWJI KS, GHORBANI S, et al. The benefits of neuroinflammation for the repair of the injured central nervous system. Cell Mol Immunol. 2019;16(6):540-546. [9] 火艺霖,林浩东.周围神经损伤后再生过程中许旺细胞与巨噬细胞的交互作用[J].中华显微外科杂志,2024,47(1):104-109. [10] GAUDET AD, POPOVICH PG, RAMER MS. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 2011;8:110. [11] BROSIUS LUTZ A, CHUNG WS, SLOAN SA, et al. Schwann cells use TAM receptor-mediated phagocytosis in addition to autophagy to clear myelin in a mouse model of nerve injury. Proc Natl Acad Sci U S A. 2017;114(38): E8072-E8080. [12] ELBERG G, LIRAZ-ZALTSMAN S, REICHERT F, et al. Deletion of SIRPα (signal regulatory protein-α) promotes phagocytic clearance of myelin debris in Wallerian degeneration, axon regeneration, and recovery from nerve injury. J Neuroinflammation.2019;16(1):277. [13] GOMEZ-SANCHEZ JA, CARTY L, IRUARRIZAGA-LEJARRETA M, et al. Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J Cell Biol. 2015;210(1):153-168. [14] CONFORTI L, GILLEY J, COLEMAN MP. Wallerian degeneration: an emerging axon death pathway linking injury and disease. Nat Rev Neurosci. 2014; 15(6):394-409. [15] FITCH MT, SILVER J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol. 2008;209(2): 294-301. [16] BUSS A, BROOK GA, KAKULAS B, et al. Gradual loss of myelin and formation of an astrocytic scar during Wallerian degeneration in the human spinal cord. Brain. 2004;127(Pt 1):34-44. [17] ZHANG K, JIANG M, FANG Y. The Drama of Wallerian Degeneration: The Cast, Crew, and Script. Annu Rev Genet. 2021;55:93-113. [18] 孙小玲,黄凯伟,万新贝,等.神经丝轻链蛋白在周围神经病中的应用价值探究[J].中国全科医学,2022,25(32):3977-3983. [19] 赖木华.NeuroD1在周围神经轴突再生过程的作用及其相关机制研究[D].广州:南方医科大学,2020. [20] PELLEGATTA M, TAVEGGIA C. The Complex Work of Proteases and Secretases in Wallerian Degeneration: Beyond Neuregulin-1. Front Cell Neurosci. 2019;13:93. [21] MATSUDA M, HUH Y, JI RR. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J Anesth. 2019;33(1): 131-139. [22] AU NPB, MA CHE. Neuroinflammation, Microglia and Implications for Retinal Ganglion Cell Survival and Axon Regeneration in Traumatic Optic Neuropathy. Front Immunol. 2022;13:860070. [23] NIEMI JP, LINDBORG JA, ZIGMOND RE. Detection of Neutrophils in the Sciatic Nerve Following Peripheral Nerve Injury. Methods Mol Biol. 2020;2143: 207-222. [24] BEIROWSKI B, ADALBERT R, WAGNER D, et al. The progressive nature of Wallerian degeneration in wild-type and slow Wallerian degeneration (WldS) nerves. BMC Neurosci. 2005;6:6. [25] KALINSKI AL, YOON C, HUFFMAN LD, et al. Analysis of the immune response to sciatic nerve injury identifies efferocytosis as a key mechanism of nerve debridement. Elife. 2020;9:e60223. [26] ZHAO XF, HUFFMAN LD, HAFNER H, et al. The injured sciatic nerve atlas (iSNAT), insights into the cellular and molecular basis of neural tissue degeneration and regeneration. Elife. 2022;11:e80881. [27] MENORCA RM, FUSSELL TS, ELFAR JC. Nerve physiology: mechanisms of injury and recovery. Hand Clin. 2013;29(3):317-330. [28] XU J, WEN J, FU L, et al. Macrophage-specific RhoA knockout delays Wallerian degeneration after peripheral nerve injury in mice. J Neuroinflammation. 2021;18(1):234. [29] KERSCHENSTEINER M, SCHWAB ME, LICHTMAN JW, et al. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat Med. 2005;11(5):572-577. [30] CUNHA MI, SU M, CANTUTI-CASTELVETRI L, et al. Pro-inflammatory activation following demyelination is required for myelin clearance and oligodendrogenesis. J Exp Med. 2020;217(5):e20191390. [31] DAVIES AJ, RINALDI S, COSTIGAN M, et al. Cytotoxic Immunity in Peripheral Nerve Injury and Pain. Front Neurosci. 2020;14:142. [32] ROTSHENKER S. Wallerian degeneration: the innate-immune response to traumatic nerve injury. J Neuroinflammation. 2011;8:109. [33] 雪若妍,杨华.周围神经损伤病理进程中巨噬细胞作用研究进展[J].中华耳科学杂志,2021,19(1):126-130. [34] 黄善敏,许林杰,谢翠梅,等.巨噬细胞在周围神经损伤中的作用研究进展[J].神经损伤与功能重建,2023,18(10):597-600. [35] CAMPANA WM. Schwann cells: activated peripheral glia and their role in neuropathic pain. Brain Behav Immun. 2007;21(5):522-527. [36] GRIFFIN JW, THOMPSON WJ. Biology and pathology of nonmyelinating Schwann cells. Glia. 2008;56(14):1518-1531. [37] 张梦媛,齐建国.周围神经损伤与轴突再生[J].四川解剖学杂志,2022, 30(4):185-188. [38] LEE HK, SHIN YK, JUNG J, et al. Proteasome inhibition suppresses Schwann cell dedifferentiation in vitro and in vivo. Glia. 2009;57(16): 1825-1834. [39] FILOUS AR, SILVER J. “Targeting astrocytes in CNS injury and disease: A translational research approach”. Prog Neurobiol. 2016;144:173-187. [40] MISHRA A, BANDOPADHYAY R, SINGH PK, et al. Neuroinflammation in neurological disorders: pharmacotherapeutic targets from bench to bedside. Metab Brain Dis. 2021;36(7):1591-1626. [41] FRATI A, CERRETANI D, FIASCHI AI, et al. Diffuse Axonal Injury and Oxidative Stress: A Comprehensive Review. Int J Mol Sci. 2017;18(12):2600. [42] KOTHUR K, WIENHOLT L, BRILOT F, et al. CSF cytokines/chemokines as biomarkers in neuroinflammatory CNS disorders: A systematic review. Cytokine. 2016;77:227-237. [43] BOHLSON SS, TENNER AJ. Complement in the Brain: Contributions to Neuroprotection, Neuronal Plasticity, and Neuroinflammation. Annu Rev Immunol. 2023;41:431-452. [44] SHEN J, ZHAO M, ZHANG C, et al. IL-1β in atherosclerotic vascular calcification: From bench to bedside. Int J Biol Sci. 2021;17(15):4353-4364. [45] MURINSON BB, ARCHER DR, LI Y, et al. Degeneration of myelinated efferent fibers prompts mitosis in Remak Schwann cells of uninjured C-fiber afferents. J Neurosci. 2005;25(5):1179-1187. [46] LIU P, PENG J, HAN GH, et al. Role of macrophages in peripheral nerve injury and repair. Neural Regen Res. 2019;14(8):1335-1342. [47] LU CY, SANTOSA KB, JABLONKA-SHARIFF A, et al. Macrophage-Derived Vascular Endothelial Growth Factor-A Is Integral to Neuromuscular Junction Reinnervation after Nerve Injury. J Neurosci. 2020;40(50):9602-9616. [48] MALFAIT AM, MILLER RE, BLOCK JA. Targeting neurotrophic factors: Novel approaches to musculoskeletal pain. Pharmacol Ther. 2020;211:107553. [49] 张世雯,王艳,支金草,等.小泛素样修饰蛋白对周围神经损伤后再生作用的研究进展[J].中国康复,2022,37(3):175-178. [50] 铁岩,李潇,杨鑫伟,等.外泌体对神经系统疾病的作用研究进展[J].医学研究杂志,2021,50(6):158-160+176. [51] GOETHALS S, YDENS E, TIMMERMAN V, et al. Toll-like receptor expression in the peripheral nerve. Glia. 2010;58(14):1701-1709. [52] 熊乐. TOLL样受体4信号对大鼠周围神经损伤后瓦勒变性及神经再生的影响[D].青岛:青岛大学,2018. [53] 熊乐,张蓓,沈若武,等.TOLL样受体4拮抗剂干预周围神经损伤后的瓦勒变性[J].中国组织工程研究,2016,20(42):6308-6316. [54] BOIVIN A, PINEAU I, BARRETTE B, et al. Toll-like receptor signaling is critical for Wallerian degeneration and functional recovery after peripheral nerve injury. J Neurosci. 2007;27(46):12565-12576.
[55] KARIKÓ K, NI H, CAPODICI J, et al. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem. 2004;279(13):12542-12550. [56] BRUNN GJ, BUNGUM MK, JOHNSON GB, et al. Conditional signaling by Toll-like receptor 4. FASEB J. 2005;19(7):872-874. [57] LEE H, JO EK, CHOI SY, et al. Necrotic neuronal cells induce inflammatory Schwann cell activation via TLR2 and TLR3: implication in Wallerian degeneration. Biochem Biophys Res Commun. 2006;350(3):742-747. [58] HUANG JK, PHILLIPS GR, ROTH AD, et al. Glial membranes at the node of Ranvier prevent neurite outgrowth. Science. 2005;310(5755):1813-1817. [59] DONG X, LIU S, YANG Y, et al. Aligned microfiber-induced macrophage polarization to guide schwann-cell-enabled peripheral nerve regeneration. Biomaterials. 2021;272:120767. [60] KRATOFIL RM, SHIM HB, SHIM R, et al. A monocyte-leptin-angiogenesis pathway critical for repair post-infection. Nature. 2022;609(7925):166-173. [61] MEYER ZU HÖRSTE G, HU W, HARTUNG HP, et al. The immunocompetence of Schwann cells. Muscle Nerve. 2008;37(1):3-13. [62] BOMBEIRO AL, PEREIRA BTN, BONFANTI AP, et al. Immunomodulation by dimethyl fumarate treatment improves mouse sciatic nerve regeneration. Brain Res Bull. 2020;160:24-32. [63] MIN Q, PARKINSON DB, DUN XP. Migrating Schwann cells direct axon regeneration within the peripheral nerve bridge. Glia. 2021;69(2):235-254. [64] MARTINI R, FISCHER S, LÓPEZ-VALES R, et al. Interactions between Schwann cells and macrophages in injury and inherited demyelinating disease. Glia. 2008;56(14):1566-1577. [65] MIZISIN AP, WEERASURIYA A. Homeostatic regulation of the endoneurial microenvironment during development, aging and in response to trauma, disease and toxic insult. Acta Neuropathol. 2011;121(3):291-312. [66] STIRLING DP, STYS PK. Mechanisms of axonal injury: internodal nanocomplexes and calcium deregulation. Trends Mol Med. 2010;16(4): 160-170. [67] 赵富生,武庚,武杨,等.瓦勒变性坐骨神经段对大鼠BMSCs向SCs分化的影响[J].中国病理生理杂志,2014,30(11):1946-1953. [68] 李月珍,武庚,武杨,等.坐骨神经瓦勒变性大鼠许旺细胞生物学特性及分泌功能变化[J].中国组织工程研究,2014,18(33):5282-5287. [69] DALAKAS MC, ALEXOPOULOS H, SPAETH PJ. Complement in neurological disorders and emerging complement-targeted therapeutics. Nat Rev Neurol. 2020;16(11):601-617. [70] DING Z, JIANG M, QIAN J, et al. Role of transforming growth factor-β in peripheral nerve regeneration. Neural Regen Res. 2024;19(2):380-386. [71] DOMOTO R, SEKIGUCHI F, TSUBOTA M, et al. Macrophage as a Peripheral Pain Regulator. Cells. 2021;10(8):1881. [72] SHUBAYEV VI, ANGERT M, DOLKAS J, et al. TNFalpha-induced MMP-9 promotes macrophage recruitment into injured peripheral nerve. Mol Cell Neurosci. 2006;31(3):407-415. [73] JANG DG, SIM HJ, SONG EK, et al. Extracellular matrixes and neuroinflammation. BMB Rep. 2020;53(10):491-499. [74] BOSCH-QUERALT M, FLEDRICH R, STASSART RM. Schwann cell functions in peripheral nerve development and repair. Neurobiol Dis. 2023;176:105952. [75] MA KH, HUNG HA, SVAREN J. Epigenomic Regulation of Schwann Cell Reprogramming in Peripheral Nerve Injury. J Neurosci. 2016;36(35): 9135-9147. [76] STIERLI S, IMPERATORE V, LLOYD AC. Schwann cell plasticity-roles in tissue homeostasis, regeneration, and disease. Glia. 2019;67(11):2203-2215. [77] BROSIUS LUTZ A, LUCAS TA, CARSON GA, et al. An RNA-sequencing transcriptome of the rodent Schwann cell response to peripheral nerve injury. J Neuroinflammation. 2022;19(1):105. [78] CHAN AH, SCHRODER K. Inflammasome signaling and regulation of interleukin-1 family cytokines. J Exp Med. 2020;217(1):e20190314. [79] GUPTA P, BARTHWAL MK. IL-1 β genesis: the art of regulating the regulator. Cell Mol Immunol. 2018;15(11):998-1000. [80] BRODERICK L, HOFFMAN HM. IL-1 and autoinflammatory disease: biology, pathogenesis and therapeutic targeting. Nat Rev Rheumatol. 2022;18(8): 448-463. [81] 郑亚妮.轴突信号Neuregulin 1在施旺细胞发育及再生修复中的作用[J].组织工程与重建外科杂志,2017,13(1):45-47. [82] 刘思阳.枸杞多糖对糖尿病大鼠周围神经病变的保护作用及mTOR/p70S6K/自噬信号通路研究[D].银川:宁夏医科大学,2018. [83] 白娟. TRPV1功能阻断对大鼠坐骨神经离断损伤后再生的干预作用[D].太原:山西医科大学,2018. [84] VOET S, SRINIVASAN S, LAMKANFI M, et al. Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol Med. 2019;11(6):e10248. [85] LI W, LIANG J, LI S, et al. Research progress of targeting NLRP3 inflammasome in peripheral nerve injury and pain. Int Immunopharmacol. 2022;110:109026. [86] PAPAYANNOPOULOS V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018;18(2):134-147. [87] MISHRA MK, RAWJI KS, KEOUGH MB, et al. Harnessing the Benefits of Neuroinflammation: Generation of Macrophages/Microglia with Prominent Remyelinating Properties. J Neurosci. 2021;41(15):3366-3385. [88] KENNEDY AD, DELEO FR. Neutrophil apoptosis and the resolution of infection. Immunol Res. 2009;43(1-3):25-61. [89] 徐筑秋,杨晓楠,祁佐良.细胞自噬在周围神经损伤及再生中研究进展[J].中国修复重建外科杂志,2017,31(1):122-125. [90] 苌彪,郭志远,全琦,等.大鼠感觉、运动神经瓦勒氏变性前后蛋白分析[J].解放军医学院学报,2017,38(1):55-58. [91] BOISSONNAS A, LOUBOUTIN F, LAVIRON M, et al. Imaging resident and recruited macrophage contribution to Wallerian degeneration. J Exp Med. 2020;217(11):e20200471. [92] TOMLINSON JE, ŽYGELYTĖ E, GRENIER JK, et al. Temporal changes in macrophage phenotype after peripheral nerve injury. J Neuroinflammation. 2018;15(1):185. [93] WCULEK SK, DUNPHY G, HERAS-MURILLO I, et al. Metabolism of tissue macrophages in homeostasis and pathology. Cell Mol Immunol. 2022; 19(3):384-408. [94] LI Y, KANG S, HALAWANI D, et al. Macrophages facilitate peripheral nerve regeneration by organizing regeneration tracks through Plexin-B2. Genes Dev. 2022;36(3-4):133-148. [95] PAN D, ACEVEDO-CINTRÓN JA, SAYANAGI J, et al. The CCL2/CCR2 axis is critical to recruiting macrophages into acellular nerve allograft bridging a nerve gap to promote angiogenesis and regeneration. Exp Neurol. 2020;331:113363. [96] BEAHRS T, TANZER L, SANDERS VM, et al. Functional recovery and facial motoneuron survival are influenced by immunodeficiency in crush-axotomized mice. Exp Neurol. 2010;221(1):225-230. [97] BALOG BM, SONTI A, ZIGMOND RE. Neutrophil biology in injuries and diseases of the central and peripheral nervous systems. Prog Neurobiol. 2023;228:102488. [98] LIU C, CHU D, KALANTAR-ZADEH K, et al. Cytokines: From Clinical Significance to Quantification. Adv Sci (Weinh). 2021;8(15):e2004433. [99] GHOSH D, MERSHA TB. Publicly available cytokine data: Limitations and opportunities. J Allergy Clin Immunol. 2022;150(5):1053-1056. [100] LAI J, WU H, QIN A. Cytokines in Febrile Diseases. J Interferon Cytokine Res. 2021;41(1):1-11. [101] REN C, CHEN M, MU G, et al. NLRP3 Inflammasome Mediates Neurodegeneration in Rats with Chronic Neuropathic Pain. Shock. 2021; 56(5):840-849. [102] WANG Y, CHE M, XIN J, et al. The role of IL-1β and TNF-α in intervertebral disc degeneration. Biomed Pharmacother. 2020;131:110660. [103] SAXTON RA, GLASSMAN CR, GARCIA KC. Emerging principles of cytokine pharmacology and therapeutics. Nat Rev Drug Discov. 2023;22(1):21-37. [104] DE SOUZA S, ROSARIO CLAUDIO J, SIM J, et al. Interleukin-10 signaling in somatosensory neurons controls CCL2 release and inflammatory response. Brain Behav Immun. 2024;116:193-202. [105] JANCALEK R, SVIZENSKA I, KLUSAKOVA I, et al. Bilateral changes of IL-10 protein in lumbar and cervical dorsal root ganglia following proximal and distal chronic constriction injury of peripheral nerve. Neurosci Lett. 2011;501(2):86-91. [106] MA SB, XIAN H, WU WB, et al. CCL2 facilitates spinal synaptic transmission and pain via interaction with presynaptic CCR2 in spinal nociceptor terminals. Mol Brain. 2020;13(1):161. [107] CHEN Y, LIU S, WU L, et al. Epigenetic regulation of chemokine (CC-motif) ligand 2 in inflammatory diseases. Cell Prolif. 2023;56(7):e13428. [108] SU H, XU F, SUN H, et al. Preparation and Evaluation of BDNF Composite Conduits for Regeneration of Sciatic Nerve Defect in Rats. J Pharm Sci. 2020;109(7):2189-2195. |
| [1] | Zhou Panpan, Cui Yinglin, Zhang Wentao, Wang Shurui, Chen Jiahui, Yang Tong . Role of cellular autophagy in cerebral ischemic injury and the regulatory mechanism of traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1650-1658. |
| [2] | Yu Jingbang, Wu Yayun. Regulatory effect of non-coding RNA in pulmonary fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1659-1666. |
| [3] | Wang Qiuyue, Jin Pan, Pu Rui . Exercise intervention and the role of pyroptosis in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1667-1675. |
| [4] | Yuan Weibo, Liu Chan, Yu Limei. Potential application of liver organoids in liver disease models and transplantation therapy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1684-1692. |
| [5] | Li Jialin, Zhang Yaodong, Lou Yanru, Yu Yang, Yang Rui. Molecular mechanisms underlying role of mesenchymal stem cell secretome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1512-1522. |
| [6] | Zhao Xiaoxuan, Liu Shuaiyi, Li Qi, Xing Zheng, Li Qingwen, Chu Xiaolei. Different exercise modalities promote functional recovery after peripheral nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1248-1256. |
| [7] | Zhang Wenhua, Li Xun, Zhang Weichao, Li Xinying, Ma Guoao, Wang Xiaoqiang . Promoting myogenesis based on the SphK1/S1P/S1PR2 signaling pathway: a new perspective on improving skeletal muscle health through exercise [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1265-1275. |
| [8] | Wu Guangtao, Qin Gang, He Kaiyi, Fan Yidong, Li Weicai, Zhu Baogang, Cao Ying . Causal relationship between immune cells and knee osteoarthritis: a two-sample bi-directional Mendelian randomization analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1081-1090. |
| [9] | Guo Zhao, Zhuang Haoyan, Shi Xuewen. Role of exosomes derived from mesenchymal stem cells in treatment of colorectal cancer [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7872-7879. |
| [10] | Shui Jing, He Yu, Jiang Nan, Xu Kun, Song Lijuan, Ding Zhibin, Ma Cungen, Li Xinyi. Astrocytes regulate remyelination in central nervous system [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7889-7897. |
| [11] | Yu Hui, Yang Yang, Wei Ting, Li Wenli, Luo Wenqian, Liu Bin. Gadd45b alleviates white matter damage in chronic ischemic rats by modulating astrocyte phenotype [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7797-7803. |
| [12] | Huang Haina, Yu Yanrong, Bi Jian, Huang Miao, Peng Weijie. Epigenetic characteristics of hepatogenic differentiation of mesenchymal stem cells in three-dimensional culture [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7848-7855. |
| [13] | Liao Qiyu, Ru Jiangying. Complications of intra-prosthetic dissociation after hip arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(33): 7241-7249. |
| [14] | Yan Laijun, Ge Haiya, Wang Zhengming, Yang Zongrui, Niu Lifeng, Zhan Hongsheng. Mechanism by which Tongdu Huoxue Decoction inhibits macrophage inflammation to delay intervertebral disc degeneration in rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6851-6857. |
| [15] | Zhang Pulian, Liu Baoru, Yang Min . Mesenchymal stem cells for treatment of aplastic anemia: inhibiting or activating relevant targets in its pathological evolution [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6800-6810. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||