Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (18): 3934-3940.doi: 10.12307/2025.678
Previous Articles Next Articles
Immune cells mediate the association between different lipids and knee osteoarthritis: a genome-wide association analysis of European individuals
Huo Jiang1, Ding Yu2, Yuan Jie1
- 1Department of Orthopedics, The Second Hospital of Shanxi Medical University, Taiyuan 030001, Shanxi Province, China; 2Shanxi Provincial Integrated TCM and WM Hospital, Taiyuan 030001, Shanxi Province, China
-
Received:2024-06-27Accepted:2024-08-21Online:2025-06-28Published:2024-11-29 -
Contact:Yuan Jie, MS, Attending physician, Department of Orthopedics, The Second Hospital of Shanxi Medical University, Taiyuan 030001, Shanxi Province, China -
About author:Huo Jiang, MS, Attending physician, Department of Orthopedics, The Second Hospital of Shanxi Medical University, Taiyuan 030001, Shanxi Province, China -
Supported by:Shanxi Province Basic Research Program Free Exploration Youth Research Project, Nos. 202203021212053 (to HJ) and 20210302124670 (to YJ); Shanxi Province Higher Education “Ten Billion Project” Science and Technology Guidance Special Project, No. BYJL031 (to YJ [project participant])
CLC Number:
Cite this article
Huo Jiang, Ding Yu, Yuan Jie. Immune cells mediate the association between different lipids and knee osteoarthritis: a genome-wide association analysis of European individuals[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(18): 3934-3940.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
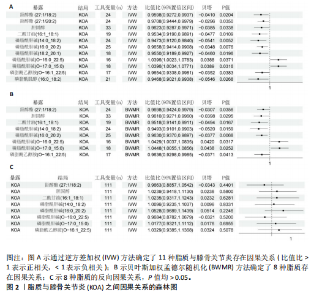
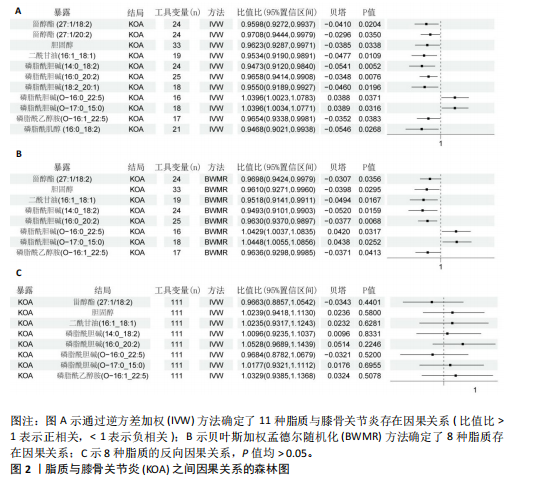
2.1 工具变量的选择 在选择全基因组独立(r2=0.001,window size=10 000 kb)和P < 1×10-5后,179个脂质的显著工具变量数量在12-44之间。在r2=0.001,window size=10 000 kb和P < 5×10-8后,731个免疫细胞的显著工具变量在1-450之间。反向孟德尔随机化分析中,膝骨关节炎的显著工具变量为136个。这些单核苷酸多态性的F值最小为19.53,表明弱仪器偏差的概率最小。 2.2 179种脂质对膝骨关节炎的影响 在逆方差加权方法和贝叶斯加权分析(P < 0.05)下,最终确定了8种脂质与膝骨关节炎之间存在因果效应(图2A,B)。结果显示,甾醇酯(27:1/20:2)、胆固醇、二酰甘油(16:1_18:1)、磷脂酰胆碱(14:0_18:2)/(16:0_20:2)、磷脂酰乙醇胺 (O-16:1_22:5)与膝骨关节炎呈负向关联趋势,即随着前者表达水平升高,后者的发生风险会降低,而磷脂酰胆碱(O-16:0_22:5)/(O-17:0_15:0)的表达水平对膝骨关节炎存在一个正向调控。此外,反映这些因果关系的贝塔估计值的方向在5种孟德尔随机化方法中是一致的,这增加了此次研究结果的稳健性。敏感性检验显示,这些因果关联中不存在异质性和水平多效性(P > 0.05;P MRPRESSO global tests > 0.05),见表1。 2.3 八种脂质对膝骨关节炎的逆向孟德尔随机化分析和Steiger方向性测试 通过前面的标准,确定了136个与膝骨关节炎相关的单核苷酸多态性,将膝骨关节炎视为暴露,与筛选出的有显著因果的脂质进行双样本孟德尔随机化,P > 0.05视为不存在反向因果关系(图2C)。Steiger方向性测试可以在正向或反向孟德尔随机化过程进行分析,如果在正向即脂质到膝骨关节炎的孟德尔随机化分析中进行测试,Correct_causal_direction的结果应显示为TRUE并统计学显著(Steiger_P < 0.05),见表1。此次结果表明膝骨关节炎对得出的8种脂质没有因果作用。 2.4 两步孟德尔随机化分析 为探索免疫细胞相关性状作为中介因素在脂质与膝骨关节炎因果效应中的介导作用,首先在P < 0.05的统计学标准下,此次研究进行了免疫细胞和膝骨关节炎之间的孟德尔随机化分析并确定了与其存在因果效应的45种免疫细胞;随后,再次进行孟德尔随机化分析评估8种脂质与45种免疫细胞性状的因果关系。 分析结果显示,一共鉴定出4种脂质与7种免疫细胞之间的8个因果关系对(图3),提示二酰甘油(16:1_18:1)与CD14 on CD14+CD16-单"
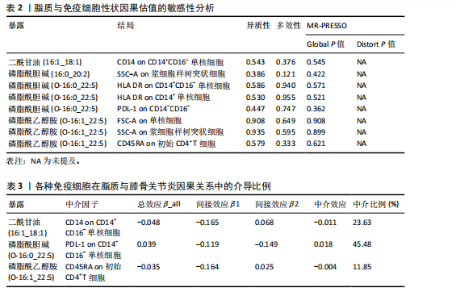
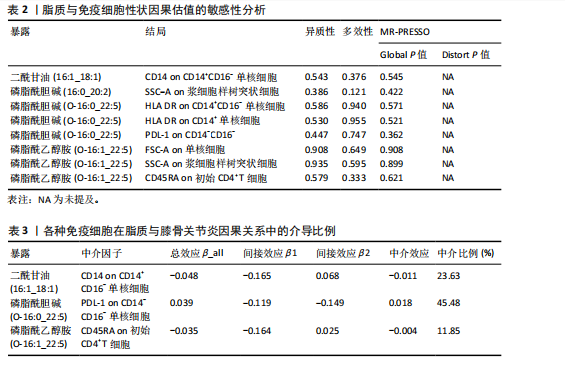
核细胞存在负向调控影响,同样的,磷脂酰胆碱(16:0_20:2)对SSC-A on浆细胞样树突状细胞,磷脂酰胆碱(O-16:0_22:5)对PDL-1 on CD14-CD16-,磷脂酰乙醇胺(O-16:1_22:5)对FSC-A on单核细胞、SSC-A on浆细胞样树突状细胞、CD45RA on初始CD4+T细胞也存在负向调控。相反,磷脂酰乙醇胺(O-16:0_22:5)对HLA DR on CD14+ CD16- 单核细胞、HLA DR on CD14+ 单核细胞则是一个正相关的关系。 在敏感性分析中,去除单个单核苷酸多态性不影响脂质与免疫细胞的因果效应。Cochran’s Q、MR-Egger截距检验和MR-PRESSO衍生的P值均大于0.05(表2),证明不存在异质性和多效性影响。 2.5 潜在中介效应计算 发挥中介作用的一个要求是脂质与免疫细胞显著相关。将两两之间都存在显著因果相关的脂质、免疫细胞、膝骨关节炎进行中介分析,分别计算步骤1(脂质-膝骨关节炎)的总效应、步骤2(免疫细胞-膝骨关节炎)的间接效应2、步骤3(脂质-免疫细胞)的间接效应1,在确定间接效应后,通过系数乘积法量化所选免疫细胞在特定因果关系中的中介效应,分析结果见表3。在膝骨关节炎中得到3个结果,CD14 on CD14+CD16-单核细胞在二酰甘油(16:1_18:1)抑制膝骨关节炎发生发展过程的介导作用占总效应的23.63%;PDL-1 on CD14-CD16-单核细胞介导磷脂酰胆碱(O-16:0_22:5)促进膝骨关节炎发生的效应占45.48%;CD45RA on 初始CD4+T细胞所占比例较少,在磷脂酰乙醇胺(O-16:1_22:5)抑制膝骨关节炎的过程中介导作用为11.85%。"
| [1] HARRELL CR, MARKOVIC BS, FELLABAUM C, et al. Mesenchymal stem cell-based therapy of osteoarthritis: Current knowledge and future perspectives. Biomed Pharmacother. 2019;109:2318-2326. [2] MANDL LA. Osteoarthritis year in review 2018: clinical. Osteoarthritis Cartilage. 2019; 27(3):359-364. [3] SACITHARAN PK. Ageing and Osteoarthritis. Subcell Biochem. 2019;91:123-159. [4] BORÉN J, CHAPMAN MJ, KRAUSS RM, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2020;41(24):2313-2330. [5] HAARTMANS MJJ, CLAES BSR, EIJKEL GB, et al. Matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI-MSI) reveals potential lipid markers between infrapatellar fat pad biopsies of osteoarthritis and cartilage defect patients. Anal Bioanal Chem. 2023;415(24):5997-6007. [6] LOEF M, VAN DE STADT L, BÖHRINGER S, et al. The association of the lipid profile with knee and hand osteoarthritis severity: the IMI-APPROACH cohort. Osteoarthritis Cartilage. 2022;30(8):1062-1069. [7] KOH JH, YOON SJ, KIM M, et al. Lipidome profile predictive of disease evolution and activity in rheumatoid arthritis. Exp Mol Med. 2022;54(2):143-155. [8] POUSINIS P, GOWLER PRW, BURSTON JJ, et al. Lipidomic identification of plasma lipids associated with pain behaviour and pathology in a mouse model of osteoarthritis. Metabolomics. 2020;16(3): 32. [9] ZHANG W, SUN G, AITKEN D, et al. Lysophosphatidylcholines to phosphatidylcholines ratio predicts advanced knee osteoarthritis. Rheumatology (Oxford). 2016;55(9):1566-1574. [10] VASSILIOU E, FARIAS-PEREIRA R. Impact of Lipid Metabolism on Macrophage Polarization: Implications for Inflammation and Tumor Immunity. Int J Mol Sci. 2023; 24(15):12032. [11] PEETERS R, JELLUSOVA J. Lipid metabolism in B cell biology. Mol Oncol. 2024;18(7):1795-1813. [12] LIM SA, SU W, CHAPMAN NM, et al. Lipid metabolism in T cell signaling and function. Nat Chem Biol. 2022;18(5):470-481. [13] ALBEITUNI S, STIBAN J. Roles of Ceramides and Other Sphingolipids in Immune Cell Function and Inflammation. Adv Exp Med Biol. 2019;1161:169-191. [14] GAO S, ZENG Q, ZENG Y, et al. High density lipoprotein inhibited group II innate lymphoid cells proliferation and function in allergic rhinitis. Allergy Asthma Clin Immunol. 2022;18(1):40. [15] LIN J, ZHOU J, XU Y. Potential drug targets for multiple sclerosis identified through Mendelian randomization analysis. Brain. 2023;146(8):3364-3372. [16] OTTENSMANN L, TABASSUM R, RUOTSALAINEN SE, et al. Genome-wide association analysis of plasma lipidome identifies 495 genetic associations. Nat Commun. 2023;14(1):6934. [17] ORRÙ V, STERI M, SIDORE C, et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat Genet. 2020;52(10):1036-1045. [18] TACHMAZIDOU I, HATZIKOTOULAS K, SOUTHAM L, et al. Identification of new therapeutic targets for osteoarthritis through genome-wide analyses of UK Biobank data. Nat Genet. 2019;51(2):230-236. [19] EMDIN CA, Khera AV, Kathiresan S, Mendelian Randomization. JAMA. 2017; 318(19):1925-1926. [20] SKRIVANKOVA VW, RICHMOND RC, WOOLF BAR, et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA. 2021;326(16):1614-1621. [21] WANG Q, DAI H, HOU T, et al. Dissecting Causal Relationships Between Gut Microbiota, Blood Metabolites, and Stroke: A Mendelian Randomization Study. J Stroke. 2023;25(3):350-360. [22] BOWDEN J, DEL GRECO MF, MINELLI C, et al. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol. 2016;45(6):1961-1974. [23] PALAZZO C, NGUYEN C, LEFEVRE-COLAU MM, et al. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016; 59(3):134-138. [24] BOWDEN J, DEL GRECO MF, MINELLI C, et al. Improving the accuracy of two-sample summary-data Mendelian randomization: moving beyond the NOME assumption. Int J Epidemiol. 2019;48(3):728-742. [25] ZHAO J, MING J, HU X, et al. Bayesian weighted Mendelian randomization for causal inference based on summary statistics. Bioinformatics. 2020;36(5): 1501-1508. [26] ZHONG Y, WANG F, MENG X, et al. The associations between gut microbiota and inflammatory skin diseases: a bi-directional two-sample Mendelian randomization study. Front Immunol. 2024;15:1297240. [27] HERRERO-MANLEY L, ALABAJOS-CEA A, SUSO-MARTÍ L, et al. Serum lipid biomarkers and inflammatory cytokines associated with onset and clinical status of patients with early knee osteoarthritis. Front Nutr. 2023; 10:1126796. [28] NANO JL, NOBILI C, GIRARD-PIPAU F, et al. Effects of fatty acids on the growth of Caco-2 cells. Prostaglandins Leukot Essent Fatty Acids. 2003;69(4):207-215. [29] WU P, HUANG Z, SHAN J, et al. Interventional effects of the direct application of “Sanse powder” on knee osteoarthritis in rats as determined from lipidomics via UPLC-Q-Exactive Orbitrap MS. Chin Med. 2020;15:9. [30] MURAKAMI C, HOSHINO F, SAKAI H, et al. Diacylglycerol kinase delta and sphingomyelin synthase-related protein functionally interact via their sterile alpha motif domains. J Biol Chem. 2020;295(10): 2932-2947. [31] HILLS BA, THOMAS K. Joint stiffness and ‘articular gelling’: inhibition of the fusion of articular surfaces by surfactant. Br J Rheumatol. 1998;37(5):532-538. [32] ROCHA B, CILLERO-PASTOR B, RUIZ-ROMERO C, et al. Identification of a distinct lipidomic profile in the osteoarthritic synovial membrane by mass spectrometry imaging. Osteoarthritis Cartilage. 2021;29(5):750-761. [33] ZHAI G, PELLETIER JP, LIU M, et al. Activation of The Phosphatidylcholine to Lysophosphatidylcholine Pathway Is Associated with Osteoarthritis Knee Cartilage Volume Loss Over Time. Sci Rep. 2019;9(1):9648. [34] KAPELLOS TS, BONAGURO L, GEMÜND I, et al. Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front Immunol. 2019;10: 2035. [35] KYTHREOTOU A, SIDDIQUE A, MAURI FA, et al. PD-L1. J Clin Pathol. 2018;71(3):189-194. [36] CHA JH, CHAN LC, LI CW, et al. Mechanisms Controlling PD-L1 Expression in Cancer. Mol Cell. 2019;76(3):359-370. [37] GARCIA-DIAZ A, SHIN DS, MORENO BH, et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. 2017;19(6):1189-1201. [38] WANG X, YANG L, HUANG F, et al. Inflammatory cytokines IL-17 and TNF-alpha up-regulate PD-L1 expression in human prostate and colon cancer cells. Immunol Lett. 2017;184:7-14. [39] EZAWA K, YAMAMURA M, MATSUI H, et al. Comparative analysis of CD45RA- and CD45RO-positive CD4+T cells in peripheral blood, synovial fluid, and synovial tissue in patients with rheumatoid arthritis and osteoarthritis. Acta Med Okayama. 1997; 51(1):25-31. |
| [1] | Zhang Yibo, Lu Jianqi, Mao Meiling, Pang Yan, Dong Li, Yang Shangbing, Xiao Xiang. Exploring the causal relationship between rheumatoid arthritis and coronary atherosclerosis: a Mendel randomized study involving serum metabolites and inflammatory factors [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [2] | Chen Jiayong, Tang Meiling, Lu Jianqi, Pang Yan, Yang Shangbing, Mao Meiling, Luo Wenkuan, Lu Wei, Zhou Jiatan. Based on Mendelian randomization, the causal relationship between 1400 metabolites and sarcopenia and the correlation analysis of cardiovascular disease were investigated [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-11. |
| [3] | Li Jiagen, Chen Yueping, Huang Keqi, Chen Shangtong, Huang Chuanhong. The construction and validation of a prediction model based on multiple machine learning algorithms and the immunomodulatory analysis of rheumatoid arthritis from the perspective of mitophagy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-15. |
| [4] | Ma Chi, Wang Ning, Chen Yong, Wei Zhihan, Liu Fengji, Piao Chengzhe. Application of 3D-printing patient-specific instruments combined with customized locking plate in opening wedge high tibial osteotomy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1863-1869. |
| [5] | Sun Yundi, Cheng Lulu, Wan Haili, Chang Ying, Xiong Wenjuan, Xia Yuan. Effect of neuromuscular exercise for knee osteoarthritis pain and function: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1945-1952. |
| [6] | Dong Tingting, Chen Tianxin, Li Yan, Zhang Sheng, Zhang Lei. Causal relationship between modifiable factors and joint sports injuries [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1953-1962. |
| [7] | Chen Shuai, Jin Jie, Han Huawei, Tian Ningsheng, Li Zhiwei . Causal relationship between circulating inflammatory cytokines and bone mineral density based on two-sample Mendelian randomization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1556-1564. |
| [8] | Wang Peiguang, Zhang Xiaowen, Mai Meisi, Li Luqian, Huang Hao. Generalized equation estimation of the therapeutic effect of floating needle therapy combined with acupoint embedding on different stages of human knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1565-1571. |
| [9] | He Guanghui, Yuan Jie, Ke Yanqin, Qiu Xiaoting, Zhang Xiaoling. Hemin regulates mitochondrial pathway of oxidative stress in mouse chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1183-1191. |
| [10] |
Zhao Wensheng, Li Xiaolin, Peng Changhua, Deng Jia, Sheng Hao, Chen Hongwei, Zhang Chaoju, He Chuan.
Gut microbiota and osteoporotic fractures #br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1296-1304.
|
| [11] | Ma Haoyu, Qiao Hongchao, Hao Qianqian, Shi Dongbo. Causal effects of different exercise intensities on the risk of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1305-1311. |
| [12] | Li Jiatong, Jin Yue, Liu Runjia, Song Bowen, Zhu Xiaoqian, Li Nianhu . Association between thyroid function levels and phenotypes associated with sarcopenia [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1312-1320. |
| [13] | Wu Guangtao, Qin Gang, He Kaiyi, Fan Yidong, Li Weicai, Zhu Baogang, Cao Ying . Causal relationship between immune cells and knee osteoarthritis: a two-sample bi-directional Mendelian randomization analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1081-1090. |
| [14] | Li Chen, Liu Ye, Ni Xindi, Zhang Yuang. Simulation analysis of real-time continuous stiffness in muscle fibers and tendons of the triceps surae during multi-joint movement [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7529-7536. |
| [15] | Yang Bo, Pan Xinfang, Chang Liuhui, Ni Yong. Correlation of echocardiographic parameters with disability at 3 months after acute ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7544-7551. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||