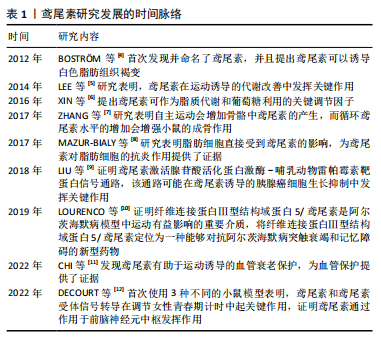
Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (18): 3897-3905.doi: 10.12307/2025.668
Previous Articles Next Articles
Irisin: a new link between exercise, disease and health
Dong Zhengqin, Zheng Qi, Wu Guanmao, Wang Wenna, Chen Leqin
- Shanxi Normal University, Taiyuan 030031, Shanxi Province, China
-
Received:2024-07-18Accepted:2024-08-31Online:2025-06-28Published:2024-11-29 -
Contact:Chen Leqin, Professor, Shanxi Normal University, Taiyuan 030031, Shanxi Province, China -
About author:Dong Zhengqin, MS, Shanxi Normal University, Taiyuan 030031, Shanxi Province, China
CLC Number:
Cite this article
Dong Zhengqin, Zheng Qi, Wu Guanmao, Wang Wenna, Chen Leqin. Irisin: a new link between exercise, disease and health[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(18): 3897-3905.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
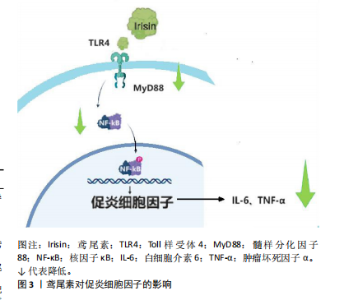
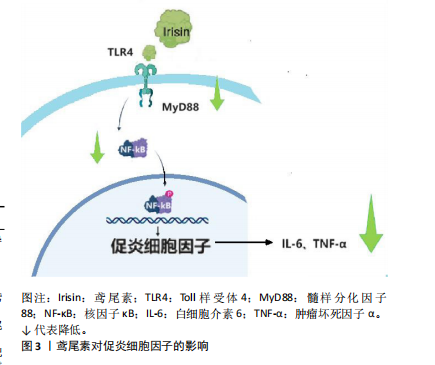
果。研究发现血清鸢尾素水平与炎症呈负相关,因此鸢尾素可作为抗炎巨噬细胞分化的新型调节剂[13]。还有研究发现,中国肥胖儿童鸢尾素的浓度明显低于正常儿童,并且与内皮活化炎症指标呈负相关,暗示鸢尾素与血管炎症有关[14]。鸢尾素还能够拮抗核因子κB活化,抑制白细胞介素6、肿瘤坏死因子α等炎症因子的水平,减少肺泡巨噬细胞M1型极化[15]。 研究证明,鸢尾素可以通过下调Toll样受体4和髓样分化因子88表达来防止缺血/再灌注诱导的脑损伤和脂多糖诱导的巨噬细胞炎症[8,16]。最近的一项研究报道,鸢尾素通过抑制Toll样受体4/核因子κB信号通路来缓解脂毒性诱导的β细胞胰岛素抵抗和炎症反应[17]。鸢尾素通过下调Toll样受体4/髓样分化因子88信号通路降低核因子κB磷酸化,减少促炎细胞因子活性[18],见图3。 综上所述,鸢尾素通过抑制Toll样受体4信号通路发挥抗炎特性,防止缺血/再灌注诱导的脑损伤和脂多糖诱导的巨噬细胞炎症,缓解脂毒性诱导的β细胞胰岛素抵抗和炎症反应。 鸢尾素还能减轻炎症性肠病相关炎症,恢复骨密度活力[19]。在非酒精性脂肪性肝病中,调节髓样分化蛋白4-Toll样受体12信号通路可减少炎症,有益代谢性疾病。运动能调节血糖和血脂,降低代谢性疾病风险、抑制慢性炎症。 2.2.2 鸢尾素与脂肪代谢的关联作用 肥胖不仅独立构成一种疾病状态,同时亦可能作为多种慢性病如高血压、"
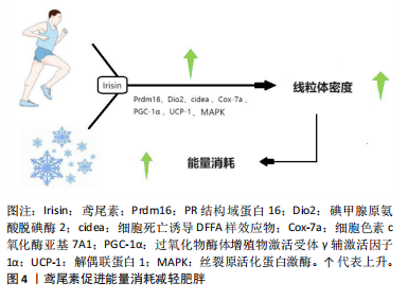
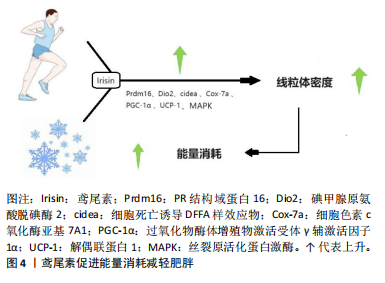
糖尿病等的重要诱因[20]。脂肪组织根据结构与功能的差异,能够被划分为白色与棕色两类。白色脂肪组织借助三酰甘油来存储能量,并且会在机体有需要的时候释放出游离脂肪酸。棕色脂肪组织在非寒战产热的情况下肩负着燃烧脂肪进而维持体温恒定的职责,该生物热能主要源于线粒体内膜上的解偶联蛋白1[21]。鸢尾素可提升白色脂肪组织中解偶联蛋白1的表达水平,从而增加棕白色脂肪细胞数量,诱导脂肪小滴形成,增加线粒体密度,增强机体能量消耗。鸢尾素的重要功能之一就是提升温度和热量,从而减轻体质量。鸢尾素通过调控丝裂原活化蛋白激酶、P38丝裂原活化蛋白激酶和细胞外调节蛋白激酶信号通路改善能量消耗并减轻肥胖。 有氧运动或寒冷环境会提升鸢尾素水平,这一过程通过增强Prdm16(一种PR结构域蛋白,它在棕色脂肪细胞分化过程中起到关键性的转录调控作用,对维持棕色脂肪细胞的形态特征和细胞功能具有不可或缺的重要性)、Ⅱ型脱碘酶、细胞死亡诱导DNA断裂因子α样效应物、细胞色素c氧化酶亚基、PGC-1α和线粒体棕色脂肪解偶联蛋白1等生热基因的表达,从而在白色脂肪组织中诱导出“褐变”过程。现有研究发现,运动可以引发PGC-1α表达上升,致使FNDC5表达增加,从而推动骨骼肌释放出更多的鸢尾素,最终提高脂肪组织中解偶联蛋白1的表达量,有助于减轻脂肪堆积[22]。鸢尾素增强白色脂肪组织中PGC-1α、解偶联蛋白1及其他棕色脂肪相关基因的表达(Prdm16是棕色脂肪细胞分化过程的重要转录因子)刺激褐变过程并促进能量消耗,进而减轻肥胖,如图4所示,其中细胞死亡诱导DFFA样效应物在增加人类脂肪细胞生热中发挥作用,碘甲腺原氨酸脱碘酶2主要影响细胞的信号传导,细胞色素c氧化酶亚基是线粒体呼吸链的末端成分。 研究表明,鸢尾素通过上调胰岛素样生长因子1/蛋白激酶B/哺乳动物雷帕霉素靶蛋白通路促进肌肉蛋白合成,实现肌肉肥大[23]。作为关键的肌肉因子,鸢尾素能"
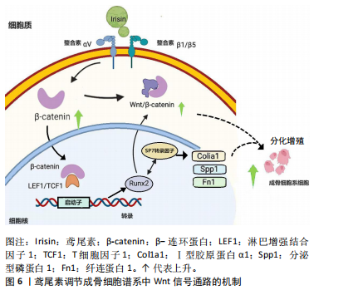
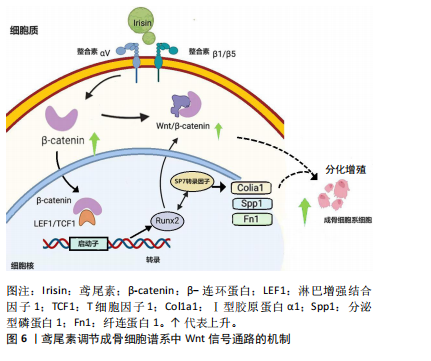
鸢尾素凭通过抑制骨细胞的凋亡来防止骨质流失及骨质疏松症。COLAIANNI等[25]的实验成果初次展露了鸢尾素对成骨细胞的直接作用,鸢尾素在体外条件下能够促进骨髓基质细胞向成骨细胞分化。另有研究表明,在小鼠体内注入鸢尾素可以引发骨骼肌显著肥大并强化肌肉力量,甚至能减少坏死和纤维化组织[26]。QIAO等[27]细胞层面的研究表明,鸢尾素通过激活P38丝裂原活化蛋白激酶和细胞外调节蛋白激酶激酶有效促进了成骨细胞的增殖,并提升了成骨细胞分化相关标志物的表达水平。这些结果均表明鸢尾素在成骨过程中扮演着重要的角色。 鸢尾素是一种由FNDC5基因编码的运动介导肽,可通过特定的αⅤ类整合素受体调节脂肪细胞和骨细胞代谢,降低破骨细胞分化和促炎细胞因子(慢性炎症性疾病会导致骨吸收过多和骨形成受损,导致关节周围和全身性骨质流失),增加合成代谢因子(如诱导成骨细胞分化的β-连环蛋白)。目前的研究表明,成骨细胞分化过程受Wnt信号通路调节,并且Runx2是成骨过程中的重要转录因子,Runx2增多可以提升Ⅰ型胶原蛋白α1、分泌型磷蛋白1和纤连蛋白1等基因的表达[28],鸢尾素在细胞内调节成骨细胞谱系细胞中Wnt信号通路的机制如图6所示。然而,关于运动或重组鸢尾素注射液与骨合成代谢之间关系的共识尚未达成[29]。"
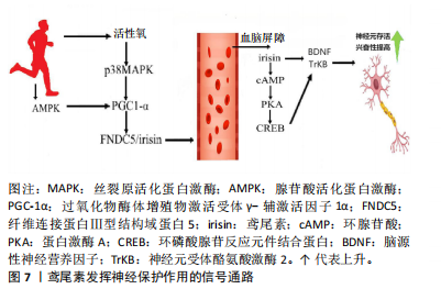
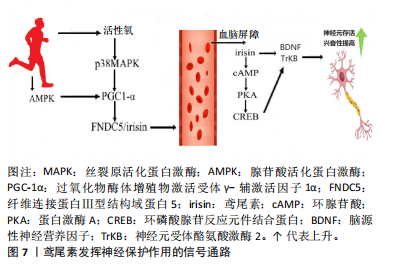
鸢尾素的抗骨质疏松机制不仅涉及成骨细胞数量和活性的增加,还与骨硬化蛋白的表达增加有关[30]。在儿童早期和青春期的骨骼快速生长阶段,鸢尾素与骨密度呈正相关,与体脂量和瘦体质量无关。高血清鸢尾素水平与健康儿童和成人的骨量、骨密度和骨转换呈正相关,而与骨质疏松性骨折风险呈负相关。这些研究表明,鸢尾素在儿童、青少年及成人骨代谢中具有重要作用[31]。 鸢尾素也被认为是肌肉骨骼系统中的生物标志物。在绝经后妇女中鸢尾素可用作肌肉减少症和髋部骨折的生物标志物,因为鸢尾素与肌肉萎缩程度和髋部骨折的风险呈负相关[32];并且在儿童的身体成分中鸢尾素也被用作生物标志物,因为与骨密度呈正相关,与瘦体质量还是胖体质量无关。值得注意的是,根据多元回归分析,鸢尾素甚至比骨碱性磷酸酶更能决定骨矿物质状态[18]。综上所述,鸢尾素-整合素-Wnt/β-连环蛋白-Runx2相互作用可能构成了一个信号轴,为鸢尾素诱导成骨细胞分化和改善骨密度的作用机制提供了可能解释。 2.2.4 鸢尾素与大脑健康的关联作用 运动可促进PGC-1α表达、刺激FNDC5分解为鸢尾素[33],鸢尾素进入血液在全身循环并通过血脑屏障,可能增加脑源性神经营养因子的表达,从而优化突触的可塑性、提升神经元的存活率、促进神经元的分化以及增进神经元的健康状况,进而提高认知能力。FNDC5的表达受多种因素调控,包括PGC-1α、运动和信号通路(如腺苷酸活化蛋白激酶和P38丝裂原活化蛋白激酶)[34]。此外,鸢尾素具有抗炎和抗氧化作用,有助于防御认知缺陷。研究表明,FNDC5/鸢尾素可能与痴呆相关疾病及预防痴呆的抑郁症存在正相关联系[34]。 鸢尾素在阿尔茨海默病的治疗和预防中可能扮演重要角色。研究发现,鸢尾素可直接在大脑中发挥作用,或通过保护大脑环腺苷酸→蛋白激酶A→环磷酸腺苷反应元件结合蛋白→脑源性神经营养因子信号通路诱导脑源性神经营养因子的产生,鸢尾素通过外周循环进入大脑,直接或间接诱导脑源性神经营养因子的产生,进而对脑功能产生保护作用[35]。鸢尾素缺乏可能导致脑源性神经营养因子/神经元受体酪氨酸激酶2水平降低,加剧认知功能障碍。 鸢尾素具有多种对抗阿尔茨海默病的机制,优化认知功能、缓解阿尔茨海默病症状、降低神经炎症水平、抑制氧化应激反应、改善心血管健康状况等[36],这些机制推动了阿尔茨海默病治疗或预防药物的研发和测试。 在大脑中检测到鸢尾素,因此推测鸢尾素具有穿越血脑屏障的能力。虽然鸢尾素跨血脑屏障转运的具体机制尚未明确,但推测它可能利用脑内皮细胞表达的转运蛋白或受体进入中枢神经系统。鸢尾素具有神经保护作用,可增强神经发生并调节突触可塑性[37]。鸢尾素在运动中产生并对神经进行保护通路如图7所示。"
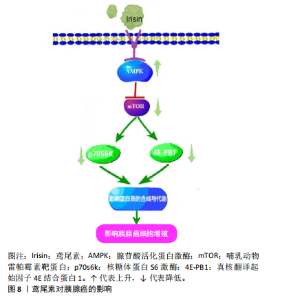
2.2.5 鸢尾素与癌症的关联作用 许多研究表明,外源性鸢尾素具有抗肿瘤和抗转移作用,因此,鸢尾素可以用作癌症的生物标志物和/或预后因素[38]。GANNON等[39]研究发现,鸢尾素可能通过抗炎反应、诱导凋亡细胞死亡或增强肿瘤对常见抗肿瘤药物(如阿霉素)的敏感性,为乳腺癌的预防和治疗提供治疗益处。另外,SHI等[40]研究也分析了鸢尾素对阿霉素治疗的影响,发现鸢尾素能够降低阿霉素在肝癌细胞中的毒性。 鸢尾素与癌症的关系备受关注。GAGGINI等[41]发现肝癌患者肝组织中鸢尾素含量异常增多,胃肠道癌组织中鸢尾素水平呈现了明显上升趋势。在肿瘤的发生发展过程中,鸢尾素作为一种旁分泌激素可以抑制脂肪的形成,在肝脏脂肪较丰富的肝癌患者体内FNDC5/鸢尾素的表达能够充当一个代偿作用机制,对由癌症进展所引发的脂肪生成予以限制[1]。 HOJMAN等[42]研究显示,运动过程中释放的肌球蛋白能够有效抑制乳腺癌细胞的增殖。MOON等[43-44]的细胞实验显示,不管是处于生理浓度还是高生理/药理浓度下,鸢尾素对与肥胖相关的癌细胞株细胞增殖及恶性潜能的作用均相对有限。乳腺癌受试者血清鸢尾素水平明显低于健康志愿者,鸢尾素含量每提高约一个单位就可以使患乳腺癌的风险减少约90%[45]。GANNON等[39]研究发现,鸢尾素可以通过诱导细胞凋亡及影响癌症细胞的迁移切实缩减恶性乳腺细胞的数量。鸢尾素还能够增强恶性肿瘤乳腺细胞对于化疗药物的敏感性,进而在治疗过程中减轻药物对正常细胞的潜在危害。这些发现为乳腺癌的医治提供了崭新的视角与策略。LIU等[9]实验成果显示,鸢尾素能够通过激活腺苷酸活化蛋白激酶来抑制胰腺癌细胞的生长,使哺乳动物雷帕霉素靶蛋白通路下调(腺苷酸活化蛋白激酶激活导致与控制细胞增殖相关的多个下游通路的调节,包括抑制哺乳动物雷帕霉素靶蛋白-p70S6K/真核翻译起始因子4E结合蛋白1信号通路,该通路是细胞生长和器官大小的主要调节因子),以此实现抑制胰腺癌发展的目的,如图8所示。"

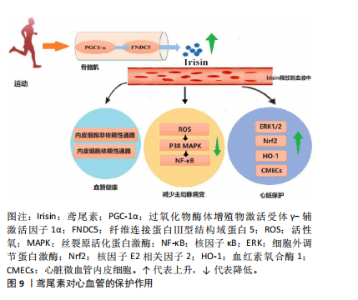
综上所述,鸢尾素可作为癌症标志物和预后因素,有抗肿瘤和抗转移作用,能影响癌细胞的增殖、迁移及化疗药物作用等。研究表明,定期运动可降低癌症复发和死亡率,但促进癌症患者运动行为存在挑战[46]。 2.2.6 鸢尾素与糖尿病的关联作用 运动可显著提高FNDC5的表达和水解,进而产生鸢尾素,鸢尾素激活解偶联蛋白1的表达,促进脂肪组织棕色化,加速能量消耗,有利于胰岛素再生和β细胞重建(鸢尾素的生成促进p38丝裂原活化蛋白激酶的磷酸化和细胞外调节蛋白激酶的激活,有助于β细胞增殖和再生)。增加鸢尾素水平可提高葡萄糖耐受量、降低胰岛素抵抗,这一特性在糖尿病治疗领域具有应用价值。 糖尿病患者鸢尾素水平明显低于糖耐量正常者[47]。目前的研究表明,鸢尾素对糖尿病心脏微血管损伤具有治疗作用,鸢尾素对糖尿病心脏的保护作用可能归因于通过激活细胞外调节蛋白激酶增加心脏微血管内皮细胞增殖和以细胞外信号调节激酶1/2/核因子E2相关因子2/血红素氧合酶1依赖性方式降低心脏微血管内皮细胞氧化应激[48]。 有研究发现,高强度间歇训练糖尿病大鼠血清鸢尾素、β-细胞营养因子和胰岛素水平升高,从而减轻胰岛素抵抗[49]。多项研究证实,鸢尾素通过直接或间接的方式参与糖脂代谢、胰岛素抵抗和炎症反应等生理过程,对糖尿病肾脏疾病的发展产生显著影响[50]。 众所周知,定期运动对2型糖尿病患者具有多种生理益处,如调节血糖和血压。各种类型的运动,包括有氧运动和阻力训练,均可有效改善2型糖尿病患者的心理健康和认知功能。而鸢尾素作为一种运动诱导的肌因子被认为是运动对糖尿病患者心血管和心理健康益处的相关机制。总之,鸢尾素在糖尿病及其并发症的防治中具有重要潜力,但具体作用机制仍需深入研究。 2.2.7 鸢尾素与心血管疾病的关联作用 KURDIOVA等[51]指出动脉粥样硬化属于心血管疾病的一种,其显著特征为脂质在动脉壁处堆积,可能引发动脉破裂和狭窄。研究表明,循环鸢尾素浓度与动脉粥样硬化参数如冠状动脉粥样硬化指数和颈动脉内膜中层厚度呈负相关[14]。此外,冠心病患者样本中鸢尾素浓度显著低于健康对照组。BOSTR?M等[4]报道骨骼肌中PGC1-α过表达会增加鸢尾素分泌,将动脉粥样硬化斑块面积减少40%。 鸢尾素可作为心力衰竭患者的治疗药物。血清鸢尾素水平与动脉粥样硬化程度之间存在明显的负相关关系。通过身体锻炼可以有效上调腺苷酸活化蛋白激酶及其下游因子PGC1-α,这一过程能够促进心肌葡萄糖和脂肪酸的转运,以及线粒体的生物合成,这些发现对进一步了解动脉粥样硬化的发病机制以及探索新的预防和治疗手段具有重要指导意义[52]。其次,鸢尾素可通过活性氧-P38丝裂原活化蛋白激酶-核因子κB信号通路减小主动脉病变,改善内皮功能;同时,鸢尾素可通过内皮非依赖性和依赖性途径治疗动脉粥样硬化。腹腔注射卵泡抑素可通过腺苷酸活化蛋白激酶-PGC1-α-鸢尾素信号通路促进鸢尾素分泌,诱导白色脂肪褐变,激活米色脂肪中的胰岛素通路,促进新陈代谢。鸢尾素也可通过激活腺苷酸活化蛋白激酶-ULK1信号通路诱导保护性自噬和自噬流,预防心肌肥大。FNDC5在骨骼肌中的表达与心力衰竭患者的有氧表现相关,αⅤ整合素受体可促进FNDC5的生理作用,p38丝裂原活化蛋白激酶通路和核因子κB可减少心力衰竭的炎症反应,鸢尾素可作为心力衰竭的生物标志物和治疗靶点[53]。鸢尾素在心血管方面的作用如图9所示。"
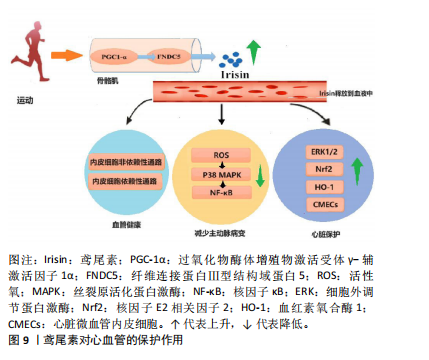

2.2.8 鸢尾素与肾脏病的关联作用 糖尿病肾脏疾病是由糖尿病所诱发的一种常见微血管并发症,正逐渐成为终末期肾脏病的第二大主导成因。鸢尾素与糖尿病肾脏疾病的发生和发展之间存在紧密的联系,并且鸢尾素通过直接或间接方式参与糖脂代谢、胰岛素抵抗和炎症反应等生理过程,对糖尿病肾脏疾病的发展产生显著影响。 研究发现,肾脏疾病患者血清鸢尾素水平存在改变[54],慢性肾脏病患者血清中鸢尾素浓度显著减少。SHELBAYA等[55]证实2型糖尿病患者血清鸢尾素水平降低,糖尿病肾病患者血清鸢尾素水平进一步降低。近期研究表明,鸢尾素具有抵消转化生长因子β诱导的代谢重编程、肾功能不全和纤维化的作用[56]。以上结果表明,鸢尾素在肾脏病理生理学中具有重要意义,为进一步研究肾脏疾病防治提供了新的方向和思路。 2.3 运动对鸢尾素分泌的影响 鸢尾素是运动诱导骨骼肌分泌的一种肌肉细胞因子,受运动频率、运动模式、运动强度等因素的影响。急性运动可以立即增加血液中鸢尾素浓度,而长期运动可以改善鸢尾素的代谢和分泌效率。阻力运动和高强度运动对于促进鸢尾素的有效分泌也很重要。同时,年龄、性别和代谢状态对鸢尾素分泌的调节因人群而异[57]。 鸢尾素与体质量指数呈负相关,与高密度脂蛋白胆固醇直接相关[58],因此运动必不可少。但是不同类型运动训练对鸢尾素的影响结果相互矛盾[59-60],大多数研究表明,中高强度和阻力练习导致鸢尾素水平显著提高;也有研究表明,有氧运动也可以提高机体鸢尾素表达水平。 不同运动强度对鸢尾素水平的影响不同,DASKALOPOULOU等[61]发现运动强度越高血浆鸢尾素水平增加越剧烈;RASHTI等[62]通过对绝经女性进行高强度间歇训练和持续有氧训练干预,发现高强度间歇训练提升鸢尾素的水平更显著,这一现象在急性运动中也被观察到。 高强度间歇训练对健康成年人血浆鸢尾素浓度的影响:干预周期≥8周的高强度间歇训练能显著提高血浆鸢尾素浓度,干预时长≥40 min的高强度间歇训练能显著提高血浆鸢尾素浓度;高强度间歇训练能显著增加女性血浆鸢尾素浓度;高强度间歇训练能增加体质量指数≥25 kg/m2人群血浆鸢尾素浓度,并且身体活动不足的人群在高强度间歇训练后血浆鸢尾素浓度显著增加。有研究表明,在一次连续耐力训练后鸢尾素水平显著增加,而间歇训练没有改变循环鸢尾素水平[63]。 TSUCHIYA等[64]比较阻力运动与耐力运动以及在同一运动时间内耐力和阻力运动组合时对鸢尾素浓度的影响,发现阻力运动导致鸢尾素浓度的显著增加。老年人循环鸢尾素基线水平低于年轻人,儿童循环鸢尾素水平显著高于成人,表明鸢尾素与年龄独立负相关[57],并且体脂率降低越多鸢尾素增加越明显。不同运动形式对鸢尾素的影响见表2所示。"

| [1] 毋江波,张勇.Irisin与疾病关系的研究进展[J].生理科学进展,2018, 49(6):465-470. [2] 袁陶燕,王荣霞,赖淑静,等.运动激素-鸢尾素的研究进展[J].中华医学杂志,2015,95(29):2411-2413. [3] MAAK S, NORHEIM F, DREVON CA, et al. Progress and Challenges in the Biology of FNDC5 and Irisin. Endocr Rev. 2021;42(4):436-456. [4] BOSTRÖM P, WU J, JEDRYCHOWSKI MP, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463-468. [5] LEE P, LINDERMAN JD, SMITH S, et al. Irisin and FGF21 Are Cold-Induced Endocrine Activators of Brown Fat Function in Humans. Cell Metabolism. 2014;19(2):302-309. [6] XIN C, LIU J, ZHANG J, et al. Irisin improves fatty acid oxidation and glucose utilization in type 2 diabetes by regulating the AMPK signaling pathway. Int J Obes (Lond). 2016;40(3):443-451. [7] ZHANG J, VALVERDE P, ZHU X, et al. Exercise-induced irisin in bone and systemic irisin administration reveal new regulatory mechanisms of bone metabolism. Bone Res. 2017;5(1):16056. [8] MAZUR-BIALY AI, POCHEĆ E, ZARAWSKI M. Anti-Inflammatory Properties of Irisin, Mediator of Physical Activity, Are Connected with TLR4/MyD88 Signaling Pathway Activation. Int J Mol Sci. 2017; 18(4):701. [9] LIU J, SONG N, HUANG Y, et al. Irisin inhibits pancreatic cancer cell growth via the AMPK-mTOR pathway. Sci Rep. 2018;8(1):15247. [10] LOURENCO MV, FROZZA RL, DE FREITAS GB, et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s. Nat Med. 2019;25(1):165-175. [11] CHI C, FU H, LI YH, et al. Exerkine fibronectin type-III domain-containing protein 5/irisin-enriched extracellular vesicles delay vascular ageing by increasing SIRT6 stability. Eur Heart J. 2022;43(43):4579-4595. [12] DECOURT C, EVANS MC, INGLIS MA, et al. Central Irisin Signaling Is Required for Normal Timing of Puberty in Female Mice. Endocrinology. 2022;164(2):bqac208. [13] TU Y, LIU J, KONG D, et al. Irisin drives macrophage anti-inflammatory differentiation via JAK2-STAT6-dependent activation of PPAR? and Nrf2 signaling. Free Radic Biol Med. 2023;201:98-110. [14] MA C, DING H, DENG Y, et al. Irisin: A New Code Uncover the Relationship of Skeletal Muscle and Cardiovascular Health During Exercise. Front Physiol. 2021;12:620608. [15] 安一娜,谭姝瑜,冯岚迪,等.鸢尾素对感染大肠埃希菌小鼠组织损伤及细菌清除的影响[J/OL].畜牧兽医学报,1-19[2024-09-02].http://kns.cnki.net/kcms/detail/11.1985.S.20240329.1708.004.html. [16] YU Q, LI G, DING Q, et al. Irisin Protects Brain against Ischemia/Reperfusion Injury through Suppressing TLR4/MyD88 Pathway. Cerebrovasc Dis. 2020;49(4):346-354. [17] ZHU W, SAHAR NE, JAVAID HMA, et al. Exercise-Induced Irisin Decreases Inflammation and Improves NAFLD by Competitive Binding with MD2. Cells. 2021;10(12):3306. [18] LIU L, GUO J, CHEN X, et al. The Role of Irisin in Exercise-Mediated Bone Health. Front Cell Dev Biol. 2021;9:668759. [19] TRETTEL CDS, PELOZIN BRA, BARROS MP, et al. Irisin: An anti-inflammatory exerkine in aging and redox-mediated comorbidities[J]. Front Endocrinol (Lausanne). 2023;14:1106529. [20] 张怡,孙易,丁树哲.新的肌肉因子:鸢尾素[J].中国生物化学与分子生物学报,2017,33(5):429-435. [21] 赵丽华,高宇.鸢尾素在糖脂代谢相关疾病中的作用研究进展[J].中国动脉硬化杂志,2023,31(8):731-736. [22] 刘子铭,于亮.Irisin:新的运动因子?[J].生理科学进展,2018,49(3): 207-211. [23] ALIZADEH PAHLAVANI H. Exercise Therapy for People With Sarcopenic Obesity: Myokines and Adipokines as Effective Actors. Front Endocrinol (Lausanne). 2022;13:811751. [24] ROGGIO F, PETRIGNA L, TROVATO B, et al. The Role of Lubricin, Irisin and Exercise in the Prevention and Treatment of Osteoarthritis. Int J Mol Sci. 2023;24(6):5126. [25] COLAIANNI G, CUSCITO C, MONGELLI T, et al. The myokine irisin increases cortical bone mass. Proc Natl Acad Sci U S A. 2015;112(39): 12157-12162. [26] REZA MM, SIM CM, SUBRAMANIYAM N, et al. Irisin treatment improves healing of dystrophic skeletal muscle. Oncotarget. 2017;8(58):98553-98566. [27] QIAO X, NIE Y, MA Y, et al. Irisin promotes osteoblast proliferation and differentiation via activating the MAP kinase signaling pathways. Sci Rep. 2016;6(1):18732. [28] PONZETTI M, RUCCI N. Osteoblast Differentiation and Signaling: Established Concepts and Emerging Topics. Int J Mol Sci. 2021;22(13): 6651. [29] NING K, WANG Z, ZHANG XA. Exercise-induced modulation of myokine irisin in bone and cartilage tissue—Positive effects on osteoarthritis: A narrative review. Front Aging Neurosci. 2022;14:934406. [30] PEREIRA LJ, ANDRADE EF, BARROSO LC, et al. Irisin effects on bone: systematic review with meta-analysis of preclinical studies and prospects for oral health. Braz Oral Res. 2022;36:e055. [31] COLAIANNI G, SANESI L, STORLINO G, et al. Irisin and Bone: From Preclinical Studies to the Evaluation of Its Circulating Levels in Different Populations of Human Subjects. Cells. 2019;8(5):451. [32] YAN J, LIU HJ, GUO WC, et al. Low serum concentrations of Irisin are associated with increased risk of hip fracture in Chinese older women. Joint Bone Spine. 2018;85(3):353-358. [33] 章森,邹勇,漆正堂,等.鸢尾素介导运动干预神经精神疾病的潜在机制[J].上海体育学院学报,2023,47(4):39-50. [34] PENG J, WU J. Effects of the FNDC5/Irisin on Elderly Dementia and Cognitive Impairment. Front Aging Neurosci. 2022;14:863901. [35] CHEN K, WANG K, WANG T. Protective effect of irisin against Alzheimer’s disease. Front Psychiatry. 2022;13:967683. [36] 韦涛.运动激素鸢尾素在阿尔茨海默病中的作用机制[J].湖北体育科技,2023,42(10):945-951,972. [37] BELLETTINI-SANTOS T, BATISTA-SILVA H, MARCOLONGO-PEREIRA C, et al. Move Your Body toward Healthy Aging: Potential Neuroprotective Mechanisms of Irisin in Alzheimer’s Disease. Int J Mol Sci. 2023;24(15): 12440. [38] VLIORA M, NINTOU E, KARLIGIOTOU E, et al. Implication of Irisin in Different Types of Cancer: A Systematic Review and Meta-Analysis.Int J Mol Sci. 2022;23(17):9971. [39] GANNON NP, VAUGHAN RA, GARCIA-SMITH R, et al. Effects of the exercise-inducible myokine irisin on malignant and non-malignant breast epithelial cell behavior in vitro. Int J Cancer. 2015;136(4):E197-202. [40] SHI G, TANG N, QIU J, et al. Irisin stimulates cell proliferation and invasion by targeting the PI3K/AKT pathway in human hepatocellular carcinoma. Biochem Biophys Res Commun. 2017;493(1):585-591. [41] GAGGINI M, CABIATI M, DEL TURCO S, et al. Increased FNDC5/Irisin expression in human hepatocellular carcinoma. Peptides. 2017;88:62-66. [42] HOJMAN P, DETHLEFSEN C, BRANDT C, et al. Exercise-induced muscle-derived cytokines inhibit mammary cancer cell growth. Am J Physiol Endocrinol Metab. 2011;301(3):E504-E510. [43] MOON HS, MANTZOROS CS. Regulation of cell proliferation and malignant potential by irisin in endometrial, colon, thyroid and esophageal cancer cell lines. Metabolism. 2014;63(2):188-193. [44] 王姝琪,王晓峰.鸢尾素临床应用研究进展[J].中国现代医学杂志,2021,31(7):50-53. [45] PARK EJ, MYINT PK, ITO A, et al. Integrin-Ligand Interactions in Inflammation, Cancer, and Metabolic Disease: Insights Into the Multifaceted Roles of an Emerging Ligand Irisin. Front Cell Dev Biol. 2020;8:588066. [46] HUDIS CA, JONES L. Promoting exercise after a cancer diagnosis: easier said than done. Br J Cancer. 2014;110(4):829-830. [47] 潘如昕,张美凤.肌肉因子鸢尾素与心脑血管疾病[J].心血管病学进展,2018,39(4):591-594.
[48] ZHU D, ZHANG X, WANG F, et al. Irisin rescues diabetic cardiac microvascular injury via ERK1/2/Nrf2/HO-1 mediated inhibition of oxidative stress. Diabetes Res Clin Pract. 2022;183:109170. [49] LEONI DE SOUSA RA, IMPROTA-CARIA AC, DE FREITAS SOUZA BS. Exercise-Linked Irisin: Consequences on Mental and Cardiovascular Health in Type 2 Diabetes. Int J Mol Sci. 2021;22(4):2199. [50] 高荣荣,刘高虹.新型脂肪因子与糖尿病肾脏疾病发生发展关系的研究进展[J].山东医药,2023,63(22):91-94. [51] KURDIOVA T, BALAZ M, VICIAN M, et al. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: in vivo and in vitro studies. J Physiol. 2014; 592(5):1091-1107. [52] 王珅,廖静雯,尹洪刚,等.鸢尾素的心血管保护作用及其在运动领域的研究进展[J].生理学报,2019,71(3):478-484. [53] LIU C, WEI A, WANG T. Irisin, an Effective Treatment for Cardiovascular Diseases? J Cardiovasc Dev Dis. 2022;9(9):305. [54] ARCIDIACONO T, MAGNI G, MACRINA L, et al. Serum Irisin May Predict Cardiovascular Events in Elderly Patients With Chronic Kidney Disease Stage 3-5. J Ren Nutr. 2022;32(3):282-291. [55] SHELBAYA S, ABU SHADY MM, NASR MS, et al. Study of Irisin Hormone Level in Type 2 Diabetic Patients and Patients with Diabetic Nephropathy. Curr Diabetes Rev. 2018;14(5):481-486. [56] LIU Y, FU Y, LIU Z, et al. Irisin is induced in renal ischemia-reperfusion to protect against tubular cell injury via suppressing p53. Biochim Biophys Acta Mol Basis Dis. 2020;1866(7):165792. [57] ZHANG Y, ZHANG X, LIN S. Irisin: A bridge between exercise and neurological diseases. Heliyon. 2022;8(12):e12352. [58] RODRÍGUEZ-PÉREZ MDC, KONTRO TK, ALMEIDA GONZÁLEZ D, et al. Irisin is more strongly associated with leisure-time physical activity than resistin and high-density lipoprotein cholesterol are. J Exerc Sci Fit. 2022;20(4):366-371. [59] BILEK F, CETISLI-KORKMAZ N, ERCAN Z, et al. Aerobic exercise increases irisin serum levels and improves depression and fatigue in patients with relapsing remitting multiple sclerosis: A randomized controlled trial. Mult Scler Relat Disord. 2022;61:103742. [60] 陈甜甜,李宁川,王鸿伟,等.有氧运动及节食干预对高脂诱导肥胖大鼠鸢尾素水平的影响[J].杭州师范大学学报(自然科学版), 2021,20(5):542-550. [61] DASKALOPOULOU SS, COOKE AB, GOMEZ YH, et al. Plasma irisin levels progressively increase in response to increasing exercise workloads in young, healthy, active subjects. Eur J Endocrinol. 2014;171(3):343-352. [62] RASHTI B, MEHRABANI J, DAMIRCHI A, et al. The influence of concurrent training intensity on serum irisin and abdominal fat in postmenopausal women. Prz Menopauzalny. 2019;18(3):166-173. [63] COSIO PL, PELAEZ M, CADEFAU JA, et al. Systematic Review and Meta-Analysis of Circulating Irisin Levels Following Endurance Training: Results of Continuous and Interval Training. Biol Res Nurs. 2023;25(3):367-381. [64] TSUCHIYA Y, ANDO D, TAKAMATSU K, et al. Resistance exercise induces a greater irisin response than endurance exercise. Metabolism. 2015;64(9):1042-1050. [65] 左崇文.功能性和传统抗阻训练对男大学生肌肉适能和血清AMPK/PGC-1α/Irisin通路的影响[D]. 北京:首都体育学院,2024. [66] 蓝雯靖.下肢抗阻训练对轻度认知功能障碍老人血液因子CRP、IL-6及Irisin的影响研究[D].成都:成都体育学院,2024. [67] AMANAT S, SINAEI E, PANJI M, et al. A Randomized Controlled Trial on the Effects of 12 Weeks of Aerobic, Resistance, and Combined Exercises Training on the Serum Levels of Nesfatin-1, Irisin-1 and HOMA-IR. Front Physiol. 2020;11:562895. [68] WANG Y, WANG M, WANG Y. Irisin: A Potentially Fresh Insight into the Molecular Mechanisms Underlying Vascular Aging. Aging Dis. 2023. doi: 10.14336/AD.2023.1112. [69] HO MY, WEN MS, YEH JK, et al. Excessive irisin increases oxidative stress and apoptosis in murine heart. Biochem Biophys Res Commun. 2018;503(4):2493-2498. [70] WAHAB F, SHAHAB M, BEHR R. Hypothesis: Irisin is a metabolic trigger for the activation of the neurohormonal axis governing puberty onset. Med Hypotheses. 2016;95:1-4. [71] BAO JF, SHE QY, HU PP, et al. Irisin, a fascinating field in our times. Trends Endocrinol Metab. 2022;33(9):601-613. |
| [1] | Chen Jiayong, Tang Meiling, Lu Jianqi, Pang Yan, Yang Shangbing, Mao Meiling, Luo Wenkuan, Lu Wei, Zhou Jiatan. Based on Mendelian randomization, the causal relationship between 1400 metabolites and sarcopenia and the correlation analysis of cardiovascular disease were investigated [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-11. |
| [2] | Sun Yundi, Cheng Lulu, Wan Haili, Chang Ying, Xiong Wenjuan, Xia Yuan. Effect of neuromuscular exercise for knee osteoarthritis pain and function: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1945-1952. |
| [3] | Zhou Panpan, Cui Yinglin, Zhang Wentao, Wang Shurui, Chen Jiahui, Yang Tong . Role of cellular autophagy in cerebral ischemic injury and the regulatory mechanism of traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1650-1658. |
| [4] | Wang Qiuyue, Jin Pan, Pu Rui . Exercise intervention and the role of pyroptosis in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1667-1675. |
| [5] | Yuan Weibo, Liu Chan, Yu Limei. Potential application of liver organoids in liver disease models and transplantation therapy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1684-1692. |
| [6] | Wang Juan, Wang Guanglan, Zuo Huiwu. Efficacy of exercise therapy in the treatment of anterior cruciate ligament reconstruction patients: #br# a network meta-analysis #br# [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1714-1726. |
| [7] | Zheng Huakun, Yin Mingyue, Liu Qian. Effects of interval and continuous training on the quality of life in physically inactive adults: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1727-1740. |
| [8] | Lou Guo, Zhang Min, Fu Changxi. Exercise preconditioning for eight weeks enhances therapeutic effect of adipose-derived stem cells in rats with myocardial infarction [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1363-1370. |
| [9] | Xie Liugang, Cui Shuke, Guo Nannan, Li Aoyu, Zhang Jingrui. Research hotspots and frontiers of stem cells for Alzheimer’s disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1475-1485. |
| [10] | Zheng Rongfa, Mo Weibin, Huang Peng, Chen Junji, Liang Ting, Zi Fangyu, Li Guofeng. Effects of electroacupuncture on the expression of metabolic enzymes and autophagy genes in gastrocnemius muscle tissues of exercising rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1127-1136. |
| [11] |
Li Tian, Ren Yuhua, Gao Yanping, Su Qiang.
Mechanism of agomelatine alleviating anxiety- and depression-like behaviors in APP/PS1 transgenic mice #br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1176-1182.
|
| [12] | Zhao Xiaoxuan, Liu Shuaiyi, Li Qi, Xing Zheng, Li Qingwen, Chu Xiaolei. Different exercise modalities promote functional recovery after peripheral nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1248-1256. |
| [13] | Chen Yilin, Jiang Xiaobo, Qu Honglin, Liu Ruilian. General pattern of GSK3/Nrf2-regulated biological rhythms in organismal aging [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1257-1264. |
| [14] | Zhang Wenhua, Li Xun, Zhang Weichao, Li Xinying, Ma Guoao, Wang Xiaoqiang . Promoting myogenesis based on the SphK1/S1P/S1PR2 signaling pathway: a new perspective on improving skeletal muscle health through exercise [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1265-1275. |
| [15] | Qian Kun, Li Ziqing, Sun Shui . Endoplasmic reticulum stress in the occurrence and development of common degenerative bone diseases [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1285-1295. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||