Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (9): 1923-1930.doi: 10.12307/2025.238
Previous Articles Next Articles
Forkhead box transcription factor O1 signaling pathway in bone metabolism
Zhao Jiyu1, 2, Wang Shaowei2
- 1Shanxi Medical University, Taiyuan 030001, Shanxi Province, China; 2Department of Orthopedics, The Second Hospital of Shanxi Medical University, Taiyuan 030001, Shanxi Province, China
-
Received:2024-01-20Accepted:2024-02-24Online:2025-03-28Published:2024-10-11 -
Contact:Wang Shaowei, MD, Professor, Department of Orthopedics, The Second Hospital of Shanxi Medical University, Taiyuan 030001, Shanxi Province, China -
About author:Zhao Jiyu, Master candidate, Shanxi Medical University, Taiyuan 030001, Shanxi Province, China; Department of Orthopedics, The Second Hospital of Shanxi Medical University, Taiyuan 030001, Shanxi Province, China
CLC Number:
Cite this article
Zhao Jiyu, Wang Shaowei. Forkhead box transcription factor O1 signaling pathway in bone metabolism[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1923-1930.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

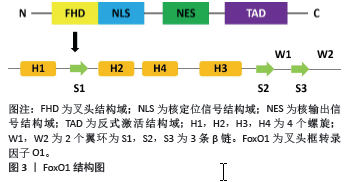
2.1 FoxO1的基本结构 FoxO1基因位于13号染色体上,翻译产生FoxO1蛋白。FoxO1蛋白包含4个功能结构域,包括核定位信号结构域(nuclear localization signal domain,NLS)、核输出信号结构域(nuclear export signal domain,NES)、反式激活结构域(transactivation domain,TAD)和叉头结构域(Forkhead domain,FHD)。FHD是发挥调节作用的主要结构域,由4个螺旋(H1,H2,H3和H4)、2个翼环(W1,W2)和3条β链(S1,S2,S3)组成,见图3。H3与DNA直接结合,起到DNA识别元件的作用,W1,S2和S3有助于稳定H3-DNA复合物的结构[6]。 FoxO1与下游DNA相互作用的复合位点是调节FoxO1活性的基础。晶体结构研究表明,FoxO1同时识别Daf-16结合元件和胰岛素反应序列[7],但其对Daf-16结合元件的亲和力更高[1]。 2.2 FoxO1的调节 上游的许多调节因子会调节FoxO1的转录活性,影响FoxO1蛋白的翻译和整合[8]。外源刺激会形成一个复杂的网络,通过多种信号通路调节FoxO1的活性。研究表明,FoxO1易受周围环境的影响,其活性与丰富度、转录后修饰、核质穿梭和亚细胞定位有关。 microRNAs是FoxO1的重要调节因子[9-10],直接影响FoxO1的mRNA水平,导致FoxO1蛋白的丰度降低。FoxO1蛋白的转录后修饰同样也可以调节FoxO1的活性,这些转录后修饰包括磷酸化和去磷酸化、乙酰化和去乙酰化、泛素化、O-GlcNA酰化和甲基化[11]。此外,它们还受到脂多糖、肿瘤坏死因子α以及蛋白伴侣的调节[12]。这些修饰通过作用于FoxO1的核输入和输出步骤,改变了FoxO1与DNA的亲和力,影响了FoxO1特异性靶基因的转录活性。 此外,FoxO1还可以调节下游的多种基因,参与氧化应激抵抗,细胞增殖、分化、衰老、凋亡、免疫和代谢调节等功能[13-14]。不同组织中FoxO1表达量的变化,可以直接或间接地引起各种生理和病理变化,从而导致疾病的发生。然而,如果FoxO1被隔离在细胞核中,就不能再调控位于细胞质中的下游靶点[15],从而阻断信号传导。FoxO1在多种细胞内广泛表达,可以维持组织稳态和细胞对各种刺激的反应,所以可能是预防和治疗疾病的重要靶点。因此,研究FoxO1的功能及其调节机制对于治疗骨相关疾病也具有非常重要的临床意义。"

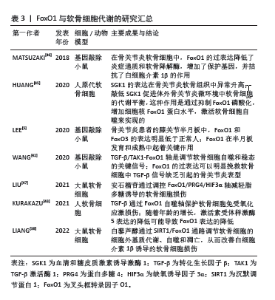
2.3 FoxO1与骨细胞 2.3.1 FoxO1与骨髓间充质干细胞 FoxO1在骨髓间充质干细胞的成骨过程中发挥着重要作用。骨髓间充质干细胞是多能基质细胞,可以产生成骨细胞、软骨细胞、肌肉细胞和脂肪细胞。骨髓间充质干细胞向成骨细胞的分化是由一系列特定的转录因子和基因调控的[16]。TEIXEIRA等[17]通过体内和体外实验证实,FoxO1是骨髓间充质干细胞向成骨细胞分化的早期正向调节因子。在小鼠胚胎间充质干细胞(C3H10T1/2 细胞)中,成骨刺激物会增强FoxO1的活性和表达。同样,过表达FoxO1会显著增加成骨标志物的表达,如 Runt相关转录因子2(runt-related transcription factor 2,Runx2)及碱性磷酸酶和骨钙素。相反,沉默FoxO1则会抑制这些成骨标志物的表达,减少钙化,影响骨骼生成和颅面部发育。此外,在离体小鼠胚胎胫骨中敲除FoxO1会导致长骨变短,骨矿化程度降低,这与在体内获得的结果一致[17]。在成骨前体细胞(如 MC3T3-E1细胞)中,FoxO1与Runx2基因启动子直接结合,其结合活性上调会在矿化结节形成过程中提高 Runx2的mRNA水平和碱性磷酸酶的活性。FoxO1与Runx2相互作用,可以促进骨髓间充质干细胞向成骨细胞分化,并触发早期祖细胞中主要骨基质蛋白基因(如Ⅰ型胶原或碱性磷酸酶)的表达[16]。因此,在某种程度上,FoxO1 是通过与Runx2或其基因启动子的相互作用来促进成骨细胞分化 的[18]。此外,骨形态发生蛋白2可以诱导FoxO1转录增加,除了通过Runx2的间接作用以外,FoxO1还可以与碱性磷酸酶基因的启动子直接结合从而提高碱性磷酸酶的活性[19],促进骨髓间充质干细胞向成骨细胞分化。 研究表明,过氧化物酶体增殖激活受体γ(peroxisome proliferator-activated receptor γ,PPARγ)可以促进脂肪细胞生成并抑制成骨细胞生成。FoxO1作为一种转录抑制因子,可以直接与PPAR响应元件(前脂肪细胞中PPARγ靶基因的启动子)结合,从而抑制 PPARγ,将骨髓间充质干细胞由成脂分化向成骨分化转化[20],从而改善骨形成。值得注意的是,在缺氧条件下,由于缺氧诱导因子的作用,也会导致PPARγ下调,但最终结果是导致成骨分化和成脂分化的共同抑制[21]。因此。关于FoxO1对PPARγ的作用仍需进一步的研究来确定,并且需要关注细胞所处的环境和条件。 2.3.2 FoxO1与成骨细胞 FoxO1对成骨细胞的影响也很复杂。有研究表明,敲除FoxO1可以增加骨形成[22],但其研究水平主要停留在动物实验层面,并没有进行深入的机制研究。目前大多数文献还是支持FoxO1促进成骨细胞形成的。适当的氧化应激可以促进成骨,但氧化应激过强则会明显抑制成骨。成骨细胞特异性缺失FoxO1会导致氧化应激明显增加,成骨细胞数量和骨量减少[23]。更准确地说,作为FoxO1的转录调控基因,当FoxO1缺失时,抗氧化基因(如超氧化物歧化酶2和过氧化氢酶)的表达降低,活性氧水平增加,过量的活性氧会对成骨细胞的存活产生有害影响。FoxO1失活会减弱其与激活转录因子4(activating transcription factor 4,ATF4)的相互作用,降低FoxO1的靶基因结合活性,阻碍ATF4介导的蛋白质合成[24]。随着蛋白质合成受阻,氧化还原平衡被打破,p19ARF(CDKN2A)和p16的表达增高,下游的靶蛋白p53被激活,成骨细胞的细胞周期被抑制,导致成骨细胞数量减少。此外,蛋白质合成受阻还会抑制谷胱甘肽的积累,进一步增强氧化应激[3],导致骨形成受阻。综上所述,FoxO1可以增强成骨细胞的活性,并通过诱导抗氧化剂的生成来保护成骨细胞,文章总结了FoxO1与骨形成的研究进展[17-20,23-26],见表1。"

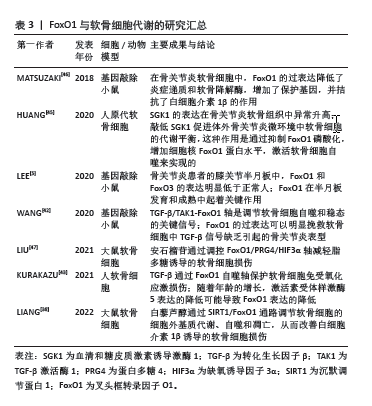
2.3.3 FoxO1与破骨细胞 骨髓单核-巨噬细胞是破骨细胞的前体细胞,破骨细胞生成受巨噬细胞集落刺激因子(macrophage colony-stimulating factor,M-CSF)和核因子κB受体激活剂配体(receptor activator of nuclear factor-κB ligand,RANKL)以及成骨细胞和破骨细胞分泌的其他细胞因子的调控,这些细胞因子控制着骨髓单核-巨噬细胞的增殖、分化和成熟[27]。在骨髓单核-巨噬细胞中,M-CSF可以刺RANKL的表达,从而促进破骨细胞分化[28],RANKL也可以间接诱导骨髓单核-巨噬细胞表达活化 T 细胞核因子1(nuclear factor of activated T cells 1,NFATc1),从而进一步促进破骨细胞的分化[29]。骨髓单核-巨噬细胞中FoxO1的缺失会减少M-CSF诱导的RANKL表达和骨髓单核-巨噬细胞的迁移[30],减少NFATc1 的表达和核定位[31],从而抑制破骨细胞的生成。因此,FoxO1是通过M-CSF和RANKL来影响破骨细胞的形成和分化的。 TAN等[32]研究表明,FoxO1是破骨细胞生成的抑制因子,在RANKL的刺激下,FoxO1的表达减少;抑制FoxO1可以促进破骨细胞分化并增强破骨细胞的活性,而激活FoxO1作用则相反;FoxO1对破骨细胞生成的抑制作用部分是由活性氧介导的,通过作用于丝裂原激活蛋白激酶(mitogen-activated protein kinases,MAPKs)、核转录因子κB和激活蛋白1(activator protein-1,AP-1)来抑制破骨细胞生成。此外,FoxO1对破骨细胞生成的抑制作用部分是通过抑制转录因子MYC来介导的。 有趣的是,FoxO1还能通过成骨细胞间接抑制破骨细胞的生成。小鼠软骨细胞中条件性缺失 FoxO1会增加破骨细胞数量[33]。成骨细胞中FoxO1活化会导致成骨细胞数量增加,成骨细胞产生骨保护素,骨保护素与RANKL结合并中和RANKL,阻止破骨细胞形成和骨吸收[34]。在小鼠体内成骨细胞中靶向敲除FoxO1可减少骨保护素的表达,在体外成骨细胞中激活FoxO1会增加骨保护素的表达[35]。因此,FoxO1可以通过增加成骨细胞中骨保护素的表达来控制破骨细胞的数量。 总之,由于FoxO1对骨髓单核-巨噬细胞以及破骨细胞作用的多样性和复杂性,导致FoxO1对破骨细胞的作用结果一直都存在争议,但大多数文献都表明FoxO1负性调节破骨细胞的生成,见表2。 2.3.4 FoxO1与软骨细胞 在人软骨细胞中,沉默FoxO1会降低细胞对活性氧的抵抗力,导致抗氧化蛋白减少,细胞内氧化应激增加,同时会导致LC3和Beclin1等自噬相关蛋白升高,增加活性氧诱导的细胞凋亡[36]。骨关节炎软骨中沉默调节蛋白1(sirtuin 1,SIRT-1)表达减少[37],下调FoxO1也可以抑制SIRT-1。SIRT-1通过调节FoxO1和p53的信号转导来调节软骨细胞的氧化应激和自噬[38],SIRT-1可以通过减少蛋白酪氨酸磷酸酶1B从而提高人软骨细胞的存活率[39],还可以通过诱导自噬相关基因ATG5,7,8的去乙酰化来直接调控软骨细胞自噬[40-41]。总之,FoxO1对软骨细胞的保护作用在一定程度上是通过SIRT-1介导的。 转化生长因子β是软骨细胞的重要保护因子,转化生长因子β可以增加FoxO1的表达,通过转化生长因子β激活酶1诱导合成代谢,增加自噬相关基因的表达,从而保护软骨细胞免受氧化应激损伤[42-43]。此外,血清和糖皮质激素诱导激酶1也可以和FoxO1直接结合并增强FoxO1的磷酸化,降低细胞核内的FoxO1蛋白水平,从而加重软骨细胞凋亡[44-45]。 总之,FoxO1通过减少软骨形成过程中的活性氧积累,改变SIRT-1蛋白水平以及减少炎症反应,从而抑制软骨细胞的凋亡[46-47],见表3。 2.4 FoxO1与骨相关疾病 2.4.1 FoxO1与骨关节炎 FoxO1可以通过调节软骨细胞的功能来延缓骨关节炎的进展。研究表明,FoxO1 在老年骨关节炎患者软骨中的表达减少,尤其是在负重区软骨表层。同样,在纤维化区和骨质增生区,FoxO1的表达也明显降低,这揭示了骨关节炎进展与FoxO1表达之间的显著相关性[5]。动物实验表明,随着小鼠年龄的增加,FoxO1的表达逐渐减少,FoxO1表达较低的软骨细胞聚集在未被半月板覆盖的软骨中[46]。除软骨退化外,半月板损伤也是膝关节骨关节炎的主要病因。在人类半月板中,FoxO1和FoxO3 的表达量高于FoxO4,而且FoxO1和FoxO3在整个半月板区域的表达量相似。在成年小鼠中,FoxO1和FoxO3的表达量较高;而在退化的半月板中,FoxO1和FoxO3的表达受到抑制,这表明它们在骨关节炎进展过程中具有保护作用[5]。 FoxO1可调节软骨细胞稳态,包括软骨细胞的凋亡和自噬、炎症、抗氧化防御和细胞外基质的降解等。蛋白多糖4是关节软骨和半月板表层滑膜软骨细胞的分泌物,起到润滑关节的作用,其表达受FoxO1的调节[47];在FoxO1 KO 小鼠中,蛋白多糖4表达量较低,导致软骨更容易受到机械应力损伤。此外,FoxO1缺陷动物模型中肥大相关基因表达异常增加,会导致软骨细胞形态肥大。因此,FoxO1在软骨细胞中的抑制可能会导致软骨降解和钙化、细胞自噬功能受损和蛋白多糖4减少,从而加重骨关节炎[48]。 2.4.2 FoxO1与骨质疏松症 大量文献表明,FoxO1可以通过调节成骨细胞的功能来影响骨质疏松症的进展,但FoxO1对于骨质疏松症的作用结果仍然存在争议。骨质疏松症是一种低骨量疾病,与成骨细胞数量减少和成骨细胞内氧化应激水平升高有关。RACHED等[25]利用细胞特异性缺失和分子分析表明,只有 FoxO1是成骨细胞增殖和氧化还原平衡所必需的。FoxO1对成骨细胞增殖的调控是通过它与ATF4和p53的相互作用来实现的。这些结果确定了FoxO1 是成骨细胞的关键调节因子,并提供了氧化应激与骨重塑调节之间的直接联系。 但也有研究得出与其相反的结论,LYER等[26]研究表明,FoxO1,FoxO3和FoxO4的条件敲除小鼠中成骨细胞的数量和骨量增加,这是Wnt/β-catenin信号和细胞周期蛋白D1表达上调的结果,这可能与FoxOs家族作用的多样性和复杂性有关。有研究表明,在幼年小鼠的成骨细胞中敲除FoxO1基因会导致骨质流失,而在老年小鼠中则会出现相反的结果。在幼年小鼠中,FoxO1基因缺失会抑制成骨细胞的分化,导致成骨细胞数量减少;除此之外,FoxO1缺失还会导致成骨细胞抵抗氧化应激的能力减弱,骨形成率降低,最终引起骨丢失和骨缺损愈合延迟。而在老年小鼠中,由于高水平活性氧的刺激,FoxO1会与T细胞因子4竞争β-catenin,从而抑制Wnt/β-catenin 信号通路,导致骨形成率降低。FoxO1的缺乏会间接促进β-catenin和T细胞因子4的结合,激活 Wnt/β-catenin信号转导,从而缓解老年小鼠的骨质流失,改善骨缺损愈合[23]。该项研究说明FoxO1对不同年龄阶段小鼠的骨代谢存在不同影响,并阐明了其可能的机制。此外,这项研究还为治疗骨质疏松症提供了新的视角,那就是造成不同年龄阶段人群骨质流失的原因可能是不同的,在治疗过程中需要采用个体化的方案。 2.4.3 FoxO1与骨折愈合 骨折愈合是一个以炎症、软骨形成、骨沉积和重塑为特征的多阶段过程,除了成骨细胞和破骨细胞以外,软骨细胞、血管内皮细胞在骨折愈合过程中也起到了重要作用。软骨内骨化是骨折愈合的重要过程。FoxO1对骨折愈合的影响也是复杂的。DING等[33]通过选择性敲除FoxO1研究了FoxO1对骨折愈合的作用。他们发现在骨折愈合的早期,FoxO1基因缺失会显著增加软骨的形成,而在后期,FoxO1基因缺失则会导致软骨的大量丢失。从机制上讲,骨折愈合早期,FoxO1基因的缺失与软骨细胞增殖增加有关;骨折愈合晚期,FoxO1基因缺失会导致小鼠软骨损失增加,破骨细胞形成增强,白细胞介素6表达增加,M2 巨噬细胞数量减少。这些结果表明FoxO1通过限制软骨的早期扩张和防止后期软骨的快速流失来调节软骨细胞的行为。在软骨内成骨过程中,软骨细胞的大量丢失和破骨细胞作用的异常增强都是不利的,因此,FoxO1的存在及其功能的正常发挥对于骨折愈合具有重要意义。 软骨细胞凋亡是骨折愈合和长骨生长过程中软骨向骨过渡的一个重要过程,这一过程由肿瘤坏死因子α和白细胞介素1β等细胞因子诱导[49]。软骨细胞可以产生RANKL,在骨形态发生蛋白2的作用下调节生长板破骨细胞生成,清除钙化基质[50]。骨折局部促炎细胞因子肿瘤坏死因子α的表达增加,会导致FoxO1的DNA结合活性以及FoxO1在体内软骨细胞中的核转位增强,最终导致软骨细胞凋亡。因此,过量的肿瘤坏死因子α会导致大量的软骨细胞凋亡,不利于骨折愈合;而适当的肿瘤坏死因子α刺激会导致软骨向骨转化,有利于骨折愈合,并且这一过程是通过FoxO1介导的[51]。 血管生成在骨折愈合过程中也发挥了重要作用。ZHANG等[52]研究表明,软骨细胞中过表达FoxO1可以增加血管内皮生长因子A的表达,并且FoxO1是与血管内皮生长因子A的启动子直接结合的。此外,去乙酰化的FoxO1突变体会增强血管内皮生长因子A的表达,而乙酰化的 FoxO1 突变体则不会。最后,他们还发现,敲除FoxO1能显著降低软骨细胞在体外刺激微血管形成的能 力[53]。鉴于血管形成在骨折修复过程中的重要作用,这些研究为骨折愈合提供了新的见解,开发针对FoxO1的药物可以从多个角度起到促进骨折愈合的作用。 "
| [1] LINK W. Introduction to FOXO biology. Methods Mol Biol. 2019;1890:1-9. [2] OREA-SOUFI A, PAIK J, BRAGANÇA J, et al. FOXO transcription factors as therapeutic targets in human diseases. Trends Pharmacol Sci. 2022;43(12): 1070-1084. [3] MA X, SU P, YIN C, et al. The roles of FoxO transcription factors in regulation of bone cells function. Int J Mol Sci. 2020;21(3):692. [4] GRAVES DT, MILOVANOVA TN. Mucosal immunity and the FOXO1 transcription factors. Front Immunol. 2019;10:2530. [5] LEE KI, CHOI S, MATSUZAKI T, et al. FOXO1 and FOXO3 transcription factors have unique functions in meniscus development and homeostasis during aging and osteoarthritis. Proc Natl Acad Sci U S A. 2020;117(6):3135-3143. [6] BYRNES K, BLESSINGER S, BAILEY NT, et al. Therapeutic regulation of autophagy in hepatic metabolism. Acta Pharm Sin B. 2022;12(1):33-49. [7] THIEL G, GUETHLEIN LA, RÖSSLER OG. Insulin-responsive transcription factors. Biomolecules. 2021;11(12):1886. [8] ADIGUZEL D, CELIK-OZENCI C. FoxO1 is a cell-specific core transcription factor for endometrial remodeling and homeostasis during menstrual cycle and early pregnancy. Hum Reprod Update. 2021;27(3):570-583. [9] CHEN J, LU Y, TIAN M, et al. Molecular mechanisms of FOXO1 in adipocyte differentiation. J Mol Endocrinol. 2019;62(3):R239-R253. [10] YU W, CHEN C, CHENG J. The role and molecular mechanism of FoxO1 in mediating cardiac hypertrophy. ESC Heart Fail. 2020;7(6):3497-3504. [11] XING YQ, LI A, YANG Y, et al. The regulation of FOXO1 and its role in disease progression. Life Sci. 2018;193:124-131. [12] GUO LT, WANG SQ, SU J, et al. Baicalin ameliorates neuroinflammation-induced depressive-like behavior through inhibition of toll-like receptor 4 expression via the PI3K/AKT/FoxO1 pathway. J Neuroinflammation. 2019;16(1):95. [13] MURTAZA G, KHAN AK, RASHID R, et al. FOXO Transcriptional factors and long-term living. Oxid Med Cell Longev. 2017;2017:3494289. [14] WANG S, XIA P, HUANG G, et al. FoxO1-mediated autophagy is required for NK cell development and innate immunity. Nat Commun. 2016;7:11023. [15] SCHÄLL D, SCHMITT F, REIS B, et al. SLy1 regulates T-cell proliferation during Listeria monocytogenes infection in a Foxo1-dependent manner. Eur J Immunol. 2015;45(11):3087-3097. [16] CHEN D, GONG Y, XU L, et al. Bidirectional regulation of osteogenic differentiation by the FOXO subfamily of Forkhead transcription factors in mammalian MSCs. Cell Prolif. 2019;52(2):e12540. [17] TEIXEIRA CC, LIU Y, Thant LM, et al. Foxo1, a novel regulator of osteoblast differentiation and skeletogenesis. J Biol Chem. 2010;285(40):31055-31065. [18] NOMURA K, KIMIRA Y, OSAWA Y, et al. Stimulation of the Runx2 P1 promoter by collagen-derived dipeptide prolyl-hydroxyproline bound to Foxg1 and Foxo1 in osteoblasts. Biosci Rep. 2021;41(12):BSR20210304. [19] SIQUEIRA MF, FLOWERS S, BHATTACHARYA R, et al. FOXO1 modulates osteoblast differentiation. Bone. 2011;48(5):1043-1051. [20] WANG D, WANG Y, ZOU X, et al. FOXO1 inhibition prevents renal ischemia-reperfusion injury via cAMP-response element binding protein/PPAR-γ coactivator-1α-mediated mitochondrial biogenesis. Br J Pharmacol. 2020; 177(2):432-448. [21] TAMAMA K, KAWASAKI H, KERPEDJIEVA SS, et al. Differential roles of hypoxia inducible factor subunits in multipotential stromal cells under hypoxic condition. J Cell Biochem. 2011;112(3):804-817. [22] XIONG Y, ZHANG Y, GUO Y, et al. 1α, 25-Dihydroxyvitamin D(3) increases implant osseointegration in diabetic mice partly through FoxO1 inactivation in osteoblasts. Biochem Biophys Res Commun. 2017;494(3-4):626-633. [23] XIONG Y, ZHANG Y, ZHOU F, et al. FOXO1 differentially regulates bone formation in young and aged mice. Cell Signal. 2022;99:110438. [24] KODE A, MOSIALOU I, SILVA BC, et al. FoxO1 protein cooperates with ATF4 protein in osteoblasts to control glucose homeostasis. J Biol Chem. 2012, 287(12):8757-68. [25] RACHED MT, KODE A, XU L, et al. FoxO1 is a positive regulator of bone formation by favoring protein synthesis and resistance to oxidative stress in osteoblasts. Cell Metab. 2010;11(2):147-160. [26] IYER S, AMBROGINI E, BARTELL SM, et al. FOXOs attenuate bone formation by suppressing Wnt signaling. J Clin Invest. 2013;123(8):3409-3019. [27] BUCK DW 2ND, DUMANIAN GA. Bone biology and physiology: Part I. The fundamentals. Plast Reconstr Surg. 2012;129(6):1314-1320. [28] UDAGAWA N, KOIDE M, NAKAMURA M, et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J Bone Miner Metab. 2021;39(1):19-26. [29] NAKASHIMA T, HAYASHI M, TAKAYANAGI H. New insights into osteoclastogenic signaling mechanisms. Trends Endocrinol Metab. 2012;23(11):582-590. [30] WANG Y, DONG G, JEON HH, et al. FOXO1 mediates RANKL-induced osteoclast formation and activity. J Immunol. 2015;194(6):2878-2887. [31] DENG W, DING Z, WANG Y, et al. Dendrobine attenuates osteoclast differentiation through modulating ROS/NFATc1/ MMP9 pathway and prevents inflammatory bone destruction. Phytomedicine. 2022;96:153838. [32] TAN P, GUAN H, XIE L, et al. FOXO1 inhibits osteoclastogenesis partially by antagnozing MYC. Sci Rep. 2015;5:16835. [33] DING Z, QIU M, ALHARBI MA, et al. FOXO1 expression in chondrocytes modulates cartilage production and removal in fracture healing. Bone. 2021;148:115905. [34] JING Z, WANG C, WEN S, et al. Phosphocreatine promotes osteoblastic activities in H(2)O(2)-induced MC3T3-E1 cells by regulating SIRT1/FOXO1/PGC-1α signaling pathway. Curr Pharm Biotechnol. 2021;22(5):609-621. [35] AMEEN O, YASSIEN RI, NAGUIB YM. Activation of FoxO1/SIRT1/RANKL/OPG pathway may underlie the therapeutic effects of resveratrol on aging-dependent male osteoporosis. BMC Musculoskelet Disord. 2020;21(1):375. [36] SHARIEH F, EBY JM, ROPER PM, et al. Ethanol inhibits mesenchymal stem cell osteochondral lineage differentiation due in part to an activation of forkhead box protein O-specific signaling. Alcohol Clin Exp Res. 2020; 44(6):1204-1213. [37] DENG Z, LI Y, LIU H, et al. The role of sirtuin 1 and its activator, resveratrol in osteoarthritis. Biosci Rep. 2019;39(5):BSR20190189. [38] LIANG C, XING H, WANG C, et al. Resveratrol protection against IL-1β-induced chondrocyte damage via the SIRT1/FOXO1 signaling pathway. J Orthop Surg Res. 2022;17(1):406. [39] ZHU S, BENNETT S, LI Y, et al. The molecular structure and role of LECT2 or CHM-II in arthritis, cancer, and other diseases. J Cell Physiol. 2022;237(1): 480-488. [40] NG F, TANG BL. Sirtuins’ modulation of autophagy. J Cell Physiol. 2013; 228(12):2262-2270. [41] SACITHARAN PK, BOU-GHARIOS G, EDWARDS JR. SIRT1 directly activates autophagy in human chondrocytes. Cell Death Discov. 2020;6:41. [42] WANG C, SHEN J, YING J, et al. FoxO1 is a crucial mediator of TGF-β/TAK1 signaling and protects against osteoarthritis by maintaining articular cartilage homeostasis. Proc Natl Acad Sci U S A. 2020;117(48):30488-30497. [43] KURAKAZU I, AKASAKI Y, TSUSHIMA H, et al. TGFβ1 signaling protects chondrocytes against oxidative stress via FOXO1-autophagy axis. Osteoarthritis Cartilage. 2021;29(11):1600-1613. [44] 黄威.SGK1调控骨关节炎软骨细胞合成和分解代谢的分子机制研究[D].合肥:安徽医科大学,2019. [45] HUANG W, CHENG C, SHAN WS, et al. Knockdown of SGK1 alleviates the IL-1β-induced chondrocyte anabolic and catabolic imbalance by activating FoxO1-mediated autophagy in human chondrocytes. FEBS J. 2020;287(1):94-107. [46] MATSUZAKI T, ALVAREZ-GARCIA O, MOKUDA S, et al. FoxO transcription factors modulate autophagy and proteoglycan 4 in cartilage homeostasis and osteoarthritis. Sci Transl Med. 2018;10(428):eaan0746. [47] LIU F, YANG H, LI D, et al. Punicalagin attenuates osteoarthritis progression via regulating Foxo1/Prg4/HIF3α axis. Bone. 2021;152:116070. [48] SUN Y, MAUERHAN DR. Meniscal calcification, pathogenesis and implications. Curr Opin Rheumatol. 2012;24(2):152-157. [49] WANG J, ZHANG Y, CAO J, et al. The role of autophagy in bone metabolism and clinical significance. Autophagy. 2023;19(9):2409-2427. [50] PARK SY, KIM KH, KIM S, et al. BMP-2 gene delivery-based bone regeneration in dentistry. Pharmaceutics. 2019;11(8):393. [51] ALHARBI MA, ZHANG C, LU C, et al. FOXO1 deletion reverses the effect of diabetic-induced impaired fracture healing. Diabetes. 2018;67(12): 2682-2694. [52] ZHANG C, FEINBERG D, ALHARBI M, et al. Chondrocytes promote vascularization in fracture healing through a FOXO1-dependent mechanism. J Bone Miner Res. 2019;34(3):547-556. [53] 张赐童.软骨细胞通过转录因子FoxO1促进骨折愈合中血管的形成[D].长春:吉林大学,2019. [54] 董书琴,卢宇,高菲,等.FoxO1在糖脂代谢机制中的研究进展[J].大连医科大学学报,2021,43(5):445-450. [55] 谭鹏.转录因子FOXO1对破骨细胞分化及功能影响的实验研究[D].武汉:华中科技大学,2017. [56] OHZONO H, HU Y, NAGIRA K, et al. Targeting FoxO transcription factors with HDAC inhibitors for the treatment of osteoarthritis. Ann Rheum Dis. 2023;82(2):262-271. |
| [1] | Ma Chi, Wang Ning, Chen Yong, Wei Zhihan, Liu Fengji, Piao Chengzhe. Application of 3D-printing patient-specific instruments combined with customized locking plate in opening wedge high tibial osteotomy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1863-1869. |
| [2] | Han Haihui, Ran Lei, Meng Xiaohui, Xin Pengfei, Xiang Zheng, Bian Yanqin, Shi Qi, Xiao Lianbo. Targeting fibroblast growth factor receptor 1 signaling to improve bone destruction in rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1905-1912. |
| [3] | Yu Shuai, Liu Jiawei, Zhu Bin, Pan Tan, Li Xinglong, Sun Guangfeng, Yu Haiyang, Ding Ya, Wang Hongliang. Hot issues and application prospects of small molecule drugs in treatment of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1913-1922. |
| [4] | Sun Yundi, Cheng Lulu, Wan Haili, Chang Ying, Xiong Wenjuan, Xia Yuan. Effect of neuromuscular exercise for knee osteoarthritis pain and function: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1945-1952. |
| [5] | Deng Keqi, Li Guangdi, Goswami Ashutosh, Liu Xingyu, He Xiaoyong. Screening and validation of Hub genes for iron overload in osteoarthritis based on bioinformatics [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1972-1980. |
| [6] | Zhou Jinhai, Li Jiangwei, Wang Xuquan, Zhuang Ying, Zhao Ying, Yang Yuyong, Wang Jiajia, Yang Yang, Zhou Shilian. Three-dimensional finite element analysis of anterior femoral notching during total knee arthroplasty at different bone strengths [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1775-1782. |
| [7] | Wang Qiuyue, Jin Pan, Pu Rui . Exercise intervention and the role of pyroptosis in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1667-1675. |
| [8] | Zhu Hanmin, Wang Song, Xiao Wenlin, Zhang Wenjing, Zhou Xi, He Ye, Li Wei, . Mitophagy regulates bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1676-1683. |
| [9] | Zhao Jiacheng, Ren Shiqi, Zhu Qin, Liu Jiajia, Zhu Xiang, Yang Yang. Bioinformatics analysis of potential biomarkers for primary osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1741-1750. |
| [10] | Chen Yueping, Chen Feng, Peng Qinglin, Chen Huiyi, Dong Panfeng . Based on UHPLC-QE-MS, network pharmacology, and molecular dynamics simulation to explore the mechanism of Panax notoginseng in treating osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1751-1760. |
| [11] | Yin Lu, Jiang Chuanfeng, Chen Junjie, Yi Ming, Wang Zihe, Shi Houyin, Wang Guoyou, Shen Huarui. Effect of Complanatoside A on the apoptosis of articular chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1541-1547. |
| [12] | Wang Wentao, Hou Zhenyang, Wang Yijun, Xu Yaozeng. Apelin-13 alleviates systemic inflammatory bone loss by inhibiting macrophage M1 polarization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1548-1555. |
| [13] | Chen Shuai, Jin Jie, Han Huawei, Tian Ningsheng, Li Zhiwei . Causal relationship between circulating inflammatory cytokines and bone mineral density based on two-sample Mendelian randomization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1556-1564. |
| [14] | Wang Peiguang, Zhang Xiaowen, Mai Meisi, Li Luqian, Huang Hao. Generalized equation estimation of the therapeutic effect of floating needle therapy combined with acupoint embedding on different stages of human knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1565-1571. |
| [15] | Liu Qi, Li Linzhen, Li Yusheng, Jiao Hongzhuo, Yang Cheng, Zhang Juntao. Icariin-containing serum promotes chondrocyte proliferation and chondrogenic differentiation of stem cells in the co-culture system of three kinds of cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1371-1379. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||